Abstract
The present study was aimed to investigate the number and size of muscle fibre and their relation to carcass quality traits in chickens (slow- and fast-growing chicken strains). A total of 40 one-day-old Arbor Acres broiler (fast-growing) and 40 Thai native chickens (slow-growing) were reared to 45 and 112 days, respectively. The Arbor Acres broilers had heavier live weight, higher breast and thigh percentage than Thai native chickens (P<0.001). In breast muscle, there was no significant difference in total number of fibres and perimysium thickness. Thai native chickens had smaller fibre diameter and fibre area (P<0.01), and thicker endomysium in comparison with Arbor Acres broiler (P<0.001). The difference between the thigh and breast muscle fibre characteristics was not significant (P>0.05). The fibre diameter was positively correlated with live weight (P<0.05) and breast percentage (P<0.01). Endomysium thickness was correlated with live weight and breast percentage (P<0.05). There was no significant difference for the correlation between muscle fibre characteristics and thigh muscle. These results suggest that muscle fibre characteristics might be related to carcass quality.
Introduction
White meat such as chicken meat is considered superior in human health aspects to red meat because of comparably low contents of fat and cholesterol. Consumers also acknowledge the relatively low price, the typically convenient portions and the lack of religious restriction against its consumption (Jaturasitha, Citation2004). In 5 years (2008 to 2013), the world’s chicken meat production and consumption increased of about 3.14 to 3.29% per year (Department of Foreign Trade, Citation2014). Chicken represents 86% of total animal production in Thailand (Department of Livestock Development, Citation2013). There are many chicken breeds for consumption. The native chicken is one of the most consumed. The meat characteristics of the native chickens are very different from the broilers as they are low in fat, have firm texture and are tasty (Wattanachant et al., Citation2004; Chuaynukool et al., Citation2007; Jaturasitha et al., Citation2008). Consequently, the native chickens are becoming popular among Thai consumers, which leads to a price increase. However, indigenous breeds have slower growth rate than the commercial broilers. Broilers have increased body weight and are slaughtered at a younger age than chickens (Rémignon et al., Citation1996). A broiler has 1.2 to 2.0 kg of live weight at 38 to 45 days, while a native chicken will get the same live weight at 4 to 5 months (Wattanachant, Citation2008). The increasing of skeletal muscle mass is influenced by the characteristics of the muscle fibre (Bee et al., Citation2006; Puolanne et al., Citation2006; Chen et al., Citation2007). As reported previously, the selection for increasing in growth rate has resulted in concomitant changes in both the number and size of muscle fibres in pigs (Choi et al., Citation2013; Kim et al., Citation2013) and chickens (Remignon et al., Citation1995). The size of muscle fibres is influenced by age, sex, muscle type and breed (Wattanachant, Citation2008). Old animals with heavier carcass weight exhibit a greater muscle fibre cross-sectional area and thicker perimysium and endomysium than the young animals with lighter carcass weight (Żochowska et al., Citation2005). The indigenous breeds have smaller muscle fibre diameter than the commercial breeds (Chaosap and Tuntivisoottikul, Citation2006; Jaturasitha et al., Citation2008). Many studies have revealed muscle fibre characteristics in chickens (Liu et al., Citation1994; Chen et al., Citation2007; Khoshoii et al., Citation2013); still, the relationships between muscle fibre characteristics and carcass quality have been little examined.
The objective of this study was to investigate the muscle fibre characteristics and their effect on carcass quality in Arbor Acres broilers and Thai native chickens. This data leads to a better understanding of muscle fibre and its response to carcass quality.
Materials and methods
Animals
The study was conducted at Kasetsart University, Bangkok, Thailand. For this trial 40 Arbor Acres broilers (fast-growing) and 40 Thai native chickens (slow-growing) – which were one day old – were used. The animals were fattened in pens of 10 birds (6 pens/breed). The animals were fed ad libitum with commercial feed. Chickens were fed to the same feeding programme: starter [21% crude protein (CP) and 3100 kcal of metabolisable energy (ME)/kg diet, 0 to 14 d], grower (20%CP and 3100 kcal of ME/kg diet, 14 to 21 d), and finisher (18%CP and 3200 kcal of ME/kg diet, 21 d to slaughtering). Each genotype was represented by 60 birds. Forty randomly selected chickens of each genotype (equivalent to 6 to 7 birds per pen) were fasted. The animals were slaughtered on 45 and 112 days for Arbor Acres broilers and Thai Native chickens (Pradu-Hangdum breed), respectively. A total of 80 chickens were fasted for 12 hours until slaughter, then weighed and slaughtered by cutting the cervical blood vessels and bled out for 7 to 10 min. After scalding in water at a temperature 56°C for 1 min, the chickens were manually plucked and eviscerated. The carcasses were stored at 4°C for 24 hours, then dressed and dissected following the method of Jaturasitha et al. (Citation2008). The live weight, dressing weight, and breast and thigh weights of each bird were recorded. Then, dressing percentage was calculated as the ratio between the carcass weight and body weight after fasting. The weight percentages of breast muscle and thigh muscle were given as the percentages of cold carcass weight.
