Abstract
Coccidiosis is one of the most common ailments in rabbits farming and is usually treated with drugs that can produce resistance; therefore, a natural alternative was sought. The objective of the current study was to evaluate the efficacy of the aqueous extract of curcumin (Curcuma longa) on the excretion of oocysts of Eimeria spp. in New Zealand white rabbits. Twenty-four eight-month-old rabbits were divided into four groups of six animals to be C. longa extract administered at 0 (Control), 10, 25 or 40 mg/kg body weight (BW). Rabbit weights were recorded and faeces samples were collected on d 0, 7, 14, 21, 28, 35 and 42. The McMaster technique was used for quantifying Eimeria spp. oocysts. Results were analysed using multivariate analysis of variance for repeated observations. Statistically significant differences (P<0.05) from d 28 were observed among the Control, the group of 25 mg/kg BW and that of 40 mg/kg BW. At d 42, statistically difference (P<0.05) among the Control group and the other three groups was observed. It could be concluded that C. longa decreased Eimeria spp. oocysts excretion efficiently at a dose of 40 mg/kg BW with 80.1, 63.7 and 64.9% for d 28, 35 and 42, respectively, with reducing concentration of eggs per gram of faeces with about 20.1, 15.6 and 17.8 for d 14, 21 and 35, respectively. However, further studies are needed to assess and confirm the antiparasitic activity of C. longa.
KeyWords:
Introduction
Digestive diseases of rabbits are of great importance and can cause economic losses resulted from growth disturbances and rabbits death, in addition to decreased body weight (BW) and drug costs (García Segura et al., Citation2014; Szkucik et al., Citation2014). It is generally accepted that intestinal damage following coccidiosis reduces nutrient absorption by the gut epithelium, thereby reducing BW and growth (Kim et al., Citation2013). Passalurus ambiguus, Encephalitozoon cuniculi, Cryptosporidium spp. and Eimeria spp. are the most prevalent endoparasites in rabbit farms worldwide; however, P. ambiguus and Eimeria spp. are the most prevalent endoparasites in Mexican rabbit farms (García Segura et al., Citation2014). Eimeria spp is one of the leading causes of diarrhea, dehydration, listlessness, mortality, decreased BW and poor feed conversion from coccidiosis (Bhat et al., Citation1996).
Traditionally, in-feed anticoccidial drugs have proven highly effective in preventing morbidity associated with coccidiosis. Currently for treatment of coccidiosis, sulfonamides, salinomycin and robenidine are mainly used (Pakandl, Citation2009), which may become toxic to infants and pregnant females. Diclazuril has proven to effectively control rabbit coccidiosis (European Food Safety Authority, Citation2015). Nevertheless, the increase in the production of organic products is currently being observed in several countries due to consumer demands. On the other hand, parasites can potentially generate resistance for chemical anti-coccidial drugs due to bad practices in use. Furthermore, drugs and antibiotic residues in meat is potentially annoyance to consumer. By virtue of the above problems, it is important to find alternatives for the treatment of coccidiosis (Cedillo et al., Citation2014, Citation2015; Mejia Hernandez et al., 2014; Olmedo-Juárez et al., Citation2014); one such alternative is the use of curcumin.
C. longa contains diferuloylmethane (curcumin), demethoxycurcumin, and bisdemethoxycurcumin as the main components (Arun Raj et al., Citation2014). As a result of these active compounds, C. longa have some pharmacology effects including anticancer, antioxidant, antimutagenic, antiinflamatory, antimicrobial agent and antiprotozoal effects (Eevuri and Putturu, Citation2013; Kim et al., Citation2013); the pharmacological activities of turmeric are considerably dependent on the bioactivity of curcumin (Jayaprakasha et al., Citation2002). C. longa have been described for Plasmodium falciparum (Cui et al., Citation2007), Leishmania spp. (Koide et al., Citation2002), Giardia lamblia trophozoites (Shahiduzzaman et al., Citation2009) and Trypanosoma spp. (Nagajyothi et al., Citation2012). Khalafalla et al. (Citation2011) in vitro studied the effect of curcumin on Eimeria tenella, and found that there is a cytotoxic effect on the sporozoite through causing morphological changes. In addition, Pérez (Citation2010) conducted an in vivo study to evaluate the antiparasitic effect of curcumin against coccidiosis in sheep, and reported 82.3% reduction in oocyst excretion at d 14 of treatment, and a reduction of 98.1% at d 21 of treatment compared to the untreated group.
