Abstract
High melatonin concentrations were expected to negatively affect Atlantic salmon appetite. Hence, individually kept Atlantic salmon postsmolts were subjected to continuous light (24:0, L:L) or natural daylight (12:12, L:D) and then given intraperitoneal implants of slow release melatonin in a 2 by 2 factorial design. Possible effects on food intake were monitored over three weeks. In addition, a plasma melatonin assessment test was run in parallel to monitor diurnal blood levels of melatonin through the trial. The surgical intervention was effective, leading to a 10x increase in mean melatonin levels when compared to control fish, but neither the implant nor the change in daylight had any significant effect on feed intake, at least in the short term.
Introduction
A good synchronism between the organism’s metabolism and the surrounding environment represents a clear advantage in the struggle for existence. The metabolic system of several animals, including salmonids, has rhythmically oscillating mechanisms controlling biological processes that repeat themselves over a period of approximately 24 h. In absence of environmental cues these endogenous oscillators should run close to 24 h, but most of the time they tune and reset in response to a specific external stimulus (Roenneberg et al., Citation2003). The most typical stimulus is the photoperiod, defined as the ratio of light to dark over a period of 24 h.
Variations in photoperiods are known to affect numerous biological functions in salmon including osmoregulation, smoltification, sexual maturation and growth hormone (Stefansson et al., Citation1991). Appetite reductions following prolonged periods of darkness and conversely increased feed intake during continuous light is a common feature in Atlantic salmon parr (Skilbrei et al., Citation1997) and postsmolts (Kråkenes et al., Citation1991; Harmon et al., Citation2012). Furthermore, the use of continuous-light regimes during winter and spring leads to enhanced growth and improved feed conversion (Nordgarden et al., Citation2003; Oppedal et al., Citation2003). However, an initial appetite and growth-rate reduction were found after the onset of a new photoperiod, probably as a consequence of the stress caused by the environment changing (Endal et al., Citation2000; Nordgarden et al., Citation2003; Oppedal et al., Citation2003).
One possible contributor to these effects is melatonin. Most of the plasma melatonin found in salmon is secreted by the pineal gland, which in turn is directly regulated by photoperiod. During periods with oscillating light and dark phases, the melatonin levels are low during daytime and gradually increase at night. However, under constant darkness the melatonin does not display the free-running rhythms as seen in many other animals and its natural nocturnal rise is abolished (Huang et al., Citation2010a, Citation2010b; Migaud et al., Citation2007; Porter et al., Citation2001; Randall et al., Citation1995). Melatonin can also be produced by other tissues like the eyes, but in fairly small quantities and it is uncertain if this melatonin reaches the systemic circulation. The low systemic contribution of the eyes has been proved by the complete abolishment of plasma melatonin level in pinealectomized fish (Porter et al., Citation1996), while ophthalmectomy has no effect (Migaud et al., Citation2007). An abrupt increase in plasma melatonin levels may modify the major signals involved in the control of an animal’s daily activity, as already observed in Mammals. For instance, single melatonin injections in rat lead to an altered transcription of molecular timing genes’ mRNA (Poirel et al., Citation2003), while a periodic treatment with this hormone causes a significant increase in these clock gene mRNA mean 24-hr levels (Mattam and Jagota, Citation2014), irrespective of photoperiod. Therefore artificially overriding natural melatonin rhythm by using slow release implants is an interesting method to study the melatonin functions in fish. Unnaturally high hormone concentration could be interpreted by the metabolism as a short daylight duration, thus leading to a decrease in food intake. To date, few experimental evidence supports this hypothesis in fish – none of each concerning specifically postsmolt salmon (Handeland et al., Citation2013; Piccinetti et al., Citation2010) – while, on the other hand, melatonin implanted salmon parr appeared to be significantly larger than the controls one month after the treatment (Porter et al., Citation1998). However, Handeland et al. (Citation2013) suggested that the cause of this discrepancy is probably due to the different experimental protocols used (e.g. temperature, fish developmental stage, time of the year). The aim of the present research was to partly fill this gap, focusing on short-term effects of melatonin implants on food intake in Atlantic salmon postsmolts reared either under normal light/dark conditions or continuous-light.
