Abstract
Algae have contributed greatly to the creation of the Earth’s environment and the development of human civilization. Currently, microalgae are considered to be among the most promising sources for biofuel. Most microalgae accumulate triacylglycerols; however, fatty acid methyl esters produced from triacylglycerols by transesterification have critical end-use issues. Hydrocarbons produced by Botryococcus and Aurantiochytrium are the most suitable algal oils for replacing existing transportation fuels and are highly compatible with existing petroleum infrastructure. Over the years, many technologies have been investigated for achieving sustainable biofuel production using oleaginous microalgae; however, existing techniques of algal fuel production are suitable mainly for small-scale procedures or for the recovery or removal of high-value products. This situation strongly influences life cycle assessment studies for algal fuel production, and published life cycle assessments show different and discrepant results – reliable data on inputs and outputs from industrial-scale experiments are needed for solving these problems. The estimated cost of algal fuel production is still high compared with that of fossil crude oils. The integration of water treatment and algal biomass production, in a coupled hybrid production system comprising of phototrophic and heterotrophic algae, has tremendous potential for improving the economy of future algal fuels.
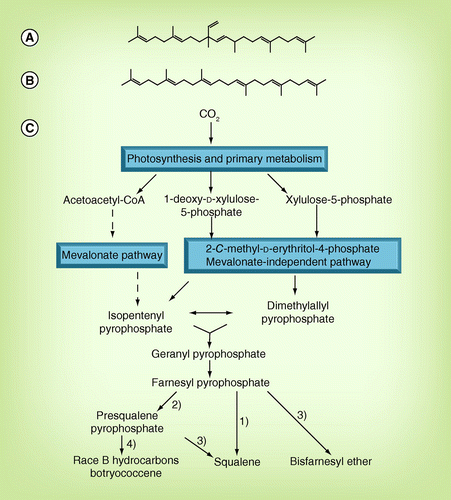
(A) C30 botryococcene, (B) squalene and (C) main biosynthetic pathway of botryococcene and squalene. 1) Squalene synthase gene, BSS; 2) squalene synthase-like gene, SSL-1; 3) squalene synthase-like gene, SSL-2; 4) squalene synthase-like gene, SSL-3.
CoA: Coenzyme A.
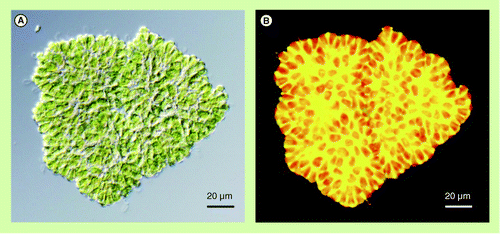
(A) Differential interference contrast microscopy. (B) Fluorescence microscopy of Nile red-stained colony. Nonpolar lipids including botryococcene show bright yellow fluorescence. Red fluorescence is autofluorescence derived from chlorophylls in chloroplasts.
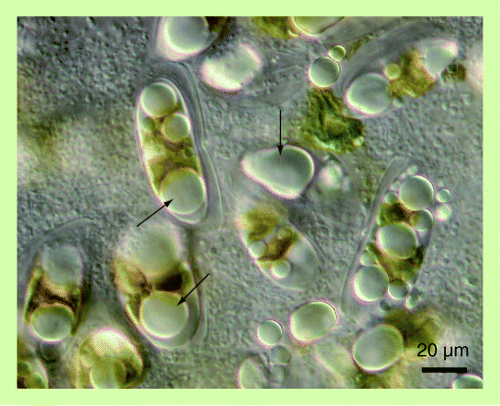
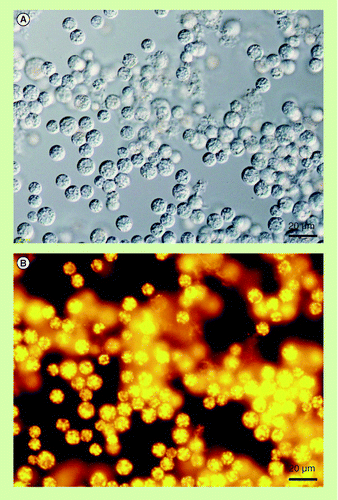
(A) Differential interference contrast microscopy. (B) Fluorescence microscopy of Nile red-stained cells. Yellow fluorescence indicates nonpolar lipids including squalene. Peripheral red fluorescence indicates phospholipids, the major component of the cell membrane.
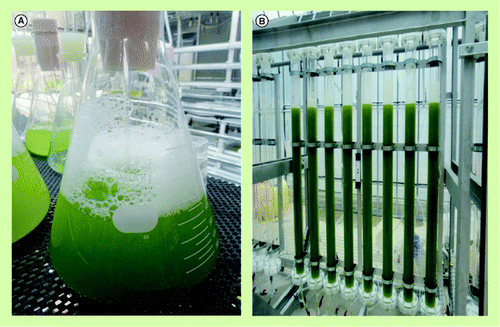
(A) Laboratory-scale seed culture. (B) The 480-l-scale tube bioreactor system.
