Abstract
In the present study we investigated the developmental expression patterns of Lbx1 gene in skeletal muscle of different genetic profile pigs after birth. A total of 28 Mashen pigs and 28 Large White pigs from seven development stages of 1, 30, 60, 90, 120, 150, 180 d were used and the expression patterns in longissimus dorsi were studied by quantitative real time-polymerase chain reaction and western blot in this study. The results showed that the breed, age, and the interaction of breed and age significantly affected the Lbx1 mRNA and protein expressions in longissimus dorsi (P<0.05). In Large White, the amount of Lbx1 protein was increased from new birth to 30 d, and decreased after 30 d to the lowest level at 180 d. In Mashen pig, Lbx1 protein level showed an increase trend with the age increase before 90 d, then decreased to the lowest level at 120 d, and then gradually increased upwards to the expression peak at 180 d. Before 120 d, the Lbx1 protein content in Large White was higher than that in Mashen pig, except for at 60 d. Afterwards, the protein level in Mashen was higher than that in Large White. The Lbx1 expression pattern was related to the age and genetic profiles of Mashen and Large White pigs, which indicates that Lbx1 could participate in the muscle development through activating the satellite cells of skeletal muscle after birth.
Introduction
Lbx (ladybird-like), originated from a part of Nk homeobox cluster of metazoan, encodes transcription factors which can regulate lots of tissues development, including skeletal muscle, heart, central nervous system and sensory organs (Wotton et al., Citation2009). Lbx family contains 4 members, which are divergent in different vertebrates. Two members (Lbx1 and Lbx2) were found expressed in bony of vertebrates, and played an essential role during muscle and neural development (Pollard and Holland, Citation2000). Lbx2 was undetectable in chicken, but a novel homeobox gene Lbx3 was detected and expressed in prospective hypaxial myoblasts at cervical and limb level (Kanamoto et al., Citation2006).
Lbx1 was mainly expressed in the embryo, central nervous system and skeletal muscle, and was essential for the development of these tissues (Chen et al., Citation1999). During myogenesis in mammals and amphibians, Lbx1 was necessary for the the migration of muscle precursor cells (Jagla et al., Citation1997; Schafer and Braun, Citation1999; Gross, et al., Citation2002; Schafer et al., Citation2003; Cheng et al., Citation2005; Martin and Harland, Citation2006; Wotton et al., Citation2008; Schmitteckert et al., Citation2011;). Previous studies have demonstrated that Lbx1 and Pax3 were co-expressed in all migration hypaxial muscle precursors (Gross, et al., Citation2000). Knockdown of Lbx1 was associated with a specific reduction of body wall muscles and hypoglossal muscles originating from the somites (Martin and Harland, Citation2006). In Lbx1 mutant mice, muscle precursor cells of limb delaminated from the ventral dermomyotome and failed to migrate laterally into the limb (Gross et al., Citation2000). In addition, Lbx1 was also involved in cell proliferation through the downregulation of myoD and p27 (Martin and Harland, Citation2006). In explanted somites in chicken, overexpression of Lbx1 and Pax3 could increase the cell proliferation dramatically (Mennerich and Braun, Citation2001). In activated satellite cells of adult mice, Lbx 1 was also detected, which might suggest its roles in the differentiation of satellite cells in mature muscle fibres (Gross et al., Citation2000; Watanabe et al., Citation2007). In six-month old pigs, Lbx 1 was also expressed in skeletal and cardiac muscles (Chao et al., Citation2011).
Mashen pig is a local breed in Shanxi Province and other regions of Northen China for thousands of years. Owing to the varied terrain, mountainous environment, natural economic and ecological conditions of Shanxi Province, the Mashen pig breed has genetic diversity, excellent adaptability and good meat quality. Furthermore, Mashen pig can maintain normal reproduction ability even while consuming a low level of nutrients and living in a cold environment (Zhang et al., Citation2001). However, growth rate and food conversion are lower compared to the western commercial pig breeds, such as Large White, Landrace, and Duroc (Yang et al., Citation2005).