Materials and methods
Fish stock and rearing conditions
The trial was run at the Institute of Marine Research (IMR) in Matre (60°N, 5°E, Western Norway). The fish (Aquagen Ltd., Kyrkæsæterøra, Norway) were hatched in autumn 2013. The smoltification was induced by employing a standard smolt-production model, subjecting parr to 6 weeks of winter signal (12:12, L:D) followed by 6 weeks summer signal (24:0, L:D) and then transferred to seawater. The postsmolts were then acclimated into a circular flow-through seawater tank (21,500 L), where they were maintained under ambient temperature (around 8°C) until use. The fish were fed a commercial extruded diet (Nutra Olympic 3; Skretting AS, Stavanger, Norway) having a high-protein/low-fat ratio [crude protein 47-50%, crude fat 23%, nitrogen-free extract (NFE) 11-13%]. Feeding was performed using feeders (ARVO-TEC T; Arvotec, Huutokoski, Finland), controlled automatically by a PC-operated system together with lighting.
On March 2014, 138 of the postsmolts were randomly sorted and moved into two new facilities, called the Individual Lab. – dedicated to food-intake measuring – and the Melatonin Unit – dedicated to assessing plasma melatonin variations. Photoperiod (LL), water salinity, temperature and feed type were kept constant until the day of the treatment. The treatment was performed when most of the fish reached a stable level of appetite. The morphometric parameters () were taken prior to the blood sampling.
Table 1. Morphometric parameters of the melatonin unit’s tanks obtained from the last groups sampled, 3 weeks after the injection.
Experiment 1: food intake trend
The Individual Lab. had 16 square, covered tanks (0.5 x 0.5 x 0.28 m). Three fish were moved into each tank on 15th March. Once they reached an acceptable level of appetite (around 0.5% of the total body mass) two fish were removed by netting following anaesthetisation with a mild sedative (0.03 mL L–1 of Aqui-s). The remaining 16 fish were fed manually to satiation, with minimal disturbance, twice a day, with the 1st feeding starting at 9:00h and the 2nd feeding at 15:30h. On 15th April, two light regimes (LL and LD) were combined with two treatments (implant or sham implant) in a 2 by 2 factorial design, obtaining 4 different groups composed of 4 fish each. Possible effects on food intake were monitored over 3 weeks. All fish were finally blood sampled on 9th of May.
Experiment 2: plasma melatonin trend
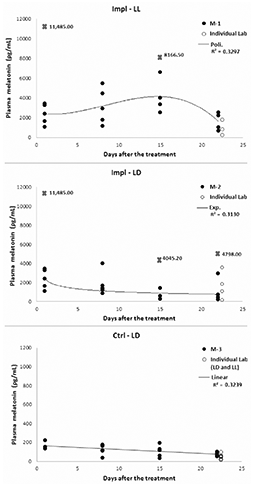
The Melatonin Unit was composed of 3 square, covered tanks (1.5 x 1.5 x 0.47 m), containing 30 fish each. Fish were fed using an automatic feeder (feeding time: 8.30-10.30 and 13.30-15.30). On 24th April, fish in two tanks were implanted with melatonin, while fish in the last tank were given a sham implant (punctured by the needle but not injected). One day (24h) later 3 fish were randomly collected from each tank, anaesthetized and blood collected for melatonin analysis. The photoperiod was then changed from LL to LD in one melatonin group and in the sham group. Further blood samples were taken every 7th day over three consecutive weeks (4 time points: 1-8-15-22 days after the treatment, as shown in ). The delayed change in the photoperiod allowed to use the control tank for both the control groups: the first sampling provided the values of the Control-LL (Ctrl-LL) treatment (whose melatonin concentration was expected not to change over the span of 3 weeks), while all the following samplings furnished the concentration of the Control-LD (Ctrl-LD) group.
Implant and blood sampling protocol
The method adopted is automated, minimally invasive and fairly similar to the procedure used when tagging fish with PIT tags. Prior to implant, all fish were firstly mildly sedated in the tanks (0.02 g/L of Finquel) and then completely anesthetised in 0.1 g/L of Finquel. The melatonin pellet (REGULIN; Schering Agrochemicals, Alexandria, Australia) was implanted in the rear peritoneum after incising with a scalpel (no.11) a thin tissue layer on the left side of the animal between the anal and ventral fins, well below the lateral line. The surgical cut was necessary to create a way for the subsequent introduction of the needle. A pre-test followed by necroscopy was performed to check possible tissue damages and to verify the absorption of the melatonin pellet by the gastrointestinal walls.
After netting and anesthetizing the fish with an overdose of anaesthesia, blood was collected via the caudal dorsal aorta in 2 mL heparinized syringes under comparable light and time regime (always between 13:00h and 14:00h). Plasma samples were separated by centrifugation (13,200 rpm for 90s at 4°C) and stored at -80°C until assayed for melatonin. The experiment was approved according to The Regulations in Animal Experimentation in Norway and carried out by certified personnel.