Traditional fuel includes oil, gas and coal. Traditional biofuels include oil and alcohol from rapeseeds, corn, palm oil and sugarcane.
Reproduced with permission from Citation[76].
![Figure 6. World energy demand and oil supply potential of algal plants.Traditional fuel includes oil, gas and coal. Traditional biofuels include oil and alcohol from rapeseeds, corn, palm oil and sugarcane.Reproduced with permission from Citation[76].](/cms/asset/39ea8bf2-5cc8-42f4-b32f-7b790d449c3d/tbfu_a_10816020_f0006.gif)
Background
▪ Algae, global environments & human life
The production of algal biomass for biofuels combined with the production of bulk chemicals, food and feed ingredients is currently attracting attention in many countries. It is expected that sustainable and industrial use of algae-based carbon could be achieved by a biorefinery concept, which embraces a wide range of technologies capable of converting algal building blocks (e.g., carbohydrates, proteins and lipids) into a range of products including transportation biofuels, chemicals, polymers, pharmaceuticals, cosmetics, food and feed Citation[1].
Algae are not newcomers to modern society, but are ancient organisms responsible for the Earth’s environment. In the Precambrian era, photosynthetic microalgal cyanobacteria (blue-green algae) whose fossils are known as stromatolites, first appeared on Earth and dramatically changed the atmospheric composition of the reductive Archaean Earth by oxygenic photosynthesis Citation[2]. The accumulated molecular oxygen diffused into primitive seawater and oxidized dissolved bivalent iron ions to insoluble trivalent iron; this phenomenon is called a great oxygenation event (GOE) Citation[3]. As a result of the GOE, a massive amount of banded ironstone formations were formed and deposited iron ores. The rise in the partial pressure of oxygen also made it possible for organisms to perform aerobic respiration, an efficient method to obtain energy by oxidation of organic compounds, leading to the rise and further diversification of multicellular organisms Citation[4].
The GOE and the consequent flourishing of photosynthetic algae also brought about the formation of an ozone layer that absorbs most of the Sun’s ultraviolet light, thereby protecting organisms. This shield allowed aquatic organisms to venture onto land, triggering the expansion of biogeocenosis and increased biodiversity.
Algae are also the largest direct contributors to the formation of fossil fuels. The most important fossil fuel, petroleum, was formed by the anaerobic decomposition of organic matter derived from algae (phytoplankton) and zooplankton that bloomed millions of years ago. In fact, the fossil of the well-known oil-producing alga Botryococcus braunii Kützing can be seen in Paleogene boghead oil shale Citation[5].
Despite their enormous contributions during the geological history of the Earth, algae are a minor player in modern human life, found only in nutrition supplements or dishes of some cuisines. Food is the most fundamental utilization of biomass as an energy source; however, the number of edible microalgal species is very small in comparison with edible land plants. Nevertheless, there may be a large number of unrecognized but potentially valuable algal species, perhaps even in culture collections around the world. Now, challenges in the production of biofuels have focused the spotlight on algae, particularly microalgae Citation[6,7]. Production of renewable fuels from microalgae has the potential to be sustainable and carbon neutral, advancing the development of human society.
▪ Algae can solve the trilemma of environmental, energy & economic development problems
Although, as described above, algae have long been used as natural resources, we consume them at an overly high rate to facilitate our modern and convenient living. In particular, the continuous increase in atmospheric CO2 coupled to an increase in fossil fuel consumption and climate change has become a major concern worldwide. Nevertheless, we cannot halt economic activity without sacrificing quality of life. We must achieve three conflicting goals, the so-called ‘trilemma’ problem: sustainable use of natural resources and energy, economic development and environmental conservation. Although these three goals are incompatible, they are intricately intertwined with each other; therefore, achievement of any goal will be impossible if we continue to lean too heavily on fossil fuels for our energy needs. A global movement toward switching to renewable energy is underway to break the deadlock.
Renewable energy is energy that comes from inexhaustible natural resources. With sunlight as the foremost, various natural resources including wind, geothermal heat and tides are being developed as commercial, stable energy sources. Algal refining is fundamentally a form of sunlight utilization and has striking merits that are not found with other technologies for harnessing solar energy, such as photovoltaic cells. Algae absorb CO2 and convert it into a broad and valuable array of organic compounds by photosynthesis and subsequent metabolic reactions. In particular, hydrocarbons produced by algae are substantially homogeneous with fossil fuels and have high compatibility with the requirements of existing combustion engines and petrochemical industries. Algal refining would ease the burden of reforming the world’s energy situation.