Previous studies related to the function of Lbx1 gene on myogenesis were mainly focused on the embryo stage, and little data was reported after birth. The objective of this study was to characterise Lbx 1 expression patterns in porcine skeletal muscle during different developmental stages and to provide basic information for further investigating its role in skeletal muscle development after birth.
Materials and methods
The use of animals and sample collection
All animal procedures were approved by the Code of Ethics of the World Medical Association (Declaration of Helsinki) for animal experiments (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm). A total of 28 healthy Mashen and 28 healthy Large White pigs were selected from Datong Pig Breeding Farm (Shanxi, China) for this study. The piglets were weaned at four weeks old and the males were castrated at weaning for both Mashen and Large White. Four pigs (2 males and 2 females) of each breed were weighed and slaughtered at each of the seven development stages at 1, 30, 60, 90, 120, 150 and 180 days after birth, respectively. Longissimus dorsi (LD) muscle was collected and snapped in liquid nitrogen for further use.
Quantitative real time-polymerase chain reaction
Total RNA was extracted from the LD muscle using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and transcripted into cDNA using the PrimeScript RT reagent Kit with gDNA Eraser (Takara, Dalian, China) following the manufacturer’s instructions.
The primers of Lbx1 gene were designed based on a specific sequence (Accession number: NM-001206336) using online Primer 3 programme. 18S rRNA was used as the internal control. Primer sequences were as follows: Lbx 1: forward, 5 -TTTAAGGGGCTGGAGGTC-3 ; reverse, 5 -CGCTTCTCCAACTCATAGA-3 ; 18S rRNA forward: 5 -CCCACGGAATCGAGAAAGAG-3 ; reverse: 5 -TTGACGGAAGGGCACCA-3 . Polymerase chain reaction products of Lbx 1 and 18S rRNA were 143bp and 117bp, respectively. Quantitative real-time PCR was performed using SYBR® PrimeScript™ RT-PCR Kit (Takara) implement on MXPro-3000P (Stratagene, La Jolla, CA, USA). Polymerase chain reaction conditions were as follows: predenaturation for 20 s, 45 cycles of 95°C for 20 s, and 60°C for 30 s, one cycle of 95°C for 20 s, 60°C for 30 s, 95°C for 30 s. The relative expression was calculated by 2-ΔACT.
Western blot analysis
Total protein was extracted from longissimus dorsi using tissue protein extraction kit (Boster, Pleasanton, CA, USA), and concentrations were measured by a NanoDrop spectrophotometer (Thermo, Waltham, MA, USA). Total 100 µg of protein extract per sample were subjected to SDS-PAGE, and transferred to nitrocellulose filter membranes (Millipore, Billerica, MA, USA). After blocking 10% skimmed milk (Boster) at room temperature for 1 h, membranes were washed with TBST and incubated with an anti-LBX1 (1:1000 [vol/vol]) or anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:3000[vol/vol]; Boster), overnight at 4°C. After that, membranes were washed with TBST and incubated with HRP-conjugated secondary antibodies (goat anti-rabbit-IgG, 1:5000; Boster) at room temperature for 1 h. Finally, the membrane was washed and a super ECL chemiluminescence plus kit (Boster) was used for visualisation. Image Lab software conjugated Bio-Rad system was used to scan and visualise the band intensities (Bio-Rad, Hercules, CA, USA). The Lbx1 content was normalised to GAPDH level in each lane.
Statistical analysis
Lbx1 gene expressions were determined with the least square method (GLM procedure, SAS version 9.0) using the following statistical model:
yijk=μ+ Bi+Dj+BDj+eijk
where: yijk is the relative expression of Lbx1 mRNA or protein, μ is the over all mean, Bi is the effect of breed (i=1, 2), Dj is the effect of age (j=1-7), BDij is the effect of the interaction between breed and age, and eijk is the random residual. P<0.05 is considered to be significant.