Muscle collection and histological processing
The breast (Pectoralis major) and thigh (Biceps femoris) muscle samples of 3 chickens per breed were randomly selected for histological analysis. Specimens measuring 0.5 cm x 0.5 cm x 1 cm were taken 15 min post-mortem after carcass bleeding. The samples were immediately fixed in 10% buffered neutral formalin solution for 24 hours, dehydrated in alcohol, cleared in xylene, infiltrated and finally embedded in paraffin (Khoshoii et al., Citation2013). The sections were cut at 3 μm thickness and stained with hematoxylin and eosin stain for general histological study. Stained cross-sections were viewed and photographed with a light microscope (Olympus FSX100; Olympus, Tokyo, Japan) at 10X objective lens and a 10X eye-piece (Tuma et al., Citation1962). Five photographs of different cross-sections from each muscle were taken. The samples were determined by using Image-J software (National Institute of Mental Health, Bethesda, MD, USA). The mean number of fibres per area was obtained by counting the total number of fibres (TNF) in five areas (each area: 593,946 µm2) per bird (Alves et al., Citation2012). The mean ≈300 fibres in five random fields for each muscle were measured to estimate the cross-sectional areas (µm2) and diameters (µm) of individual muscle fibres (Rémignon et al., Citation1996). Thickness of the endomysium and perimysium were determined on each sample according to the procedures outlined by Liu et al. (Citation1996) with modifications by Rahaman et al. (Citation2010) and ochowska et al. (Citation2005). The structural elements were measured in an area of fibre bundle. Forty measurements of the thickness (µm) of endomysium, and 10 measurements of the thickness (µm) of primary perimysium were made on each picture. The mean thickness was estimated from the measured values.
Statistical analyses
The data were analysed by paired t tests of SAS (SAS Inst. Inc., Cary, NC, USA). Values of P<0.05 were considered to indicate statistically significant differences. Pearson’s correlation coefficients were used for testing the correlations between muscle fibre characteristics and carcass quality.
Results
Carcass quality
The carcass quality traits were presented in . There were significant differences in live weights, breast and thigh percentages of genotypes (P<0.001). It was determined that Arbor Acres broilers had higher live weight, breast and thigh percentage than Thai native chickens (P<0.001). The dressing percentage did not differ between the genotypes (P>0.05).
Table 1. Carcass quality traits of the Arbor Acres broilers and Thai native chickens (n=40 per genotype).
Muscle fibre characteristics
Total number of fibres per area and perimysium thickness of the breast muscle of the genotypes were not significantly different (P>0.05) (). The fibre diameter and fibre area in breast muscle of Arbor Acres broilers were higher than in Thai native chickens (P<0.01). The breast muscle of Arbor Acres broiler had lower endomysium thickness in comparison with Thai native chickens (P<0.01). There were not significantly differences between groups in term of TNF, fibre diameter, fibre area, perimysium thickness and endomysium thickness in thigh muscle. The fibre characteristics of breast and thigh muscles were represented in .
Table 2. Muscle fibre characteristics of the Arbor Acres broilers and Thai native chickens (n=3 per genotype).
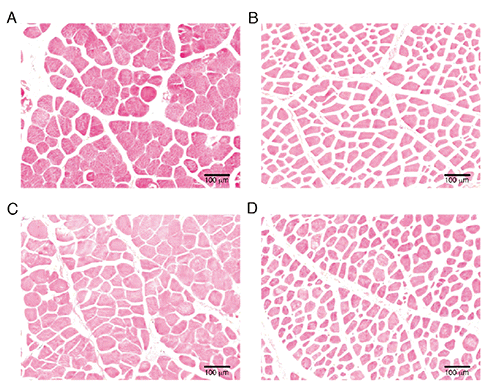
Table 3. Correlation coefficients between muscle fibre characteristics and carcass quality traits in breast muscle (n=6 in total).