▪ Advantages of algal feedstocks
There has been a recent surge of interest in algae and their potential capacity as sources of biomass for the following reasons:
▪ Typical photosynthetic (autotrophic) algae require only water, light, CO2 and trace amounts of inorganic salts for their growth. It should be noted that nonphotosynthetic heterotrophic algae require organic carbon for their growth; | |||||
▪ Algae have high biomass productivity in comparison with land plants, both per unit area and per unit time; | |||||
▪ Algal production poses no competition with existing food production; | |||||
▪ Algae produce multiple products and byproducts. Many high value-added chemicals, such as polyunsaturated fatty acids (PUFAs), antioxidant carotenoids and functional polysaccharides, which are unavailable in terrestrial crops, can be extracted from algal cells. Moreover, hydrogen or CH4 could be recovered through specific fermentation or digestion of residual algal biomass Citation[8,9]. | |||||
Despite these advantages, the technology for development of algal feedstocks is immature. For example, although breeding of superior strains is conventional in agriculture, the screening of favorable wild-type strain remains an efficient strategy in algal cultivation, partly because sexual reproduction is known only in certain species of algae. Technical standards and common frameworks for the algae industry are still under development. As matters stand, individual companies or institutes accumulate knowledge and protect it with patents. Economically, neither the distribution system nor markets for algal feedstocks are well established. Extensive development of both suppliers and distributors will be necessary for the success of algae in the biofuel market.
Oleaginous algae & their products
▪ Lipid content & productivities
To date, hundreds of algae have been screened worldwide for biofuel production. From 1978 to 1996, the Aquatic Species Program was conducted at the initiative of the National Renewable Energy Laboratory, which is an affiliate of the US Department of Energy, and identified approximately 300 algal species that have potential for high oil yields Citation[10,11]. The authors of the National Renewable Energy Laboratory report asserted that five major groups of algae were of primary importance for biofuel production: diatoms, green algae, golden-brown algae, haptophytes and eustigmatophytes. In fact, most oil-producing algae belong to or are neighbors of these phylogenetic groups. Oil content levels of representative oleaginous algae are 20–50%, actual oil productivity depends on the physiological specifications of the algae (growth rate and oil content level) and on cultivation system (open raceway pond or photobioreactorCitation[6]). Nevertheless, productivities of algal oil are much higher than those of terrestrial crops in general Citation[6].
▪ Triacylglycerol
A triacylglycerol (TAG) is an ester derived from glycerol and three fatty acid moieties. TAGs, the main constituents of vegetable oil and animal fats, represent the most common and widespread storage lipid among living organisms. Microalgae store TAGs in high concentrations, making them the key targets for a biorefinery.
Under normal growing conditions, algae synthesize lipids as membrane lipids, phospholipids, glycerophospholipids and glycolipids. Phospholipids and glycerophospholipids, such as phosphatidylethanolamine and phosphatidylglycerol, comprise phospholipid bilayers and thus constitute the prime component of biological membranes such as the plasma membrane or endoplasmic reticulum Citation[12]. Glycolipids such as galactosyl diacylglycerol and sulfoquinovosyl diacylglycerol are components of the chloroplast membrane of autotrophic algae Citation[12]; these membrane lipids constitute approximately 5–20% of dry cell weight. Under growth-limiting conditions, such as nitrogen starvation, algae switch their lipid synthesis pathway and begin to accumulate TAGs. Because these TAGs cannot be used for synthesizing membranes, they are stored as lipid bodies in the cytoplasm .
Fatty acid synthesis by autotrophic algae is conducted in the chloroplast. The precursor of the synthetic pathway is acetyl-coenzyme A (acetyl-CoA) derived from glycolysis. Acetyl-CoA is first converted by acetyl-CoA carboxylase into malonyl-CoA, which is transferred onto an acyl carrier protein (ACP). Subsequently, it is processed by a series of enzymes, including 3-ketoacyl-ACP synthetase, 3-ketoacyl-ACP reductase, 3-hydroxyacyl-ACP dehydrase and enoyl-ACP reductase, leading to an elongated carbon chain. The chain is finally hydrolyzed to ACP and saturated fatty acids by thioesterase. Various fatty acid desaturases act on the saturated fatty acids and convert it to unsaturated fatty acids (UFAs). Synthesized UFA is activated as an acyl-CoA, which is transferred to glycerol 3-phosphate to synthesize phosphatidic acid, followed by dephosphorylation to diacylglycerol by a specific phosphatase. In the final step of TAG synthesis, diglyceride acyltransferase catalyzes the formation of triglycerides from diacylglycerol and another acyl-CoA. The composition of fatty acids in TAG depends on the characteristics of the acyltransferases in the pathway.