Appetite and melatonin assessment
The feed intake in the individual tanks was calculated as the difference between fed-pellets and recovered uneaten-pellets (pellets collected in a sieve from outlet water). The daily fish biomass was calculated according to the following formula, based on Bagenal and Tesch studies (Citation1978):
where (Wi) and (Wf) are the initial and final weights respectively, and day n and day n-1 indicates a particular day and its preceding day. After having converted the number of eaten-pellets into grams and having calculated the Daily individual appetite as ![]() the Deviation from the average appetite (i.e. the average value of the last 5 days prior the treatment) was determined. Feed Intake was therefore calculated as the positive or negative deviation from this value.
the Deviation from the average appetite (i.e. the average value of the last 5 days prior the treatment) was determined. Feed Intake was therefore calculated as the positive or negative deviation from this value.
The hormone concentration was assessed using the Melatonin ELISA (RE54021; IBL International GMBH, Hamburg, Germany), an enzyme immunoassay for the in-vitro diagnostic quantitative determination of melatonin in plasma. The kit was used as outlined by the producer and the optical density (OD) was measured with a photometer at 405 nm. The Standards were analysed in duplicates. All samples from implanted fish were diluted with diluted Wash Buffer prior to analysis. All data are given as melatonin pg mL-1 of plasma.
Statistical analysis
Statistical analysis of the melatonin data were performed using IBM SPSS Statistics (IBM Corp., Citation2013), while analysis of the feed-intake was done with R software (R CORE TEAM, Citation2013). Mann-Whitney U test was used to check possible significant differences within the 5 melatonin values of each time-point. The average intake values of each treatment were compared 3 weeks after the treatment through an ANOVA test, while within each group the intake-variation (that is the comparison of the average appetite prior to the treatment with the appetite 3 weeks later) was assessed with a paired t-test. The initial weight homogeneity of the four fish groups was tested with an ANCOVA test (combining a regression analysis and an ANOVA). This calculation was necessary to make sure that the initial weight did not affect the individual treatment reaction and, consequently, the feed intake performance.
Results
Plasma melatonin trend
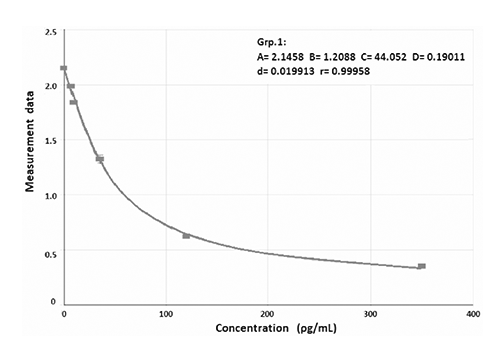
A good fit of the immunoassay Standards obtained was provided by a four parameters Marquardt curve, as shown in . Individual Lab. values 3 weeks after the treatment had a good fit with the results from the Melatonin Unit (). It can therefore be assumed that the release rates of the individual fish would be the same. The implant treatment lead to melatonin values between 1500 and 4500 ρg mL-1 in fish subjected to continuous light (LL) and between 500 and 3500 ρg mL-1 in the LD group. Control fish always showed melatonin concentrations below 220 ρg mL-1. In all charts there was a slight downward trend over time indicating exhaustion of the implants. The melatonin concentration of the Ctrl-LL group was assumed not to change over time due to the absence of both an artificial hormone raise and a light-darkness alternation.
Food intake trend
With the exception of a few tanks, the deviation from the average intake (average of the last 5 days prior the treatment) appeared to be located within a small range: -1% immediately after the implant and +1% 22 days later (). The general trend showed an initial small appetite decrease due to surgery followed by a quick recovery and a food intake stabilisation, particularly evident in the two control groups. Only two fish (n. 5 and n. 14) did not recover and their appetite gradually diminished after 2 weeks.
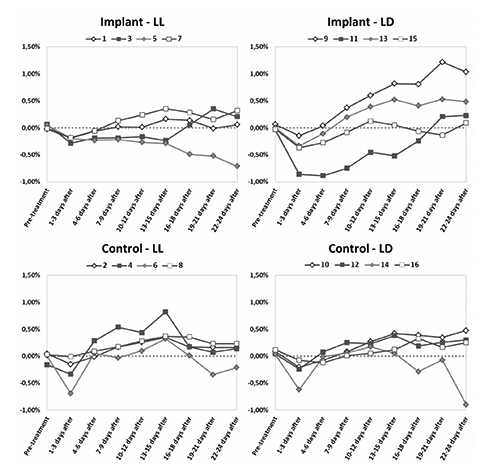
There were no statistical significant differences in appetite between the 4 treatments except for the Implant-LD group, where there was a tendency to strong variation in appetite. There did not appear to be any relationship between plasma melatonin at final sampling and feeding history of the fish.