Therefore, the objective of the current study was to evaluate the in vivo antiparasitic efficacy of different doses of the aqueous extract of C. longa on the intestinal coccidiosis caused by Eimeria spp. in New Zealand white rabbits, with the hypothesis that the inclusion of C. longa causes a reduction in oocyst excretion.
Materials and methods
Preparation of aqueous extract of Curcuma longa
About 500 g of macerated C. longa rhizome mash were weighed and mixed in 1 liter of bidistilled water and allowed to boil for 20 minutes. The mixture was allowed to stand for 24 h, then the supernatant was removed and introduced in a Buchi rotavapor at 60°C under vacuum for sample drying, where 350 mg of aqueous extract were obtained. To determine the concentration of curcuminoids, the UV-Vis method (Ultraviolet-visible spectroscopy) at 421 nm (Thermo Scientific spectrophotometer; Thermo Scientific, Inc.Waltham, MA, USA) was used according to Sharma et al. (Citation2012). The obtained concentration of curcumins was 4.61 mg/100 mg of aqueous extract of C. longa.
Animals and management
Twenty four NZW rabbits with eight-months-old and an average BW of 3 kg, from the Agricultural School, Autonomous University of Chapingo, Chapingo, State of Mexico, Mexico were used. The rabbits were housed in San Mateo Huexotla, Texcoco, State of Mexico, Mexico. Rabbits were naturally infected and belonged to a farm with a history of intestinal coccidiosis; with no experimental infection performing. Rabbits were kept in individual galvanized cages with 45 cm wide×30 cm long×30 cm high. Rabbits were fed on commercial diet (PURINA®, Lausanne, Switzerland) at 100 g daily. Diet contained 88% dry matter (DM), 90% organic matter (OM), 16% crude protein (CP), 3% crude fat, 17% crude fibre (CF), 42.5% of nitrogen-free extract, 1% calcium and 0.55% phosphorus. Water was offered ad libitum. For avoiding selection and decreasing feed waste, rabbits were offered pelleted feed at 07:00 and 17:00 h.
Experimental design and sampling
One-hundred g of a mixture containing an aqueous extract of 36.8 g of C. longa mixed with oatmeal and a non-nutritional apple flavoring additive were individually administered per os using a syringe. The untreated Control rabbits only received the mixture of oatmeal with the powdered artificial apple flavouring. The mixture was fed twice daily at 07:00 and 17:00 h. The composition of the mixture was: 91% DM, 98% OM, 13% CP, 6% crude fat and 12% CF. As rabbits were naturally infected, the stage of the life cycle of the parasite was uncertain at the time the first sample was taken. Therefore, we waited until all rabbits shed oocysts in faeces to start the experiment by speculating that the parasite had just finished the gametogony phase in most of the animals. As the life cycle is completed in 7 d (prepatent period), rabbits were sampled every 7 d to allow a life cycle to be completed. Thereafter, 7 d were skipped to check for efficacy after reinfection, considering that at least two life cycles of the parasites were completed by that time point. Therefore, the treatment of rabbits was administered from d 1 to 7, and allowed to rest in rabbits from d 8 to 14 and re-administer at d 15 to 21. Untreated Controls received the apple-flavored oatmeal mixture and the same commercial diet.
Before the beginning of the experiment (pre-treatment), faeces samples from each rabbit were collected and examined by performing flotation technique quantitation of oocysts per gram faeces (OPG) with the technique of McMaster in order to confirm the natural infection of Eimeria spp. Based on the results of rabbits according to the load of OPG only be ordered, and randomly assigned to one of four treatments to make stockings for OPG similar groups (Kummuru et al., Citation2014). Oocyst counts ranged from 150 to 1100 OPG. Four groups of six rabbits each were formed where C. longa extract was administered at 0 (Control), 10, 25 or 40 mg/kg BW. As explained previously, every 100 g of the Curcuma/oatmeal/apple flavoring mixture contained: 36.8 g of the aqueous extract of C. longa; hence, for the 10 mg dose 27.1 mg of the mixture were used. The 25 mg dose included 67.9 mg of the mixture, whereas the 40 mg dose included 108.6 mg of the mixture. During the experiment, the weight of each rabbit was recorded and faeces as well as cecotrope samples were obtained and weighed on d 7, 14, 21, 28, 35 and 42 as the daily sampling procedure stresses the animals, which would have interfered with results and might have even caused mortality. A wire mesh was adapted at the cage floor to avoid urine to mix with faeces. Nevertheless, faeces did not fit into this mesh, so rabbits were in contact with their faeces. However, faeces from rabbits belonging to different experimental or Control groups did not have contact among them. Faeces samples were collected directly from the floor immediately after excretion and kept in plastic bags. Samples were kept refrigerated at -4°C until analysis and McMaster technique performing for quantifying Eimeria spp.