For use as biodiesel, TAGs must be converted into fatty acid methyl esters (FAMEs) by transesterification. FAMEs are a type of fatty acid ester that may be produced by a reaction between TAG and methanol in the presence of alkali catalysts. Common catalysts for transesterification are sodium hydroxide or potassium hydroxide. After the reaction, the glycerol byproduct, residual methanol and catalysts should be removed through a purification process. These residual materials, if not removed, cause failure of engines and other combustion devices. Oil quality of FAMEs is affected by their fatty acid composition. At room temperature, long-chain saturated fatty acids and UFAs are solid and liquid, respectively. The fluidity of unsaturated FAMEs makes them suitable for use as biodiesel oil. However, PUFAs are easily oxidized, become viscous polymers and/or form oxygen-containing fatty acids containing epoxides and aldehydes. These compounds produce oxygen radicals that corrode diesel engines Citation[13]. Even nonoxygenated, paraffinic kerosene produced by hydrotreating TAGs or FAMEs Citation[14] presents cold-weather performance problems unless heavily isomerized Citation[15].
Because FAMEs have different solvent properties from petrodiesel they are not direct substitutes for petrodiesel. Biodiesel standards such as EN 14214 define requirements for FAMEs and limit the blending percentage of FAME in transportation fuel.
▪ Hydrocarbons
Hydrocarbons are among the preferable algal fuels because of their high energy density and availability as materials for chemical engineering. Although many oleaginous algae have been reported, few hydrocarbon-producing species are known to date. Although traces of hydrocarbons are produced by major green algae and blue-green algae, their exploitation remains unrealistic because of their extremely low content Citation[16]. The most promising species appears to be the green alga B. braunii, which produces high amounts of triterpenoid hydrocarbons known as botryococcenes. Another remarkable alga is Aurantiochytrium, which is unalterably heterotrophic and accumulates high amounts of squalene. Detailed characteristics of these algae are described later in this article. Besides these, an undescribed green alga Pseudochoricystis ellipsoidea is reported to produce hydrocarbons Citation[101]. Although most of the storage lipids of P. ellipsoidea consist of TAGs, this alga also accumulates hydrocarbons with carbon numbers ranging from C17 to C20. These hydrocarbons are different from botryococcene or squalene, possessing a short carbon chain and, thus, may possess solvent properties similar to those of petrodiesel.
One of the most studied algal hydrocarbons is botryococcene, which is derived from B. braunii. B. braunii is further classified into four chemical groups; namely races A, B, L and S, on the basis of different hydrocarbon molecules, as shown in the section entitled ‘Botryococcus braunii’ Citation[17,18]. Botryococcenes are considered to be produced by race B through the 2-C -methylerythritol 4-phosphate (MEP) pathway rather than the mevalonate pathway . The precursors of the MEP pathway are pyruvic acid and glycerol 3-phosphate. These precursors are coupled to form 1-deoxy-d-xylulose 5-phosphate, which is then converted to MEP and subsequently to isopentenyl pyrophosphate (IPP). IPP and dimethyl diphosphate, an isomer of IPP, are coupled to form geranyl pyrophosphate. C30 botryococcene is finally synthesized from farnesyl pyrophosphate via presqualene pyrophosphate Citation[19]. Squalene is also produced in B. braunii by squalene synthase (BSS) from farnesyl pyrophosphate . Squalene (2,6,10,15,19,23–hexamethyltetracosa-2,6,10,14,18,22-hexane; C30H50) is a polyunsaturated triterpene hydrocarbon that is a precursor of steroids in living organisms. Because B. braunii cells contain only a trace level of squalene, a novel squalene-producing alga Aurantiochytrium was reported in 2010. Recently three squalene synthase-like (SSL) genes, SSL-1, SSL-2 and SSL-3, were isolated Citation[19]. When SSL-1 and SSL-3 were mixed together, robust botryococcene was synthesized, whereas when SSL-1 and SSL-2 were mixed, squalene was synthesized .
As hydrocarbons, botryococcene and squalene can be used as feedstocks for producing octane and kerosene by hydrocracking instead of transesterification. Incidentally, some properties of squalene and botryococcene, such as kinematic viscosity, are similar to those of fossil diesel Citation[20]; hence, untreated squalene can be mixed with fossil diesel for driving diesel engines. The University of Tsukuba (Tsukuba, Japan) and the Mazda Motor Corporation (Fuchu, Japan) succeeded in their joint project for utilizing squalene produced from Aurantiochytrium as a diesel fuel in 2011. Squalene accounted for 70% of the total volume of fuel.
