Abstract
The domestic guinea pig is a valuable genetic resource because it is part of local folklore and food tradition in many South American countries. The economic importance of the guinea pig is due to its high feed efficiency and the quality of animal protein produced. For these reasons, our study is aimed to design a complete dinucleotide microsatellite marker set following international recommendation to assess the genetic diversity and genealogy management of guinea pigs. We selected a total of 20 microsatellites, looking for laboratory efficiency and good statistical parameters. The set was tested in 100 unrelated individuals of guinea pigs from Ecuador, Peru, Colombia, Bolivia and Spain. Our results show a high degree of polymorphisms with a total of 216 alleles and a mean number of 10.80±3.49 for markers with a combined exclusion probability of 0.99.
Introduction
The guinea pig (Cavia porcellus), also called cavy, is originally from the Andean regions of southern Colombia, Ecuador, Peru, and Bolivia, where the species was domesticated between 7000 and 5000 BC (Morales, Citation1995). Today, a stable population of 35 million animals is reared in this area (DAD-IS, Citation2014). The guinea pig has several uses and is a valuable economic resource for indigenous populations in the South American marginal areas where they originate. The guinea pig is a unique source of food due to their ability to convert poor vegetable resources to protein. Additionally, the guinea pig has a strong presence in local folklore and in popular medicine and is an important resource in the cultural patrimony of local nations, especially the Quechuas and Aymaras. The guinea pig has been introduced to other countries since the Spanish colonisation of the American continent, and today, they are used as exotic pets or for scientific experimentation (Guerrini, Citation2003). Owing to it great capacity of growing and the poor feeding needs, many efforts have also been made to promote guinea pig husbandry in developing countries. The guinea pig was introduced in several West African countries. Even if no official statistics are available (Manjeli et al., Citation1998), there are some stable reared populations in Cameron, Democratic Republic of Congo and Tanzania (Maass et al., Citation2005, Citation2010; Matthiesen et al., Citation2011). To date, no complete genetic study has been carried out on the domestic guinea pig although great advances have been reached with the completion of the genomic sequence (http://www.ensembl.org/Cavia_porcellus/Info/ Index; Broad Institute, Citation2015). Only a few studies have been conducted looking at microsatellites in guinea pigs, and they have centred on wild subspecies of the Cavia genus such as Cavia aperea and Cavia magna (Kanitz et al., Citation2009) or have been limited to a small marker panel (Burgos-Paz et al., Citation2011). The large number of guinea pig animals and breeds reared in South America necessitated the development of molecular tools to perform genetic characterizations and population structure studies as well as a parentage testing strategy for modern breeding approaches. To respond to this demand, the aims of our study were to design a polymorphic set of dinucleotide microsatellites useful both for analysing the genetic diversity of the domestic Cavia and as for parentage control, following the Food and Agriculture Organization (FAO) and International Society for Animal Genetics (ISAG) recommendations on this type of research in domestic animals.
Materials and methods
Samples used and DNA extraction
Hair samples from a total of 100 unrelated animals belonging to several domestic guinea pig populations were used in our study. Some samples were collected from several breeding lines from Ecuador (40) divided in 10 sample for type/line (Andina, Peru, Inti and commercial local type) and others from Colombia (15), Bolivia (13) and Perú (15); also, some samples were collected in Spain from commercial lines (20) reared as pets. DNA was obtained by incubating 3 hair roots in the presence of 100 µL of 5% Chelex® (Biorad, Göttingen, Germany) resin suspension at 95°C for 10 minutes and 99°C for 3 min.
In silico identification of microsatellites and primer design
The cavPor3 (high-coverage 6.79X assembly) genome release of the guinea pig (Cavia porcellus) was used to search for microsatellite sequences (http://www.ensembl.org/Cavia_porcellus/Info/Index) using the NCBI finder tool (). Sequence repeat motifs of ≥18 bp including poly AG, AC, AT, TC, CA, and GT were searched. A total of 25 sequences were selected. The primer pairs used for polymerase chain reaction (PCR) amplification were designed using Primer3 software version 0.4.0 (Rozen and Skaletsky, Citation2000). Our parameter sets included an optimum primer size of 20±5 bp, an optimum melting temperature of ~60±5°C and a GC content between 20 and 80%. The software was allowed to design primer pairs with expected PCR product sizes of 80 to 350 bp.