Data statistical analysis
Data were analyzed in a completely randomized design using a single factor-univariate analysis (ANOVA) with four levels of extract for each time. Multivariate analysis of variance for repeated measures was performed. Oocyst and eggs counts were natural log transformed, prior to analysis.
Results
Oocyst quantification
The multivariate analysis of variance showed a significant interaction (P<0.001) between time and treatment, which means that the number of OPG for each treatment did not exhibit homogeny in behavior over time. As a consequence of the above result, a single factor-univariate analysis (ANOVA) with four levels for each time was performed. The Welch test was used because the variances of the groups were heterogeneous. Statistically differences (P<0.003) between groups from d 28 were found (). Dunnet T3 test was used for multiple comparisons (). At d 28, statistically differences (P<0.05) in the log of average number of oocysts per gram of faeces in rabbits between the Control group and the groups receiving 25 mg/kg and 40 mg/kg BW of aqueous extract of C. longa, and between these two groups (). At d 35, decreased (P<0.05) numbers of oocysts were obtained with groups receiving 25 and 40 mg C. longa extract/kg BW compared to Control group (). At d 42, rabbits receiving C. longa extract at 10, 25 and 40 mg/kg BW had decreased (P<0.05) oocysts numbers compared to Control ().
Egg counts
During the oocysts counting, analysis of P. ambiguus egg was performed. The multivariate analysis of variance showed a significant interaction between time and treatment (P<0.01) which means that the log average number of eggs per gram of faeces (HPG) for each treatment are not homogeneous behavior over time (). As a consequence of the above result, univariate analysis of single factor with four levels for each time was performed. Welch test was used to analyze from d 0 to d 14. Statistically differences (P<0.002) were found up to d 14 and multiple comparisons were performed using the Dunnet T3 test. Statistically significant difference (P<0.05) was found between the Control group and the group receiving 40 mg/kg BW aqueous extract of C. longa (). At d 21 and 42, analysis of univariate with the test F was performed. The group receiving a dose of 10 mg/kg BW aqueous extract of C. longa was discarded from the analysis because at these days there was no presence of egg, and therefore dose worked best to remove it (i.e., hypothesis tests for each of the other treatments at each time to determine if the mean of the logarithms was made zero. All were significantly greater than zero (P<0.0004) at the d 7 except for treatments 25 and 40 mg/kg BW, therefore, the treatment 10 mg/kg BW was the most efficient in removing eggs in a shorter time). At d 21, statistically significant differences (P<0.05) among the three groups (Control, 25 BW and 40 mg/kg BW aqueous extract of C. longa) were found, with loweres values with the group receiving 25 mg/kg BW (). At d 28, statistically differences between the Control group with the groups receiving 25 and 40 mg/kg BW aqueous extract of C. longa were found. At d 35, statistically differences between the Control and the group treated with 25 mg/kg BW aqueous extract of C. longa were found, with lowered value with the group receiving 25 mg/kg BW (). At d 42, no statistically differences among the three groups (Control, 25 and 40 mg/kg BW aqueous extract of C. longa) were found ().
Live weight, feed intake, faecal and cecotrope production
During experiment and for all treatments, no significant difference in live weight was observed between rabbits at different days (). Significant differences (P<0.0001) were found in feed intake and faecal production between weeks 1 and 2 versus weeks 3 and 4, with a mean intake and faecal production of 98.32 and 40.88 g/d, respectively for the former two weeks and 162.15 and 78.67 g/d, respectively for weeks 3 and 4 ().