Squalene is currently used in cosmetics, foods and the medical and pharmaceutical sectors. Conventionally, the major commercial source of squalene has been the liver oil of deep-sea sharks (Centrophorus spp.) Citation[21]; however, the supply of squalene is unstable because of the vulnerability of threatened shark species. Although some yeasts have been reported as sources of squalene, the contents are low (maximum 0.34 g/l after 120 h; 68 mg/l/day) Citation[22]. It is hoped that squalene from algal biomass will reduce the production cost and stabilize the supply.
Since algal hydrocarbons, such as botryococcenes and squalene, are unsaturated triterpenes with few conjugated dienes , they have high oxidative stability compared with TAGs or FAMEs. In addition to this oxidative stability, the mixture of hydrocarbons and carotenoids obtained at the final step of the hydrocarbon extraction process from algae can achieve a stable state for long-term storage of hydrocarbons, since the contaminating carotenoids function as antioxidants. Therefore, we anticipate that algal hydrocarbons can be used for transportation fuels without any hydrotreating processes. At the same time, we can expand the versatility of hydrocarbon products by the hydrocracking process, as mentioned above, at the expense of energy efficiency. Improved cracking methods have been reported, and some have been applied to algal hydrocarbons Citation[23]. Sophisticated hydrotreating techniques will reduce the energy consumption required for these processes and greatly expand the availability of algal hydrocarbons.
▪ Other biofuel products
Algae can provide several different types of biofuels besides liquids. One such fuel is CH4 produced by anaerobic digestion of the algal biomass Citation[9]. Hydrogen is another fuel of great importance as a gasiform renewable energy resource Citation[8]. Algae may also be converted into solid fuels that can be directly combusted for energy production. These include simple dried pellets and biochar produced by a thermochemical process Citation[24]. Biochar is a stable form of carbon and is now promoted as a carbon-negative technology.
Culture, growth & oil productivity of oleaginous algae under laboratory conditions
A number of oil-producing algae have been investigated for biomass and oil production . Here, we outline the growth and oil-producing characteristics of these algae.
▪ B. braunii
B. braunii is a colony-forming microalga that accumulates high amounts of hydrocarbons (up to 75% of dry weight) inside colony matrices . This microalga is considered to be one of the algal sources that contributed to petroleum generation over geological time. Taxonomically, B. braunii belongs to the family Trebouxiophyceae in the phylum Chlorophyta. This microalga is usually found at low concentration in freshwater environments worldwide, although occurrences of blooms of B. braunii in eutrophic reservoirs have been sporadically reported Citation[25].
Like other phototrophic algae, B. braunii needs light, CO2 and nutrients. B. braunii is often cultured in liquid Chu-13 and related media, and less frequently in BG11 and AF6 media Citation[26]. Although the optimum culture conditions differ from strain to strain, most strains show the fastest growth rate at approximately 25°C under a light intensity of 50 µE/m2/s. The doubling time of typical B. braunii under common culture conditions is 7 days, which is much longer than that of other commercially used microalgae, hindering the instant commercial application. However, aeration with 1% CO2 may dramatically reduce the doubling time of B. brauniiCitation[27]. A further growth increment can be achieved under mixotrophic (phototrophic plus heterotrophic) culture where a carbon source, such as glucose, is added to the media Citation[28].
B. braunii can be classified into three groups (races A, B and L) on the basis of different hydrocarbon products Citation[17]. Race A strains contain n -alkadiene and/or n -alkatrienes and their derivatives with odd carbon numbers (C23–C33), whereas race B strains contain CnH2n–10 triterpenes, called botryococcenes (C30–C37), and methylated squalenes (C31–C34). Race L strains contain a tetraterpenoid hydrocarbon called lycopadiene (C40H78) and its derivatives. Recently, a novel race comprising epoxy-n -alkane and saturated n -alkane chains with carbon numbers 18 and 20 was found and tentatively named race S Citation[18]. This classification is supported by phylogenetic analyses using the 18S rRNA gene marker, suggesting that the production of different hydrocarbons has a genetic basis Citation[18]. Indeed, comparative gene expression analyses revealed the involvement of different biosynthetic pathways between race A and B strains. In race A strains, the acyl-ACP elongation pathway is the major route leading to race A hydrocarbon production, whereas in race B strains, the reductive pentose phosphate pathway and mevalonate-independent pathway are primarily used to synthesize race B hydrocarbons Citation[29]. The maximum production of hydrocarbons in B. braunii is achieved at logarithmic growth phase, which is less affected by nutrient (e.g., nitrogen and phosphorus) limitation.
▪ Aurantiochytrium
Aurantiochytrium, formerly one linage of Schizochytrium, is a genus belonging to the family Thraustochytriaceae of the class Labyrinthulomycetes . The members of this group are uniformly colorless, heterotrophic and fungus-like algae. They are mostly marine decomposers, found on the surface of organic debris, macroalgae and seagrass. Vegetative cells are typically spherical to fusiform and sluggish in movement; however, they have a motile life cycle stage in which the flagellate cells possess two differently shaped heterokont-type flagella.