Microsatellite locus selection
Our primer pairs were synthesised by Stabvida, Costa de Caparica (Portugal) without further modifications. PCR was performed separately for each locus in a reaction volume of 25 µL containing ~10-30 ng of genomic DNA, 0.2 µM each primer pair, 1X NH4 SO4 PCR buffer, 2.5 mM MgCl2, 200 µM each dNTP, and 1U Taq polymerase (AIDLAB, Beijing, China). The annealing temperature was 56°C for 35 cycles. PCR products were visualised on a 3% agarose gel, stained with ethidium bromide, in TBE buffer at 150 V/cm, using a 100-bp ladder as a reference (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Based on the amplification efficiency and the absence of a nonspecific PCR product, the samples were sequenced using the BigDye cycle sequencing kit 2.0 (Life Technologies, Carlsbad, CA, USA), and the sequences were deposited in GenBank () after sequencing a control sample from the original clone (). Additionally, four microsatellite loci (Kanitz et al., Citation2009) were included in our study with some modifications and discarding tetranucleotide repeat motifs loci ().
Table 1. Summary of the general characteristics of the twenty selected microsatellite loci.
Microsatellite typing
A final set of 20 polymorphic microsatellites was selected from the microsatellites we tested. The forward primer for each locus was 5’ end labelled with fluorescent dye (). PCR was performed separately for each locus in a final reaction volume of 25 µL containing ~10-30 ng of genomic DNA, 0.2 µM each primer pair, 1X NH4 SO4/KCl PCR buffer, 3 mM MgCl2, 200 µM each dNTP, and 1U Taq polymerase (AIDLAB, Beijing, China). Multiplex reactions were performed following the size range and dye availability using ABI dye set D (). The optimal annealing temperature was established by a gradient amplification of 8 samples (annealing temperature from 50 to 62°C) on a Biometra Tgradient Thermal cycler (Biorad).
The sizes of the microsatellite alleles were visualised using an ABI PRISM 3130 Genetic Analyzer (Life Technologies), using a POP7 polymer and the internal size standard GeneScan500-Rox (Life Technologies). Genotypes were read with the ABI PRISM GeneScan 3.1.2 software (Applied Biosystems, Carlsbad, CA, USA) and interpreted with the ABI PRISM Genotyper 3.7 NT software (Applied Biosystems).
Statistical analysis
The mean number of alleles, observed and unbiased expected estimates of gene diversity, and their standard deviations, together with the polymorphic information content (PIC) were obtained using MICROSATELLITE TOOLKIT software (Park, Citation2001). We estimated non-exclusion probabilities considering the first (NE-1P), second (NE-2P) or parent pairs (NE-PP) and individual (NE-I) and sib identity (NE-SI) as well as the Hardy Weinberg Equilibrium (HWE), using Cervus software version 3.0.3 (Kalinowski et al., Citation2007). The combined posterior probability (PEC) was calculated with the algorithm of Jamieson (Citation1994). Deviations from HWE and Fis based on locus by locus AMOVA calculations were assessed using ARLEQUIN 3.5.1.3 (Excoffier and Lischer, Citation2010).
Results
Fluorescent polymerase chain reaction design and microsatellite genotyping
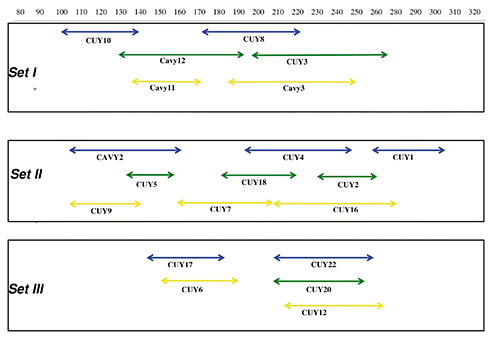
Based on amplification efficiency, success rate, and the absence of non-specific amplification of our primer pairs, a total of 16 microsatellites were selected for the panel design. We named these microsatellites CUY1, CUY2, CUY3, CUY4, CUY5, CUY6, CUY7, CUY8, CUY9, CUY10, CUY12, CUY16, CUY17, CUY18, CUY20, and CUY22. Additionally, 4 dinucleotide markers were selected from the Kanitz et al. (Citation2009) based on sequence length and marker polymorphisms () with no modification except for Cavy11 and Cavy 12, where the primer sequence was re-designed to improve the melting temperature parameter. A 4 colour system (ABI D Dye set) and a ~20 bp minimum predicted distance between loci was used to design the electrophoresis pattern. The unusually large distance between loci was designed because of a lack of references about this species, specifically information about expected allelic range. The panel of PCR amplification resulted in four PCR multiplexes divided into three electrophoresis sets (). The gradient amplification resulted in an optimal hybridisation temperature, based on the broadness of the band, of 55±0.5°C for all of the multiplexes, with the exception of the CUY 16 maker (60±0.5°C).