Discussion
The results indicate that the aqueous extract of C. longa at doses of 25 and 40 mg/kg BW decreased the parasite load of oocysts of Eimeria spp. with 24.2 and 80.1%, respectively compared with the Control group at d 28. These results are similar to those found by Pérez (Citation2010), at a dose of 30 mg/kg BW; curcumin extract was able to reduce the parasite burden of Eimeria spp. in sheep. The dose of 10 mg/kg BW aqueous extract of C. longa decreased the burden of Eimeria spp. with 19.5% compared to the Control group at d 42. C. longa was reported to suppress the development of coccidiosis in chickens in diets at different concentrations (Allen et al., Citation1998). The reduction in sporozoite burden may be precipitated by curcumin surface especially at high concentrations; in an in vitro study on Eimeria tenella curcumin was reported to affect the morphology, viability and adhesion ability of the sporozoite that induces apoptosis (Chattopadhyay et al., Citation2004). These changes depend on both the extract dose and the time of delivery (Khalafalla et al., Citation2011). The same morphological changes were reported by Pérez-Arriaga et al. (Citation2006) in Giardia lamblia trophozoites. Furthermore, it was observed at d 14 that the aqueous extract of C. longa at a dose of 40 mg/kg BW reduced the parasite burden of P. ambiguus eggs with 20.1%. At d 21, the dose of 10 mg/kg BW aqueous extract of C. longa decreased parasite load whole egg plus P. ambiguous; the Control group and the groups treated with 25 and 40 mg/kg BW showed a significant difference with 26.6 and 15.6% reduction with 25 and 40 mg/kg BW levels, respectively. At d 35, only statistically significant difference was found between the Control and the group receiving a dose of 25 mg/kg BW with 35% reduction compared to Control. Kiuchi et al. (Citation1993) observed nematocidal activity caused by the synergistic action of curcuminoids (demethoxycurcumin and bisdemetho xycurcumin) larval 2 (L2) of Toxocara cannis. The decrease in parasite load could be because curcumin decreases the viability of the parasite producing a dose-dependent effect, inhibiting egg production in adult parasites; these alterations were reported in Schistosoma mansoni (Magalhães et al., Citation2009). It has been observed that in Pheretima posthuma, the alcoholic extract of C. longa causes paralysis and death of the parasite at 10 mg/mL (Singh et al., Citation2011). But the mechanism by which the aqueous extract of C. longa exerts its effect is still not clear enough. However, the anticoccidial effect of C. longa extract due to its antioxidant effect is suggested. The antioxidant-rich plants, such C. longa, may be lethal to the parasites by inducing oxidative stress and neutralize reactive oxygen species. Antioxidant compounds have the ability to control Eimeria infections due to the association of coccidial infection with host cell destruction which being associated with oxidative stress and lipid peroxidation of the intestinal mucosa (Allen et al., Citation1998). In addition, the presence of the phenolic compound curcumin in C. longa extract is a possible reason. Phenols have the ability to interact with cytoplasmic membranes and change their cation permeability, causing impairment of crucial processes in the coccidia cells and, finally, their death (Sikkema et al., Citation1995).
Obtained results, specially reduced oocyst output, suggest that C. longa can inhibit and impair invasion, replication and development of Eimeria parasites species before the relatively inert oocysts are formed and finally released, through affecting intracellular stages of the Eimeria specially at the second schizogony stage as well as those of the sexual stage of Eimeria at the lumen of the intestine.
Feed intake was increased between the first and second two weeks which shows an adaptability of rabbits for C. longa administration. Adaptability for C. longa presence in the diet may improve feed digestion with time advancing resulting increased intake during the second two weeks compared to the first two weeks. Patel and Srinivasan (Citation2000) reported that curcumin increased digestibility of nutrients as it elevates the activity of pancreatic lipase, amylase, trypsin and chymotrypsin. Faecal production followed the same trend as feed intake.
In the current study, no effects for C. longa extract on BW were observed at different concentrations and different days. Basavaraj et al. (Citation2010) reported that average daily BW gain did not differ as a result of C. longan administration in the diet of commercial broiler chickens. However, Kim et al. (Citation2013) found that chickens fed a diet supplemented with C. longa extract showed increased BW gain following infection with E. maxima or E. tenella compared with chickens given diet without extract.
Experimental flaws could have resulted from this work, even though every effort was made not to bias the results towards or away our hypothesis. Undoubtedly, the age of the rabbits and the sample size could have influenced the present results. It would be therefore interesting to repeat the experiment using a larger sample size and growing rabbits to confirm the anticoccidial efficacy of C. longa.
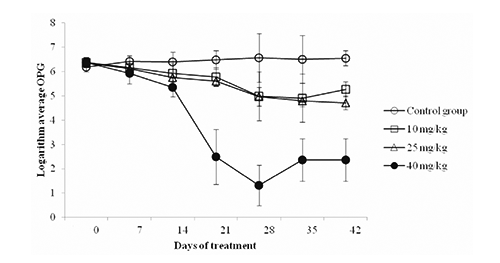
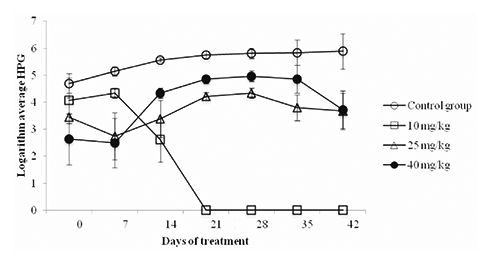
Table 1. Concentration of oocysts in faeces of adult New Zealand white rabbits treated with aqueous extract of Curcuma longa at different days (n=6 rabbits per treatment).