The genus Aurantiochytrium was distinguished from Schizochytrium in 2007 on the basis of cell structure and composition of carotenoids and PUFAs Citation[30]. They possess astaxanthin, phoenicoxanthin, canthaxanthin, and β-carotene as carotenoids, and minor quantities of arachidonic acid and major quantities of docosahexaenoic acid (DHA) as PUFAs. Even years after differentiating the genus, there is some confusion about the genus name in articles and patent descriptions. Only two species of Aurantiochytrium, Aurantiochytrium limacinumCitation[31] and Aurantiochytrium mangroveiCitation[32], have been described and are valid at present, although a number of unnamed strains are under experiment. Schizochytriumsensu lato including Aurantiochytrium have been studied for the purpose of harvesting PUFAs, especially DHA Citation[33–35]. In 2010, a novel strain of Aurantiochytrium that accumulates high amounts of squalene, 18W-13a, was reported Citation[36,37]. The way has been paved to utilize Aurantiochytrium as a source of biofuel.
Squalene is a polyunsaturated triterpene hydrocarbon and is currently used in cosmetics and foods and for medical purposes as described above. Under a standard culture condition, at 25°C in peptone, yeast extract and glucose medium prepared with diluted seawater and shaking at 100 rpm, the squalene content of the Aurantiochytrium sp. strain 18W-13a reached 1.29 g/l after 4 days (322.5 mg/l/day) Citation[36]. This productivity is 3.8-times higher than that of yeast Citation[37]. The squalene productivity of Aurantiochytrium will improve with further optimization of culture conditions and continuous screening of additional superior strains.
Because of heterotrophy, the choice of carbon source for culture media is an important issue. Although, peptone, yeast extract and glucose medium is a convenient and acceptable medium for laboratory-scale culture, its cost is too high to support commercial large-scale biofuel production. Efforts to slash cultivation costs of Aurantiochytrium should be pursued. Some autotrophic algae, such as Chlorella and B. braunii, excrete fair amounts of polysaccharides into the culture medium. These polysaccharides would be an appropriate carbon source for Aurantiochytrium for media recycling and carbon neutralizing of heterotrophic cultures. In fact, by feeding a wide variety of plant-based sugars to oil-producing heterotrophic microalga in standard industrial fermentation equipments, photosynthetic plant sugars are converted into oils Citation[201]. This indirect photosynthetic technology platform on an efficient scale can accelerate algal oil production time to just a few days. The exploitation of residual dross or wastewater from food industries would be another particularly promising approach for heterotrophic algal cultivation. Combined with primary and secondary wastewater treatment processes, R&D breakthroughs for hybrid production systems, comprising photosynthetic and heterotrophic algae, could greatly increase oil productivity.
▪ Other algae
Besides the two major candidates described above, some oil-producing algae also accumulate TAGs in general. These include the green algal species Chlorella spp., Dunaliella spp., Neochloris oleoabundans and Nannochloropsis spp. Chlorella spp. are known to achieve higher biomass productivity under mixotrophic culture. Considering the general difficulty of mass cultivation of algae, Dunaliella spp. may have an advantage over other algae, given that one species of Dunaliella, Dunaliella salina, has been successfully cultivated on a large scale in some commercial plants for decades. A marine diatom Phaeodactylum tricornutum that is known to produce eicosapentaenoic acid also accumulates TAG to a substantial level (up to 60%). Recently, another promising algal species that accumulates TAG as well as smaller amounts of hydrocarbons under nitrogen deficient condition has been isolated from a hot spring in Japan. This species showed low phylogenetic affinity to described algal species and has been tentatively named P. ellipsoideaCitation[101]. Given the vast diversity of undescribed microalgae on earth, there is no doubt that promising novel microalgae will continue to be discovered by screening for oil-producing algae from natural environments.
Algae & wastewater
Wastewater is usually discharged into the environment after physical, chemical, and biological treatments. In biological treatment, microalgae play an important role of removing high concentration nutrients such as dissolved nitrogen and phosphorus, chemical oxygen demand (COD), and metal ions from wastewater. This microbial ability has been studied since the 1950s Citation[38]. In recent years, a considerable number of studies have been performed for coupling wastewater treatment with algae cultivation for algal fuel production . In this section, we show the advantages of using wastewater and describe recent progress in research on algal fuel production coupled with treatment of sewage, agricultural and industrial wastewater.