Relationship between muscle fibre characteristics and carcass quality traits
The correlation between muscle fibre characteristics and carcass quality are presented in and . In breast muscle, fibre diameter was positively correlated with live weight (P<0.05) and breast percentage (P<0.01). The breast percentage was positively correlated with fibre area (r=0.94, P<0.01). Moreover, endomysium thickness was correlated with live weight (r=-0.88, P<0.05), dressing percentage (r=0.81, P<0.05) and breast percentage (r=-0.90, P<0.05). It showed no association in the correlation between muscle fibre characteristics and carcass quality traits in thigh muscle (P>0.05).
Table 4. Correlation coefficients between muscle fibre characteristics and carcass quality traits in thigh muscle (n=6 in total).
Discussion
Carcass quality
The indigenous chicken (slow-growing) generally has slower growth rate than the commercial broiler (fast-growing) when raised under the same commercial conditions (Wattanachant, Citation2008). Especially, the indigenous chicken strains from Thailand (Black-boned chickens) have lower growth rate than the imported chicken breeds (Bresse and Rhode Island Red) at 16 wk of age (Jaturasitha et al., Citation2008). Our study has revealed the carcass quality of broilers (45 days) and native chickens (112 days). The breast and thigh percentage of Arbor Acres broilers is higher than in Thai native chickens. The broilers are fast growing and specifically bred for meat production compared with the native chickens (Van Marle-Köster and Webb, Citation2000; Dyubele et al., Citation2010). The native chickens are extremely active and aggressive even under captivity resulting in more energy dissipation (Khalid et al., Citation2012). Wattanachant (Citation2008) stated that the appropriate age for consumption of the indigenous chicken was 16 to 20 weeks with 1.2 to 1.5 kg live weight. Chen et al. (Citation2007) reported that the breast muscle percentage was significantly positively correlated to body weight. According to Olawumi (Citation2013), there is a positive correlation between breast weight (r=0.89) and thigh weight (r=0.95) with live weight.
Muscle fibre characteristics
Characteristics of the growth and time course for full growth of each muscle in the chicken are unique (Chen et al., Citation2007). Postnatal growth of skeletal muscle is accompanied by increased size of individual myofibres, diameter and elongation (Smith, Citation1963; Chen et al., Citation2007). In the current study, the total numbers of fibre per area in the breast and thigh muscles of Arbor Acres broilers at day 45 were in a range of 170-183 fibres per 593,946 µm2. Alves et al. (Citation2012) revealed that the Sartoirus muscles of broiler had 617 and 597 fibres per 892,967 m2 at 28th and 42nd day, respectively. The muscle fibre area of chicken at 55 days of age was 2755 µm2 and 1946 µm2 in rapid-growing and slow-growing chicken, respectively (Dransfield and Sosnicki, Citation1999). This might depend on different muscle types, ages, breeds and muscle fibre sizes. The fast-growing chickens show a rapid increase in the muscle area and in body weight. However, the total number of fibres per area of breast muscle did not show differences between breeds in this study, although the broiler had greater fibre diameter and muscle area than the native chickens. The larger body weight of chickens is based on larger muscle fibre diameter and area and less muscle fibre density (Dransfield and Sosnicki, Citation1999; Chen et al., Citation2007). A previous study revealed that the imported breed chickens have larger fibre diameter in breast and thigh muscles than the Thai native chicken (Jaturasitha et al., Citation2008). The muscle fibre diameter in broilers ranged from 31 to 50 µm, while it ranged from 27 to 35 µm for the native chickens (Chen et al., Citation2007; Khoshoii et al., Citation2013).
The thickness of connective tissue in the breast muscle showed that Arbor Acres broilers had thinner endomysium than muscles from Thai native chicken. In the current study, the perimysium thicknesses of breast and thigh muscles of Arbor Acres broilers were 15.60 and 19.48 μm, while the perimysium thicknesses of breast and thigh muscles of Thai native chickens were 20.05 and 19.52 μm. Larger diameter fibres have less connective tissue, which resulted in more tender meat (Musfiroh et al., Citation2013). The development of connective tissue and thickness of collagen fibrils was slower in chickens than in many other fast growing species (Shiba et al., Citation2006; Fernandez et al., Citation2001). The perimysium of indigenous chicken muscles was thicker than in broilers, which led to an extremely tough meat (Wattanachant et al., Citation2005). However, the increasing of endomysium and perimysium thickness was paralleled by a significant increase in muscle fibre diameter (Lachowicz et al., Citation2007; Wojtysiak, Citation2013).