Results
Large White and Mashen pigs possess different body weight and growth rate at different stages
The body weights of Large White and Mashen at different stages were measured (). Large White were more heavy than those of Mashen at each stage except for the birth weight (P<0.05). For Large White pig, the growth rate before weaning was 195.67 g/d, 541 g/d during 30 to 90, and above 1070.50 g/d during 90-150 d. For Mashen pigs, the growth rate was also lower before weaning and became faster during 30 to 60 d, with the growth rate of 410.33 g/d, then grew slower again with the growth rate of 180.33 g/d during 60 to 90 d, and afterwards, the growth rate was 583.22 g/d.
Lbx1 mRNA contents are different in Mashen and Large White longissimus dorsi muscle
Our data shows that Lbx 1 mRNA relative expression was affected significantly by the effects of breed, age, and the interaction of breed and age (P<0.05) (). The developmental expression pattern of Lbx1 mRNA in LD muscle of both Large White and Mashen pigs is shown in . The mRNA content in Large White was significantly higher than in Mashen before 30 d, and lower after 30 d. In Mashen pigs, the Lbx1 mRNA contents kept a stable level before 120 d and there was no difference among these stages. After 120 d (with the increase of age), the mRNA showed an increase trend, and reached the highest value – which was greater than those at other stages – at 180 d (P<0.05). In Large White, the mRNA amounts were higher at 30 and 180 d than those at other stages (P<0.05), while was the least at 60 d.
Differential expression of Lbx1 protein in Mashen and Large White longissimus dorsi muscle
To further analyse Lbx1 protein expression at different developmental stages, western blot was performed. In accordance with the Lbx1 mRNA expression, the Lbx1 protein level was also affected significantly by the breed, age, and the interaction of breed and age (P<0.05) ().
The developmental expression pattern of Lbx1 protein in LD muscle is shown in . In Large White, the Lbx1 protein level showed an up-down-up-down trend; the expression was increased with the increase of age before 60 d, which was greater than those in Mashen pigs at the same age. After that, its contents was decreased dramatically and reached the lowest level at 60 d, and then increased to reach the second expression peak at 90 d, and subsequently gradually decreased with the age. In Mashen pig, Lbx1 protein level showed an increase trend with the age increase before 90 d, then decreased to the lowest level at 120 d, and then increased gradually up to the expression peak at 180 d.
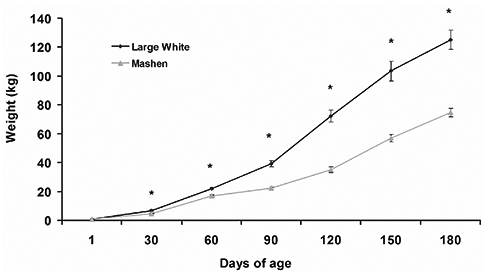
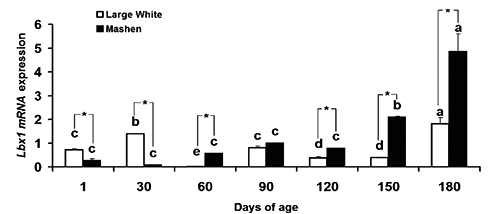
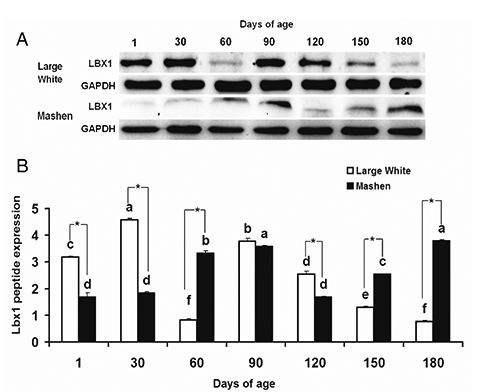
Table 1. Analysis of variance table for Lbx1 mRNA relative expression.