▪ Advantages & disadvantages of using wastewater for algal fuel production
Wastewaters derived from municipal, agricultural and industrial activities potentially provide cost-effective and sustainable means of algal growth for biofuels Citation[39]. Culturing of microalgae at an industrial scale for biofuel production requires substantial amounts of nutrients, particularly nitrogen (usually in the form of nitrate) and phosphorus (usually in the form of orthophosphate) Citation[40]. Heterotrophic and mixotrophic microalgae require organic nutrients such as glucose as the carbon source added to inorganic nutrients. If chemical fertilizers and glucose are used for algae-based industrial biofuel production, the feedstocks account for 60–75% of the total cost of biodiesel Citation[41]. In fact, a study using glucose as a feedstock for heterotrophic microalgae gave disappointing results owing to the high cost Citation[42]. Thus, in the long run, using chemical fertilizers to culture microalgae for biofuel production is unsustainable Citation[40]. However, wastewater has the potential to provide nutrients or to replace the culture medium. In general, municipal sewage contains inorganic nitrogen and phosphorus; agricultural wastewater such as livestock abounds in ammonium nitrogen, nitrate and nitrite; and some industrial wastewaters contain high concentrations of organic nutrients such as sugars, proteins and amino acids. Thus, coupling wastewater treatment with algae cultivation for algal fuel production may offer an economically viable and environmentally friendly means of sustainable, renewable algal-based energy production Citation[39,40,43]. To date, however, fewer than 30% of published studies on microalgae culture have used wastewater as a nutrient source, while the remaining studies have used chemical fertilizers Citation[40]. Furthermore, it should be noted that most of the studies using wastewater have been conducted on a small laboratory scale.
A potential disadvantage of wastewater utilization is the need to remove undesirable organisms and harmful chemicals that would damage algal growth and biomass accumulation. To overcome these problems, diluted wastewater or algal strains tolerant of harmful chemicals should be used Citation[44,45], if necessary, in combination with chemical (antibiotics, fungicides and herbicides) or biological (bacteriovorous or herbivorous predators) additives. Since most studies on the removal of undesirable organisms and harmful chemicals have been on a small scale, a definitive solution to these problems has not yet been found.
Thus, pilot-scale investigations and the further accumulation of reliable data are required to evaluate the advantages and disadvantages of using wastewater for algal biofuel production.
▪ Growth & oil productivity in municipal sewage wastewater
Conventional municipal sewage is discharged into the environment mainly through a three-stage process: a primary treatment phase to remove solid materials by sedimentation, a secondary treatment phase to remove suspended and dissolved organic materials, and a tertiary treatment phase to remove stubborn contaminants not removed by the secondary treatment. Commonly, the secondary and tertiary treated wastewaters have been used in studies for algal fuel production because these wastewaters contain high amounts of nitrate and orthophosphate that are not removed during primary treatment Citation[40].
Recently, many studies have been conducted using municipal sewage wastewater Citation[46–50]. All of the 14 algal strains of the genera Chlorella, Haematococcus, Scenedesmus, Chlamydomonas and Chlorococcum were able to grow in concentrated wastewater. The highest net biomass accumulation (2.01 g/l) was observed with Chlorella kessleri followed by Chlorella protothecoides (1.31 g/l), and both proved to be capable of mixotrophic growth when cultivated in concentrated wastewater Citation[46]. When B. braunii was cultured using secondary treated sewage from domestic wastewater, it showed high lipid productivity (61.7 mg/l/day) Citation[47]. Five strains of Chlorella sp., Heynigia sp., Hindakia sp., Micractinium sp. and Scenedesmus sp. showed higher lipid productivities (74.5–77.8 mg/l/day) in concentrated municipal wastewater Citation[48]. Twenty algal strains, including Spirulina platensis, Anacystis nidulans, Chlorella vulgaris, Chlorella minutissima, C. kessleri, B. braunii, Neochloris oleoabundans and unidentified species, were tested for their growth capacity using effluents from secondary domestic wastewater treatment Citation[49]. The strain of B. braunii produced the best combined results; biomass production (1.88 g/l) from this strain was associated with the best nitrogen and phosphorus removal (79.63 and 100%, respectively) and lipid accumulation (36.14%) among all the strains. Auxenochlorella protothecoides was cultured heterotrophically on concentrated municipal wastewater and then autotrophically with CO2 supplementation (air, 1 and 5%) Citation[50]. A. protothecoides was harvested by self-sedimentation after the heterotrophic stage, and the supernatant containing the residual cells was aerated with different levels of CO2 to facilitate autotrophic growth in the second stage. The maximal biomass concentration and lipid content at the first and second stages reached 1.12 g/l and 28.90%, and 1.16 g/l and 33.22%, respectively. The nutrient removal efficiencies for total phosphorus, ammonia, nitrogen and COD at the end of the two-stage cultivation were 98.48, 100, 90.60 and 79.10%, respectively. All these experiments showed that the use of municipal sewage wastewater resulted in comparatively higher nutrient uptake and biomass productivity. Most of these studies, however, used autoclaved wastewater samples, and such treatment is not feasible for future large-scale commercialization because of high energy consumption and cost. Thus, appropriate treatments for large-scale cultures must be addressed.