Marker polymorphism and quality
The allelic range (a region of the electropherogram where a locus specific allele can be found) we obtained was generally high. The mean difference between two alleles in the same individual ranged from 1.5 in CUY7 to 10.91 Cavy2.
A total of 216 alleles were found with a mean value of 10.80±3.49. All microsatellites were highly polymorphic with a minimum of 6 alleles (CUY6) and a maximum of 19 (Cavy12). The allelic richness ranged from a minimum of 4.002 for CUY9 and a maximum of 9.969 for Cavy12. We found observed and expected heterozygosity to have an average mean value of 0.590±0.115 and 0.778±0.080, respectively, which is considered high (). To evaluate the polymorphisms of each marker, the PIC value was calculated and found to range from 0.503 for CUY9 and 0.902 for Cavy12. Deviations from HWE were found in 9 of the 20 loci (); Cavy12 and CUY7 were found in disequilibrium in 6 populations, CUY2, CUY10 and CUY17 (P<0.05). The sample from Bolivia showed the highest number markers in disequilibrium (8) while the Spanish population showed the lowest ones (2). Fis values with a total mean value of 0.173.
Table 2. Descriptive statistics of the twenty designed microsatellite marker loci.
Panel set power statistics
In , the non-exclusion probability values are shown. The first two values (NE-1P and NE-2P) give the non-exclusion probability when the parents were considered one by one (the first parent and then the second parent of the opposite sex, respectively). In both cases, the higher value was for CUY9 (0.84 and 0.68), and the lower value was for Cavy12 (0.31 and 0.18). When parent pairs were considered, the results were comparable for identity and sibling identity non-exclusion probability, with a maximum value obtained for CUY9 (0.50, 0.25, and 0.54, respectively) and a lower probability for Cavy12 (0.05, 0.02 and 0.30, respectively).
Following the Jamieson (Citation1994) algorithm the combined posterior probability (PEC) was calculated (). The results show a high value for all types (0.99) but a smaller value for the sibling identity exclusion combined probability (0.84).
Table 3. Summary statistics for the non-exclusion probability values.
Discussion
The aim of our study was to construct a polymorphic marker panel of microsatellites that would be useful for both genetic diversity studies and kinship and parentage analysis in Cavia porcellus populations. Microsatellites are very powerful genetic markers that can be used for identifying the genetic structure, pedigree analysis and genetic variation of closely related species. Until the present work, only a few studies had been carried out on wild guinea pigs using either a reduced microsatellite loci panel (Asher et al., Citation2008; Kanitz et al., Citation2009; Kouakou et al., Citation2015) or AFLP loci (Burgos-Paz et al., Citation2011). Some biodiversity studies have been carried out in Africa using the Kanitz et al. (Citation2009) marker panel, such in Côte d’Ivoire (Kouakou et al., Citation2015) although these authors did not find clear genetic differences among the three analysed populations. The most complete study on the genus Cavia was performed on mitochondrial DNA (Dunnum and Salazar-Bravo, Citation2010). Domestic guinea pigs were included in these studies as an out-group. Our main objective was to compare the genetic diversity of the domestic guinea pig to the overall rearing area of the species. For this reason, we designed a panel of microsatellite markers to examine recent evolutionary events to infer the population structure and the genetic differentiation among different commercial lines and locally recognised guinea pig breeds. In addition, the importance of the guinea pig for the rural economy of several Latin American countries increases the need for molecular tools to further initiatives for their genealogical management and breeding design (Mommens et al., Citation1998; Tozaki et al., Citation2001; Bonnet et al., Citation2002). Despite the diffusion into local communities and the low technological level needed for guinea pig farming, there exists intense commercial activity for these animals. Dinucleotide microsatellites are being used as genetic markers for the identification of population structure, genome mapping, and pedigree analysis and to resolve taxonomic ambiguities in many other animals in addition to the guinea pig (Xu and Liu, Citation2011; Martinez et al., Citation2012; Gama et al., Citation2013; Abdul-Muneer, Citation2014).