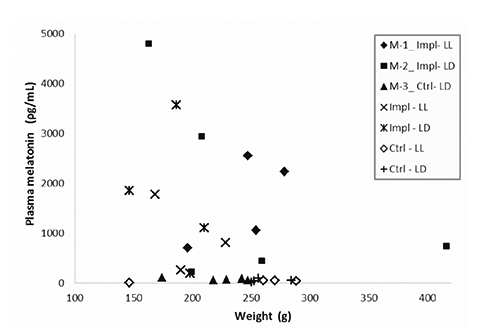
Relationship biomass-melatonin
All Control fish had melatonin values below 220 ρg mL-1, independently from their biomass (). No relationships between weight and melatonin were observable within the Implant groups as well.
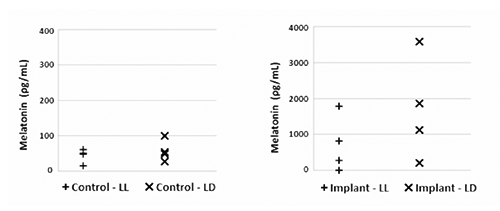
Relationship photoperiod-melatonin
Photoperiod did not appear to have any effect on the melatonin level over the 3 weeks of observation (): comparing the Individual Lab. melatonin concentrations through the ANOVA test, the two Control groups resulted to be perfectly comparable P=0.50). The comparison between the two Implanted groups furnished a similar result (P=0.47), even if their values fell into a wider range (200-1800 ρg mL-1).
Discussion
Melatonin trend
The diurnal melatonin concentration is known not to change over time (Migaud et al., Citation2006; Porter et al., Citation2001; Randall et al., Citation1995), therefore hormone levels during both the feeding sessions are well represented by the levels assessed between 13:00h and 14:00h. Moreover the comparison of the current results with previous studies on Atlantic salmon postsmolts is possible, since – according to both the results of the present study and the finding of Porter et al. (Citation2001) – the three critical variables photoperiod, fish size and temperature profiles show to have no effect on melatonin concentration in the daylight. In both Control groups the diurnal melatonin levels were, indeed, below 200 ρg mL-1 irrespective of photoperiod, confirming previous results in Atlantic salmon parr (Porter et al., Citation2001) and postsmolts (Migaud et al., Citation2006; Randall et al., Citation1995). Implanted fish show melatonin concentrations ten times higher, but no research has been published on this topic yet.
Appetite trend
The present results show that postsmolt metabolism did not respond as expected neither to artificial melatonin increase nor to changes in light conditions: rather than lowering their appetite, all groups had a similar stable intake over the 3 weeks of study.
These findings confirm the results of Porter et al. (Citation1998) and contrast with the other two publications mentioned in the Introduction (Handeland et al., Citation2013; Piccinetti et al., Citation2010). One plausible explanation is that the present work represents the first study about effects of melatonin implant on Atlantic salmon postsmolts, while Porter et al. (Citation1998) and Handeland et al. (Citation2013) researches concerned smoltifying subjects and Piccinetti et al. (Citation2010) focused on zebra fish. Therefore the observed similarities and discrepancies might be due to both the different species and protocols used.
Conclusions
The surgical method adopted was successful: the implant was highly effective and almost all the subjects recovered well after the treatment. However, both implanted and control fish quickly regained their pre-test appetite, showing a similar intake over the 3 weeks of study. The present research proved that both photoperiod and diurnal plasma melatonin concentration do not affect food-intake in post-smolt Atlantic salmon in the short term. However, researches over a longer observation period are required before drawing any kind of conclusion.
Acknowledgements
Thanks are due to the whole staff of Matre Aquaculture Station for their kindness and skilled technical assistance during the entire work period, to Stian Morken, Marita Larsen, Marsela Alvanopoulou and Anna Concollato for their willingness to help whenever needed, and to Erasmus placement scholarship that financially supported the stage of Silvia Maiolo at Matre.
References
- BagenalT.B. Tesch F.W., 1978. Age and growth. In: BagenalT.B. (ed.) Methods for assessment of fish production in freshwaters, 3rd ed. Blackwell Scientific Publications, Oxford, UK, pp 101–136.
- EndalH.P. Taranger G.L. Stefansonn S.O. Hansen T., 2000. Effects of continuous additional light on growth and sexual maturity in Atlantic salmon, Salmo salar, reared in sea cages. Aquaculture 191:337–349.