Table 2. Concentration of eggs per gram of faeces of adult New Zealand white rabbits treated with aqueous extract of Curcuma longa at different days (n=6 rabbits per treatment).
Table 3. Live weight (kg) of adult New Zealand white rabbits treated with different levels of aqueous extract of Curcuma longa.
Table 4. Mean weekly feed intake, faecal and cecotrope production.
Conclusions
It is important to note that the average weight of the animals was not affected by the application of aqueous extract of C. longa as no statistically difference was found with respect to the average weight of the Control group, even though a significant difference was observed regarding faecal and cecotrope output, as well as a possible result of an increased feed intake. In vivo feeding of adult rabbits with an organic aqueous extract of C. longa decreased the elimination of oocysts of Eimeria spp. more effectively at a dose of 40 mg/kg BW. The dose of 40 mg/kg DM reduced oocysts concentration in faeces with 80.1, 63.7 and 64.9% at d 28, 35 and 42, respectively, together with reducing the concentration of P. Ambiguus eggs per gram of faeces of about 20.1, 15.6 and 17.8 at d 14, 21 and 35, respectively. Further studies in rabbits and other species at different growth stages are suggested to assess and confirm the antiparasitic activity of C. longa spice.
Acknowledgments
This work was supported by the Support Programme for Projects of Research and Techonological Innovation (PAPIIT; IN215209, IN216309).
References
- Allen P.C. Danforth H.D. Augustine P.C., 1998. Dietary modulation of avian coccidiosis. Int. J. Parasitol. 28:1131-1140.
- Arun Raj G.R. Shailaja U. Prasanna N. Ajayan S. Thomas N.P., 2014. Review on the contribution of Ura-Marunnu, a traditional baby care practice in southern India. Pharm. Innovat. 2:42-70.
- Basavaraj M. Nagabhushana V. Prakash N. Mallikarjunappa S. Appannavar M.M. Wagmare P., 2010. Effect of dietary supplementation of Pulvis Curcuma Longa on the voluntary feed intake, nutrient digestibility and growth performance of broiler rabbits under summer stress. Vet. World 3:369-372.
- Bhat T.K. Jithendran K.P. Kuraden N.P., 1996. Rabbit coccidiosis and its control: a review. Word Rabbit Sci. 4:37-41.
- Cedillo J. Kholif A.E. Salem A.Z.M. Elghandour M.M.Y. Vázquez J.F. Alonso M.U. Barbabosa A. Chagoyán J.C.V. Reyna A.G., 2015. Oral administration of Sauce llorón extract to growing lambs to control gastrointestinal nematodes and Moniezia spp. Asian Pac. J. Trop. Med. ( in press).
- Cedillo J. Vázquez-Armijo J.F. González-Reyna A. Salem A.Z.M. Kholif A.E. Hernández-Meléndez J. Martínez-González J.C. Jiménez R.M. Rivero N. López D., 2014. Effects of different doses of Salix babylonica extract on growth performance and diet in vitro gas production in Pelibuey growing lambs. Ital. J. Anim. Sci. 13:609-913.
- Chattopadhyay I. Biswas K. Bandyopadhyay U. Banerjee R.K., 2004. Tumeric and curcumin: biological actions and medicinal applications. Curr. Sci. India 87:44-53.
- Cui L. Miao J. Cui L., 2007. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob. Agents Ch. 51:488-494.
- Eevuri T.R. Putturu R., 2013. Use of certain herbal preparations in broiler feeds. A review. Vet. World 6:172-179.
- European Food Safety Authority, 2015. Scientific opinion on the safety and efficacy of Coxiril®(diclazuril) for rabbits for fattening and breeding. EFSA J. 13:3968-3988.
- García Segura F. Aranda Aburto M.S. Espino Barros O.A. Hernández Hernández J Camacho Ronquillo J.C. Portillo Monroy A., 2014. Prevalence study of external and internal parasites in the municipality of rabbits in Libres, Puebla, Mexico. pp 344-351 in Proc. of 5th Am. Congr. Rabbit Farming, Mexico City, Mexico.