Table 2. Analysis of variance table for Lbx1 protein relative expression.
Discussion
In vertebrates, skeletal muscle is formed by the fusion of mononuclear precursor cells termed myoblasts. In adult skeletal muscle, some myoblasts remain undifferentiated as satellite cells. Satellite cells are located between the sarcolemma and the basement membrane of terminally differentiated muscle fibres (Campion et al., Citation1979; Bischoff and Heintz, Citation1994). The number of satellite cell was abundant when the animal was young and was decreased after aging. Normally, satellite cells are mitotically quiescent and replicate very slowly to self-renewal (Schultz, Citation1996; Decary et al., Citation1997). When muscles were damaged, these cells were activated and underwent multiple rounds of cell division, and then migrated to the damage sites, formed multinucleate myofibres either de novo or by fusion with preexisting muscle fibres (Seale and Rudnicki, Citation2000; Watanabe et al., Citation2007). In pig, the number of myofibres was fixed in postnatal growth of skeletal muscle, and only the fibre size was increased (Swatland and Cassens, Citation1973; Swatland and Kieffer, Citation1974). Muscle hypertrophy depended on the increasing of myofibrils and of the nucleus which relayed on fusion of satellite cells (Campion et al., Citation1981).
Functionally, Lbx1 could induce myogenesis through guiding muscle precursor migration to the proper regions (Schafer and Braun Citation1999; Schmitteckert et al., Citation2011) and controlling myoblasts proliferation (Mennerich and Braun, Citation2001; Martin and Harland, Citation2006). Furthermore, Lbx1 played important roles in satellite cells differentiation and self-renew (Watanabe et al., Citation2007). Lbx1 gene was specifically expressed in central nervous system and muscles during embryogenesis in mouse (Jagla et al., Citation1995), and it was expressed in myoblasts that contributed to the body wall musculature (Martin and Harland, Citation2006). Previous studies demonstrated that in Meishan pigs, the Lbx1 gene was moderately expressed in muscle and heart, and weakly or hardly expressed in other organs at 120 days old (Chao et al., Citation2011).
In the current study, Lbx1 was detectable at seven post-natal stages, including 1, 30, 60, 90, 120, 150 and 180 d in Large White and Mashen pigs of LD muscle. In Large White, both the mRNA and protein amounts of Lbx1 were greater at birth and 30-d than those at other stages, which is consistent with previous reports (Chao et al., Citation2011).
In our study, all piglets were weaned at four weeks old. After weaning, the Lbx1 expression was decreased to the lowest level at 60 d, and then showed a sharp increase at 90-d following a gradually decrease to the lowest level again at 180 d in Large White. Lbx1 is mainly expressed in activated satellite cells, the fusion of satellite cells with myofibres and myofibrils increasing can result in myofibres hypertrophy after birth (Mesires and Doumit, Citation2002). The percentage of activated satellite cells was highest in 1 week after birth and was significantly decreased in 7 weeks age in pigs; between 7 to 21 weeks, the percentage of satellite cells slightly declined (Mesires and Doumit, Citation2002). Because of the decline of activated satellite cells, it was possible that Lbx1 expression was gradually decreased. In addition, the Lbx1 expression pattern in Large White also corresponded to its growth and development. The fast development of muscle during the first month is most likely attributed to the Lbx1 higher expression level in activated satellite cells.