We successfully isolated, by scaffold genome sequencing, 25 microsatellite sequences, of which 16 were selected for the final panel based on their technical quality. All markers proposed here can be easily amplified in multiplex PCR reactions using crude sample lysates. Generally, all of the loci had a very high number of alleles (10.8±3.40), which was higher than the values found by Kanitz et al. (Citation2009) and Kouakou et al. (Citation2015), as well as a high mean allelic range (25 bp). Even if only 11 loci out of 20 were in HWE in overall sample, the F index values were very high (0.173). These findings, despite the high number of alleles, can be explained by the small sample number used in this preliminary study, possibly leading to the maximization of heterozygous excess values (Wahlund, Citation1928), as highlighted also by the HW disequilibrium calculated by separated populations that showed a significant value for the sixth population only in the markers Cavy12 and CUY7. These results can be due by the particular mating system based on using inbreeding animals added to the great interchange of males and females in the country markets. The total combined exclusion probability highlighted that the 20 loci are enough to obtain a good efficiency for parentage testing and traceability purposes in this species.
Conclusions
We have identified a set of 16 microsatellite loci for domestic Cavia porcellus genetic diversity research, and we have also established their standardised genotype analysis parameters. These markers could potentially resolve parentage and individual assignment cases. The high degree of genetic diversity and polymorphisms indicate the potential of this microsatellite panel to be employed in future extended studies on the biodiversity of the cavy population. Therefore, genotype analyses with these standardised microsatellite panels will enhance cavy genetic selection by providing individual identification to increase the precision of measured phenotypes and for the construction of pedigrees to support the measurement of genetic estimates of phenotypic variation across generations.
Acknowledgements
The authors wish to express thanks to the different breeders and research groups who kindly provided biological samples: Angelika Stemmer (University of San Simon, Cochabamba, Bolivia), Niltón Gómez (Universidad Nacional del Altiplano, Puno, Perú), Luz Angela Franco (Universidad Nacional de Colombia, Palmira, Colombia) and D. Carlos San José Marqués (BioDonostia, Spain). The authors gratefully thank the members of the CONBIAND network for valuable cooperation over the years.
Additional information
Funding
References
- Abdul-MuneerP.M., 2014. Application of microsatellite markers in conservation genetics and fisheries management: recent advances in population structure analysis and conservation strategies. Genet. Res. Int. 2014:691759.
- AsherM. LippmannT. EpplenJ.T. KrausC. TrillmichF. SachserN., 2008. Large males dominate: ecology, social organization, and mating system of wild cavies, the ancestors of the guinea pig. Behav. Ecol. Sociobiol. 62:1509–1521.
- BonnetA. ThevenonS. MaudetF. MaillardJ.C., 2002. Efficiency of semi-automated fluorescent multiplex PCRs with 11 microsatellite markers for genetic studies of deer populations. Anim. Genet. 33:343–350.
- Broad Institute, 2015. Guinea pig genome project. Available from: http://www.broadinstitute.org/science/projects/mammals-models/guinea-pig/guinea-pig
- Burgos-PazW. Ceron-MunozM. Solarte-PortillaC., 2011. Genetic diversity and population structure of the Guinea pig (Cavia porcellus, Rodentia, Caviidae) in Colombia. Genet. Mol. Biol. 34:711–718.
- DAD-IS, 2014. DAD-IS: domestic animal diversity information system. Available from: http://dad.fao.org/cgibin/EfabisWeb.cgi?sid=d856f41036885944dea1b5f0c1525bab,reports
- DunnumJ.L. Salazar-BravoJ., 2010. Molecular systematics, taxonomy and bio-geography of the genus Cavia (Rodentia: Caviidae). J. Zool. Syst. Evol. Res. 48:376–388.