- HandelandS.O. Imsland A.K. Björnsson B.T. Stefansson S.O. Porter M., 2013. Physiology during smoltification in Atlantic salmon: effect of melatonin implants. Fish Physiol. Biochem. 39:1079–1088.
- HarmonP.R. Peterson R.H. Glebe B.D., 2012. The effect of photoperiod on growth and maturation of Atlantic salmon (Salmo salar) in the Bay of Fundy. Available from: http://publications.gc.ca/pub?id=476394&sl=0
- HuangT.S. Ruoff P. Fjelldal P.G., 2010a. Diurnal expression of clock genes in pineal gland and brain and plasma levels of melatonin and cortisol in Atlantic salmon parr and smolts. Chronobiol. Int. 27:1697–1714.
- HuangT.S. Ruoff P. Fjelldal P.G., 2010b. Effect of continuous light on daily levels of plasma melatonin and cortisol and expression of clock genes in pineal gland, brain, and liver in Atlantic salmon postsmolts. Chronobiol. Int. 27:1715–1734.
- IBM Corp., 2013. IBM SPSS statistics for Windows, Version 22.0. IBM Corporation, Armonk, NY, USA.
- KråkenesR. Hansen T. Stefansson S.O. Taranger G.L., 1991. Continuous light increases growth rate of Atlantic salmon (Salmo salar L.) postsmolts in sea cages. Aquaculture 95:281–287.
- MattamU. Jagota A., 2014. Differential role of melatonin in restoration of age-induced alterations in daily rhythms of expression of various clock genes in suprachiasmatic nucleus of male Wistar rats. Biogerontology 15:257–268.
- MigaudH. Davie A. Martinez ChavezC.C. Al-Khamees S., 2007. Evidence for differential photic regulation of pineal melatonin synthesis in teleosts. J. Pineal Res. 43:327–335.
- MigaudH. Taylor J.F. Taranger G.L. Davie A. Cerdá-Reverter J.M. Carrillo M. Hansen T. Bromage N.R., 2006. A comparative ex vivo and in vivo study of day and night perception in teleosts species using the melatonin rhythm. J. Pineal Res. 41:42–52.
- NordgardenU. Oppedal F. Taranger G.L. Hemre G.I. Hansen T., 2003. Seasonally changing metabolism in Atlantic salmon (Salmo salar L.) I. Growth and feed conversion ratio. Aquacult. Nutr. 9:287–293.
- OppedalF. Taranger G.L. Hansen T., 2003. Growth performance and sexual maturation in diploid and triploid Atlantic salmon (Salmo salar L.) in seawater tanks exposed to continuous light or simulated natural photoperiod. Aquaculture 215:145–162.
- PiccinettiC.C. Migliarini B. Olivotto I. Coletti G. Amici A. Carnevali O., 2010. Appetite regulation: the central role of melatonin in Danio rerio. Horm. Behav. 58:780–785.
- PoirelV.J. Boggio V. Dardente H. Pevet P. Masson-Pevet M. Gauer F., 2003. Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei. Neuroscience 120:745–755.
- PorterM.J.R. Duncan N. Handeland S.O. Stefansson S.O. Bromage N.R., 2001. Temperature, light intensity and plasma melatonin levels in juvenile Atlantic salmon. J. Fish Biol. 58:431–438.
- PorterM.J.R. Randall C.F. Bromage N.R., 1996. The effect of pineal removal on circulating melatonin levels in Atlantic salmon parr. J. Fish Biol. 48:1011–1013.
- PorterM.J.R. Randall C.F. Bromage N.R. Thorpe J.R., 1998. The role of melatonin and the pineal gland on development and smoltification of Atlantic salmon (Salmo salar) parr. Aquaculture 168:139–155.
- R Core Team, 2013. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: http://www.r-project.org/
- RandallC.F. Bromage N.R. Thorpe J.E. Miles M.S. Muir J.S., 1995. Melatonin rhythms in Atlantic salmon (Salmo salar) maintained under natural and out-of-phase photoperiods. Gen. Comp. Endocr. 98:73–86.
- RoennebergT. Daan S. Merrow M., 2003. The art of entrainment. J. Biol. Rhythms 18:183–194.
- SkilbreiO.T. Hansen T. Stefansson S.O., 1997. Effects of decreases in photoperiod on growth and bimodality in Atlantic salmon Salmo salar L. Aquac. Res. 28:43–49.
- StefanssonS.O. Björnsson B.T. Hansen T. Haux C. Taranger G.L. Saunders R.L., 1991. Growth, parr-smolt transformation, and changes in growth hormone of Atlantic Salmon (Salmo salar) reared under different photoperiods. Can. J. Fish. Aquat. Sci. 48:2100–2108.