Our data suggests that the Lbx1 expression pattern in Mashen pig is different from that in Large White, which might be due to the different genetic profiles between these two breeds. The Lbx1 mRNA expression in Mashen pig was increased from new birth to 180 d, with the peak appearing at 180 d. The protein content was also increased with age, except for 120 d, indicating that the percentage of activated satellite cells in Mashen skeletal muscle was higher. Regarding the expression pattern in these two pig breeds, Lbx1 expression in Large White was higher before weaning and lower after weaning than that in Mashen pig. The proliferation ability of muscle precursor in Large White is most likely greater than that in Mashen during embryogenesis, and this potential remains at a higher level after birth. Thus, the Large White grew faster than Mashen among whole stages. However, Mashen pig is a Chinese indigenous pig, one of its the biological characteristics is being very active. Previous studies show that exercise could induce satellite cell proliferation both in animal models (Li et al., Citation2006) and humans (Kadi et al., Citation2005). In addition, as a Chinese indigenous pig, the proliferative potential of satellite cells in Mashen pig was higher than in western commercial breed. (Wang et al., Citation2012). The percentage of activated satellite cells in Mashen pig could be greater than in Large White at the same stage. As Lbx1 was mainly expressed in activated satellite cells after birth, so the expression content in Mashen pig was higher than in Large White after weaning. To demonstrate this, however, more studies need to be done in the future.
Conclusions
The expression patterns of Lbx1 mRNA and peptide showed that Lbx1 expression level is related to age and genetic profiles of Mashen and Large White pigs. Growth rate is associated with Lbx1 expression levels. Furthermore, Lbx1 was expressed in activated satellite cells and the differentiation of satellite cells results in muscle development after birth. We then hypothesise that Lbx1 may promote skeletal muscle development by activating satellite cell after birth.
Acknowledgements
This research was supported by the projects of Science & Technology of Shanxi Province of China (20140311020-5, 20080311031), Shanxi Scholarship Council of China (2012-055), Shanxi Graduate Students Outstanding Innovation Project (20123057), Shanxi Provincial Foundation for Leaders of Disciplines in Science in Higher Education Institutions of China, and Foundation for Leaders of Disciplines in Science in Shanxi Agricultural University.
References
- BischoffR.HeintzC., 1994. Enhancement of skeletal muscle regeneration. Dev. Dynam. 201:41-54.
- CampionD.R.RichardsonR.L.KraelingR.R.ReaganJ.O., 1979. Changes in the satellite cell population in fetal pig skeletal muscle. J. Anim. Sci. 48:1109-1115.
- CampionD.R.RichardsonR.L.ReaganJ.O.KraelingR.R., 1981. Changes in the satellite cell population during postnatal growth of pig skeletal muscle. J. Anim. Sci. 52:1014-1018.
- ChaoZ.WuJ.ZhengR.LiF.E.XiongY.Z.DengC.Y., 2011. Molecular characterization and expression patterns of Lbx1 in porcine skeletal muscle. Mol. Biol. Rep. 38:3983-3991.
- ChenF.LiuK. C.EpsteinJ.A., 1999. Lbx2, a novel murine homeobox gene related to the Drosophila ladybird genes is expressed in the developing urogenital system, eye and brain. Mech. Develop. 84:181-184.
- ChengL.SamadO.A.XuY.MizuguchiR.LuoP.ShirasawaS.GouldingM.MaQ., 2005. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat. Neurosci. 8:1510-1515.
- DecaryS.MoulyV.HamidaC.B.SautetA.BarbetJ.P.Butler-BrowneG.S., 1997. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum. Gene Ther. 8:1429-1438.
- GrossM.K.DottoriM.GouldingM., 2002. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron 34:535-549.
- GrossM.K.Moran-RivardL.VelasquezT.NakatsuM.N.JaglaK.GouldingM., 2000. Lbx1 is required for muscle precursor migration along a lateral pathway into the limb. Development 127:413-424.
- JaglaK.DolleP.MatteiM.G.JaglaT.SchuhbaurB.DretzenG.BellardF.BellardM., 1995. Mouse Lbx1 and human LBX1 define a novel mammalian home-obox gene family related to the Drosophila lady bird genes. Mech. Develop. 53:345-356.
- JaglaK.FraschM.JaglaT.DretzenG.BellardF.BellardM., 1997. Ladybird, a new component of the cardiogenic pathway in Drosophila required for diversification of heart precursors. Development 124:3471-3479.