Relationship between muscle fibre characteristics and carcass quality traits
This study indicated that the live weight and breast percentage of the chickens increased with the larger muscle fibre diameter. The higher live weight and breast percentage could be affected on larger size of muscle fibres, since postnatal growth is achieved almost by muscle fibre hypertrophy (Larzul et al., Citation1997; Bolink et al., Citation2000; Chen et al., Citation2007; Orzechowska et al., Citation2008; Dai et al., Citation2009; Kim et al., Citation2013). The body weight and growth rate of chicken was the most important factor determining muscle fibre size (Duclos et al., Citation2005; Chen et al., Citation2007). The differences in animal breeds could be associated with growth and changed in muscle fibre characteristics due to the growth selection (Dransfield and Sosnicki, Citation1999). This study revealed that the endomysium thickness was negatively correlated with live weight and breast percentage. The dressing percentage was positively correlated with the endomysium thickness in breast muscle. Kleczek et al. (Citation2009) revealed that the male breast muscle thickness measured after slaughter was positively correlated with the carcass weight (r=0.63) and breast weight (r=0.75). The muscle grew through rapid accumulation of protein for muscle fibre growth and had a relatively slower deposition of collagen around muscle fibres (Swatland, Citation1990; Shiba et al., Citation2006). Das et al. (Citation2010) found that the collagen concentration was higher in the late-growth restricted broilers than the compensatory growth broilers. The perimysium collagen fibres seemed to have retarded development in the compensatory growth broilers. The thickness of collagen fibres increased with aging (Fang et al., Citation1999; ochowska et al., Citation2005; Das et al., Citation2010). The increasing of the crosslinking demonstrates a positive correlation with insoluble collagen (Wojtysiak, Citation2013).
Conclusions
Chicken breed affects the carcass quality traits and muscle fibre characteristics in the breast muscle. The live weight, breast and thigh percentage in Arbor Acres broilers are higher than in Thai native chickens. Moreover, the fibre size of breast muscle in Arbor Acres broilers is higher than in Thai native chickens, but smaller as for the endomysium thickness. The increase of live weight and breast percentage is positively correlated with fibre diameter and muscle area.
Acknowledgements
This work was partially supported by Kasetsart University’s Project for Excellence and Sustainability Building of Academic and Research Group provided through the Faculty of Agriculture, Kasetsart University, Thailand. Authors are grateful to the Department of Animal Science, Faculty of Agriculture and Histological Preparation LAB, Faculty of Veterinary Medicine, Kasetsart University for the utilisation of laboratory facilities. Authors are also indebted to Ms. Pakawadee Pongket for her technical assistance during experiments.
References
- AlvesM.R. Abe F.R. Boleli I.C., 2012. Influence of enclosure size on growth of breast and leg muscle fibers in domestic fowl. Int. J. Poult. Sci. 11:361–367.
- BeeG. Calderini M. Biolley C. Guex G. Herzog W., 2006. Changes of the histochemical properties and meat quality traits of porcine muscles during growth. II. Effect of feed restriction in pigs slaughtered at the same body weight and varying age. Arch. Tierzucht 49:62–66.
- BolinkA.H.H. Kranen R.W. Klont R.E. GerritsenC.L.M de GreefK.H., 2000. Fibre area and capillary supply in broiler breast muscle in relation to productivity and ascites. Meat Sci. 56:397–402.
- ChaosapC. Tuntivisoottikul K., 2006. The study of meat quality of Burmese native, broiler and Thai native chicken. pp 230–239 in Proc. 44th Kasetsart Univ. Annu. Conf. Anim. Vet. Med., Bangkok, Thailand.
- ChenX.D. Ma Q.G. Tang M.Y. Ji C., 2007. Development of breast muscle and meat quality in Arbor Acres broilers, Jingxing 100 crossbred chickens and Beijing fatty chickens. Meat Sci. 77:220–227.