- Jayaprakasha G.K. Jena B.S. Negi P.S. Sakariah K.K., 2002. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z. Naturforsch. C 57:828-835.
- Khalafalla R.E. Müller U. Shahiduzzaman M. Viktor D. Desouky Y.A. Gottfried A. Daugschies A., 2011. Effects of curcumin (diferuloymethane) on Eimeria tenella sporozoites in vitro. Parasitol. Res. 108:879-886.
- Kim D.K. Lillehoj H.S. Lee S.H. Jang S.I. Lillehoj E.P. Bravo D., 2013. Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poultry Sci. 92:2635-2643.
- Kiuchi F. Goto Y. Sugimoto N. Akao N. Kondo K. Tsuda Y., 1993. Nematocidal activity of turmeric: synergistic action of curcuminoids. Chem. Pharm. Bull. 41:1640-1643.
- Koide T. Nose M. Ogihara Y. Yabu Y. Ohta N., 2002. Leishmanicidal effect of curcumin in vitro. Biol. Pharm. Bull. 25:131-133.
- Kummuru D.S. Barker T. Desai S. Burke J.M. Ramsay A. Mueller-Harvey I. Miller J.E. Mosjidis J.A. Kamisetti N. Terrill T.H., 2014. Use of pelleted sericea lespedeza (Lespedeza cuneata) for natural control of coccidia and gastrointestinal nematodes in weaned goats. Vet. Parasitol. 204:191-198.
- Magalhães L.G. Machado C.B. Morais E.R. Bueno de Carvalho-Moreira É. Soares C.S. da Silva S.H. Rodrigues V., 2009. In vitro schistosomicidal activity of curcumina against Schistosoma mansoi adult worms. Parasitol. Res. 104:1197-1201.
- Mejía Hernández P. Salem A.Z.M. Elghandour M.M.Y. Cipriano-Salazara M. Cruz-Lagunas B. Camacho L.M., 2014. Anthelmintic effects of Salix babylonica L. and Leucaena leucocephala Lam. extracts in growing lambs. Trop. Anim. Health Pro. 46:173-178.
- Nagajyothi F. Zhao D. Weiss L.M. Tanowitz H.B., 2012. Curcumin treatment provides protection against Trypanosoma cruzi infection. Parasitol. Res. 110:2491-2499.
- Olmedo-Juárez A. Rojo-Rubio R. Arece-García J. Salem A.Z.M. Kholif A.E. Morales-Almaraz E., 2014. In vitro activity of Pithecellobium dulce and Lysiloma acapulcensis on exogenous development stages of sheep gastrointestinal strongyles. Ital. J. Anim. Sci. 13:303-307.
- Pakandl M., 2009. Coccidia of rabbit: a review. Folia Parasit. 56:153-166.
- Patel K. Srinivasan K., 2000. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Nahrung 44:42-46.
- Pérez F.A., 2010. Evaluación de la eficacia anticcocidiana de los extractos de curcumina y naringenina administrados a borregos infectados naturalmente con coccidias del género Eimeria spp. Degree Diss. Universidad Nacional Autónoma de México, Mexico City, Mexico.
- Pérez-Arriaga L. Mendoza-Magaña M.L. Cortés-Zárate R. Corona-Rivera A. Bobadilla-Morales L. Troyo-Sanromán R. Ramírez-Herrera M.A., 2006. Cytotoxic effect of curcumin on Giardia lamblia trophozoites. Acta Trop. 98:152-161.
- Shahiduzzaman M. Dyachenko V. Khalafalla R.E. Desouky A.Y. Daugschies A., 2009. Effects of curcumin on Cryptosporidium parvum in vitro. Parasitol. Res. 105:1155-1161.
- Sharma K. Agrawal S.S. Gupta M., 2012. Development and validation of UV spectrophotometric method for the stimation of curcumin in bulk drug and pharmaceutical dosage forms. Int. J. Drug Dev. Res. 4:375-380.
- Sikkema J. De Bont J.A.M. Poolman B., 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222.
- Singh R. Mehta A. Mehta P. Shukla K., 2011. Anthelmintic activity of rhizome extracts of Curcuma longa and Zingiber officinale (zingiberaceae). Int. J. Pharm. Sci. 3:236-237.
- Szkucik K. Pyz-Łukasik R. Szczepaniak K.O. Paszkiewicz W., 2014. Occurrence of gastrointestinal parasites in slaughter rabbits. Parasitol. Res. 113:59-64.