- ExcoffierL. LischerH.E., 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Res. 10:564–567.
- GamaL.T. MartinezA.M. CarolinoI. LandiV. DelgadoJ.V. VicenteA.A. Vega-PlaJ.L. CortesO. SousaC.O., 2013. Genetic structure, relationships and admixture with wild relatives in native pig breeds from Iberia and its islands. Genet. Sel. Evol. 45:18.
- GuerriniA., 2003. Experimenting with humans and animals: from Galen to animal rights. Johns Hopkins University Press, Baltimore, MD, USA.
- JamiesonA., 1994. The effectiveness of using co-dominant polymorphic allelic series for (1) checking pedigrees and (2) distinguishing full-sib pair members. Anim. Genet. 25:37–44.
- KalinowskiS.T. TaperM.L. MarshallT.C., 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16:1099–1106.
- KanitzR. TrillmichF. BonattoS., 2009. Characterization of new microsatellite loci for the South-American rodents Cavia aperea and C. magna. Conserv. Genet. Resour. 1:47–50.
- KouakouP.K. SkiltonR. ApollinaireD. AgatheF. BeatriceG. ClémentA.S., 2015. Genetic diversity and population structure of cavy (Cavia porcellus L) in three agro ecological zones of Côte d’Ivoire. Int. J. Agron. Agr. Res. 6:27–35.
- MaassB.L. JamnadassR.H. HansonJ. PengellyB.C., 2005. Determining sources of diversity in cultivated and wild Lablab purpureus related to provenance of germplasm by using amplified fragment length polymorphism. Genet. Resour. Crop. Ev. 52:683.
- MaassB.L. Katunga-MusaleD. ChiuriW.L. ZozoR. PetersM., 2010. Livelihoods of smallholders in South Kivu depend on small livestock: the case of the ‘cobaye’. Available from: www.tropentag.de/2010/abstracts/full/491.pdf
- ManjeliY. TchoumboueJ. NjweR.M. TeguiaA., 1998. Guinea-pig productivity under traditional management. Trop. Anim. Health Pro. 30:115–122.
- MartinezA.M. GamaL.T. CanonJ. GinjaC. DelgadoJ.V. DunnerS. LandiV. Martin-BurrielI. PenedoM.C. RodellarC. Vega-PlaJ.L. AcostaA. AlvarezL.A. CamachoE. CortesO. MarquesJ.R. MartinezR. MartinezR.D. MelucciL. Martinez-VelazquezG. MunozJ.E. PostiglioniA. QuirozJ. SponenbergP. UffoO. VillalobosA. ZambranoD. ZaragozaP., 2012. Genetic footprints of Iberian cattle in America 500 years after the arrival of Columbus. PLoS One 7:e49066.
- MatthiesenT. NyameteF. MsuyaJ.M. MaassB.L., 2011. Importance of guinea pig husbandry for the livelihood of rural people in Tanzania: a case study in Iringa region. Available from: http://www.tropentag.de/2011/abstracts/links/Matthiesen_llDdf2DY.pdf
- MommensG. Van ZeverenA. PeelmanL.J., 1998. Effectiveness of bovine microsatellites in resolving paternity cases in American bison, Bison bison L. Anim. Genet. 29:12–18.
- MoralesE., 1995. The guinea pig: healing, food, and ritual in the Andes. University of Arizona Press, Tucson, AZ, USA.
- ParkS.D.E., 2001. Trypanotolerance in west african cattle and the population genetics effects of selection. University of Dublin, Dublin, Ireland.
- RozenS. SkaletskyH.J., 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000:365–386.
- TozakiT. KakoiH. MashimaS. HirotaK. HasegawaT. IshidaN. MiuraN. Choi-MiuraN.H. TomitaM., 2001. Population study and validation of paternity testing for Thoroughbred horses by 15 microsatellite loci. J. Vet. Med. Sci. 63:1191–1197.
- WahlundS., 1928. Zusammensetzung von Population und Korrelationserscheinung vom Standpunkt der Vererbungslehre aus betrachtet. Hereditas 11:65-106.
- XuQ. LiuR., 2011. Development and characterization of microsatellite markers for genetic analysis of the swimming crab, Portunus trituberculatus. Biochem. Genet. 49:202–212.