- KadiF.CharifiN.DenisC.LexellJ.AndersenJ.L.SchjerlingP.OlsenS.KjaerM., 2005. The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Arch. 451:319-327.
- KanamotoT.TeradaK.YoshikawaH.FurukawaT., 2006. Cloning and expression pattern of lbx3, a novel chick homeobox gene. Gene Expr. Patterns 6:241-246.
- LiP.AkimotoT.ZhangM.WilliamsR.S.YanZ., 2006. Resident stem cells are not required for exercise-induced fiber-type switching and angiogenesis but are necessary for activity-dependent muscle growth. Am. J. Physiol.-Cell Physiol. 290:1461-1468.
- MartinB.L.HarlandR.M., 2006. A novel role for lbx1 in Xenopus hypaxial myogenesis. Development 133:195-208.
- MennerichD.BraunT., 2001. Activation of myogenesis by the homeobox gene Lbx1 requires cell proliferation. EMBO J. 20:7174-7183.
- MesiresN.T.DoumitM.E., 2002. Satellite cell proliferation and differentiation during postnatal growth of porcine skeletal muscle. Am. J. Physiol.-Cell Ph. 282:899-906.
- PollardS.L.HollandP.W., 2000. Evidence for 14 homeobox gene clusters in human genome ancestry. Curr. Biol. 10:1059-1062.
- SchaferK.BraunT., 1999. Early specification of limb muscle precursor cells by the homeobox gene Lbx1h. Nat. Genet. 23:213-216.
- SchaferK.NeuhausP.KruseJ.BraunT., 2003. The homeobox gene Lbx1 specifies a subpopulation of cardiac neural crest necessary for normal heart development. Circ. Res. 92:73-80.
- SchmitteckertS.ZieglerC.KartesL.RolletschekA., 2011. Transcription factor lbx1 expression in mouse embryonic stem cell-derived phenotypes. Stem Cells Int. 2011:130970.
- SchultzE., 1996. Satellite cell proliferative compartments in growing skeletal muscles. Dev. Biol. 175:84-94.
- SealeP.RudnickiM.A., 2000. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev. Biol. 218:115-124.
- SwatlandH.J.CassensR.G., 1973. Prenatal development, histochemistry and innervation of porcine muscle. J. Anim. Sci. 36:343-354.
- SwatlandH.J.KiefferN.M., 1974. Fetal development of the double muscled condition in cattle. J. Anim. Sci. 38:752-757.
- WangX.Q.YangW.J.YangZ.ShuG.WangS.B.JiangQ.Y.YuanL.WuT.S., 2012. The differential proliferative ability of satellite cells in Lantang and Landrace pigs. PLoS One 7:e32537.
- WatanabeS.KondoS.HayasakaM.HanaokaK., 2007. Functional analysis of homeodomain-containing transcription factor Lbx1 in satellite cells of mouse skeletal muscle. J. Cell Sci. 120:4178-4187.
- WottonK.R.WeierudF.K.DietrichS.LewisK.E., 2008. Comparative genomics of Lbx loci reveals conservation of identical Lbx ohnologs in bony vertebrates. BMC Evol. Biol. 8:171.
- WottonK.R.WeierudF.K.Juarez-MoralesJ.L.AlvaresL.E.DietrichS.LewisK.E., 2009. Conservation of gene linkage in dispersed vertebrate NK homeobox clusters. Dev. Genes. Evol. 219:481-496.
- YangW.P.CaoG.Q.ShiJ.Z.LiuJ.H.ZhouZ.X., 2005. Study on the finishing ability of different cross combination in pig. Chinese J. Anim. Sci. 41:48-49.
- ZhangJ.G.WangX.DuM.H.ZhouZ.X., 2001. Species diversity and the way to protect Mashen pig. J. Shanxi Agric. Univ. 21:188-191.