- ChoiY.M. Nam K.W. Choe J.H. Ryu Y.C. Wick M.P. Lee K. Kim B.C., 2013. Growth, carcass, fiber type, and meat quality characteristics in Large White pigs with different live weights. Livest. Sci. 155:123–129.
- ChuaynukoolK. Wattanachant S. Siripongvutikorn S., 2007. Chemical and properties of raw and cooked spent hen, broiler and Thai indigenous chicken muscles in mixed herbs acidified soup (Tom Yum). J. Food Technol. 5:180–186.
- DaiF. Feng D. Cao Q. Ye H. Zhang C. Xia W. Zuo J., 2009. Developmental differences in carcass, meat quality and muscle fibre characteristics between the Landrace and a Chinese native pig. S. Afr. J. Anim. Sci. 39:267–273.
- DasC. Roy B.C. Oshima I. Miyachi H. Nishimura S. Iwamoto H. Tabata S., 2010. Collagen content and architecture of the pectoralis muscle in male chicks and broilers reared under various nutritional conditions. Anim. Sci. J. 81:252–263.
- Department of Foreign Trade, 2014. Chicken product. Available from: http://www.dft.go.th/Default.aspx?tabid=444&ctl=DetailUserContent&mid=908&contentID=5724
- Department of Livestock Development, 2013. Statistics of livestock population in Thailand. Available from: http://ict.dld.go.th/th2/index.php/th/report/276-report-thailand-livestock/reportsurvey56/479-report-survey56
- DransfieldE. SosnickiA.A., 1999. Relationship between muscle growth and poultry meat quality. Poultry Sci. 78:743–746.
- DuclosM.J. Berri C. Hattab N.H., 2005. Selection for growth rate alters the expression of rapid myosin heavy chain isoforms in chicken breast muscle. Arch. Tierzucht 48:76 (abstr.).
- DyubeleN.L. Muchenje V. Nkukwana T.T. Chimonyo M., 2010. Consumer sensory characteristics of broiler and indigenous chicken meat: a South African example. Food Qual. Prefer. 21:815–819.
- FangS.H. Nishimura T. Takahashi K., 1999. Relationship between development of intramuscular connective tissue and toughness of pork during growth of pigs. J. Anim. Sci. 77:120–130.
- FernandezX. Sante V. Baeza E. Lebihan-Duval E. Berri C. Remignon H. Babile R. Le PottierG. Millet N. Berge P. Astruc T., 2001. Post mortem muscle metabolism and meat quality in three genetic types of turkey. Brit. Poultry Sci. 42:462–469.
- JaturasithaS., 2004. Meat management. Mingmuang Press, Chiang Mai, Thailand.
- JaturasithaS. Srikanchai T. Kreuzer M. Wicke M., 2008. Differences in carcass and meat characteristics between chicken indigenous to northern Thailand (Black-Boned and Thai native) and imported extensive breeds (Bresse and Rhode Island Red). Poultry Sci. 87:160–169.
- KhalidA.M. Yousif I.A. Omer M.I. Elamin K.M., 2012. Genetic variability of body composition traits in Sudanese native large Beladi chicken. Agric. Biol. J. North Am. 3:69–76.
- KhoshoiiA.A. Mobini B. Rahimi E., 2013. Comparison of chicken strains: muscle fibre diameter and numbers in Pectoralis superficialis muscle. Glob. Vet. 11:55–58.
- KimG.D. Kim B.W. Jeong J.Y. Hur S.J. Cho I.C. Lim H.T. Joo S.T., 2013. Relationship of carcass weight to muscle fiber characteristics and pork quality of crossbred (Korean native black pig × Landrace) F2 pigs. Food Bioprocess Tech. 6:522–529.
- KleczekK. Wawro K. Wilkiewicz-Wawro E. Makowski W. Konstantynowicz D., 2009. Relationships between breast muscle thickness measured by ultrasonography and meatiness and fatness in broiler chickens. Arch. Tierzucht 52:538–545.
- Lachowicz K. Kamieniecki H. Gajowiecki L. Wójcik J. Szarkowski K. Sobczak M. ochowska-Kujawska J. Kotowicz M. ych A., 2007. Comparison of texture and structure of ST (Semitendinosus) muscle of black-white cattle crossbreds with Charolaise, Marchigiana, Piemontese and Chianina and its susceptibility to massaging. Polish J. Food Nutr. Sci. 57:63–68.
- Larzul C. Lefaucheur L. Ecolan P. Gogué J. Talmant A. Sellier P. Le RoyP. Monin G., 1997. Phenotypic and genetic parameters for longissimus muscle fiber characteristics in relation to growth, carcass, and meat quality traits in large white pigs. J. Anim. Sci. 75:3126–3137.
- LiuA. Nishimura T. Takahashi K., 1994. Structural changes in endomysium and perimysium during post-mortem aging of chicken semitendinosus muscle-contribution of structural weakening of intramuscular connective tissue to meat tenderization. Meat Sci. 38:315–328.
- LiuA. Nishimura T. Takahashi K., 1996. Relationship between structural properties of intramuscular connective tissue and toughness of various chicken skeletal muscles. Meat Sci. 43:43–49.
- MusfirohA.F. Janisch S. Bintoro V.P. Wicke M. Pramono Y.B., 2013. The correlation of muscle fiber and perimysium thickness to the quality of turkey breast meat. J. Appl. Food Technol. 2:121–125.
- OlawumiS.O., 2013. Phenotypic correlations between live body weight and carcass traits in Arbor Acre breed of broiler chicken. Int. J. Sci. Nature 4:145–149.
- OrzechowskaB. Wojtysiak D. Migdał W. Tyra M., 2008. Relationships between muscle fibre characteristics and physico-chemical properties of longissimus lumborum muscle and growth rate in pig fatteners of three breeds. Anim. Sci. Pap. Rep. 26:277–285.
- PuolanneE. Ruusunen M. Voutila L. Yläajos M., 2006. Growth rate, muscle physiology, carcass traits and meat quality in pigs. A collage of studies on pigs at the University of Helsinki. Arch. Tierzucht 49:126–131.
- RahamanM.T. Rahman M.S. Hoque M.F. Parvez N.H., 2010. Age related muscle texture variation between Cobb-500 and Ross broiler strain. J. Bangladesh Agril. Univ. 8:265–269.
- RémignonH. Desrosiers V. Marche G., 1996. Influence of increasing breast meat yield on muscle histology and meat quality in the chicken. Reprod. Nutr. Dev. 36:523–530.
- RemignonH. Gardahaut M.F. Marche G. Ricard F.H., 1995. Selection for rapid growth increases the number and the size of muscle fibers without changing their typing in chickens. J. Muscle Res. Cell M. 16:95–102.
- ShibaN. Nakamura Y.N. Matsuzaki M. Tabata S. Nishimura S. Tsuneishi E. Iwamoto H., 2006. Comparative study of the collagen content and architecture of the longissimus muscle in concentrate- and forage-fed male goats. J. Fac. Agr. Kyushu Univ. 51:105–109.
- SmithJ.H., 1963. Relation of body size to muscle cell size and number in the chicken. Poultry Sci. 42:283–290.
- SwatlandH.J., 1990. A note on the growth of connective tissues binding turkey muscle fibers together. Can. Inst. Food Sci. Technol. J. 23:239–241.
- TumaH.J. Venable J.H. Wuthier P.R. Henrickson R.L., 1962. Relationship of fiber diameter to tenderness and meatiness as influenced by bovine age. J. Anim. Sci. 21:33–36.
- Van Marle-Köster E. Webb E.C., 2000. Carcass characteristics of South African native chicken lines. S. Afr. J. Anim. Sci. 30:53–56.
- WattanachantS., 2008. Factors affecting the quality characteristics of Thai indigenous chicken meat. Suranaree J. Sci. Technol. 15:317–322.
- WattanachantS. Benjakul S. Ledward D.A., 2004. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poultry Sci. 83:123–128.
- WattanachantS. Benjakul S. Ledward D.A., 2005. Microstructure and thermal characteristics of Thai indigenous and broiler chicken muscles. Poultry Sci. 84:328–336.
- WojtysiakD., 2013. Effect of age on structural properties of intramuscular connective tissue, muscle fibre, collagen content and meat tenderness in pig longissimus lumborum muscle. Folia Biol.-Prague 61:221–226.
- ŻochowskaJ. Lachowicz K. Gajowiecki L. Sobczak M. Kotowicz M. Żych A., 2005. Effects of carcass weight and muscle on texture, structure and myofibre characteristics of wild boar meat. Meat Sci. 71:244–248.