▪ Growth & oil productivity in agricultural wastewater
Agricultural wastewater used for algal fuel production is usually a livestock wastewater derived from swine and cattle manures Citation[51–54]. These wastewaters usually have high concentrations of biological oxygen demand (40–48,000 mg/l), COD (80–95,000 mg/l) Citation[55], nitrogen and phosphorus, leading to environmental problems such as eutrophication of water bodies worldwide.
Many studies have shown that when undiluted wastewater or a high concentration of wastewater was used for algal culturing, the nutrient uptake was comparatively high, but algal biomass concentration and lipid productivity were lower than those when synthetic media was used Citation[52,54]. Mixotrophic cultivation of Chlorella pyrenoidosa with primary-treated piggery wastewater showed a maximum lipid productivity of 6.3 mg/l/day when the wastewater was diluted to an initial COD of 1000 mg/l Citation[54]. Nutrients in the piggery wastewater were removed efficiently with an ammonium removal rate of 90% in all diluted samples, suggesting a convenient way to reduce the high organic content of piggery waste by the production of algal lipids Citation[54]. When growth, lipid production and nutrient removal of C. vulgaris were investigated under three different concentrations (20, 50 and 80%) of original wastewater using either synthetic media or distilled water Citation[52], the lipid content of microalgae accounted for 28 ± 2% of dried biomass in all treatment conditions, which is relatively lower than that when synthetic media was used. The C. vulgaris grown on a 20% concentration of wastewater effluent was more promising than other culture conditions for generating high-efficiency biodiesel. The highest removal of inorganic nutrients was also achieved at the same dilution condition. Another study showed that microalgae grew well and produced oil under different conditions as well Citation[51]. Secondary-treated piggery wastewater greatly enhanced the growth and hydrocarbon production of B. braunii. A high dry cell weight of 8.5 g/l and hydrocarbon productivity of 0.95 g/l were obtained, and nitrate was removed at a rate of 620 mg N/l. This was confirmed by our recent investigation [Watanabe MM, Unpublished Data]. Therefore, it is recommended that algal fuel production be developed as part of piggery wastewater treatment processes.
▪ Growth & oil productivity in industrial wastewater
Several types of industrial wastewaters have been investigated, including toxic materials from chemical plants, highly concentrated organic materials and salt from food factories.
A study was conducted to evaluate carpet industry wastewater as a medium for algal biomass and biodiesel production Citation[56]. A consortium of 15 native algae cultivated in treated wastewater produced 9.2–17.8 tons/ha/year of biomass with 6.82% lipid content and showed >96% nutrient removal in treated wastewater. Approximately 63.9% of algal oil obtained from the consortium could be converted into biodiesel. Treated and untreated wastewater dominated by carpet mill effluent (85–90%) provided 1.8–1.9-times higher biomass productivity and 1.2–1.7-times higher oil productivity than the synthesis medium for B. braunii, Chlorella saccharophila, Dunaliella tertiolecta, Pleurochrysis carterae and the consortium of algal isolates.
Two studies using soybean factory wastewater have been conducted to date. In one study, B. braunii was cultivated in soybean curd wastewater in batch culture Citation[44]. Growth and hydrocarbon production were approximately two-times higher in cultures with 2% soybean curd wastewater than in AF-6 artificial medium. It was postulated that proteins and/or reducing sugars in soybean curd wastewater could enhance growth and hydrocarbon production. In the other study, C. pyrenoidosa was cultivated in soybean processing wastewater in batch and fed-batch cultures without supply of additional nutrients Citation[57]. The alga was able to remove 77.8 ± 5.7%, 88.8 ± 1.0%, 89.1 ± 0.6% and 70.3 ± 11.4% of soluble COD, total nitrogen, NH4+-N and total phosphate, respectively, after 120 h in fed-batch culture. C. pyrenoidosa attained an average biomass productivity of 0.64 g/l/day, an average lipid content of 37.00 ± 9.34%, and a high lipid productivity of 0.40 g/l/day. These studies suggest that food industry wastewater is more appropriate for algal growth and oil production than chemical industry wastewater.
One interesting study demonstrated that the utilization of distillery wastewater as a sole nitrogen source enabled a marine thraustochytrid, Schizochytrium sp., to synthesize and accumulate valuable lipids including DHA and astaxanthin Citation[58]. The supernatant of distillery waste contains organic matters such as proteins (approximately 2.5%) and amino acids (approximately 0.2%). The culture medium was prepared by adding various amounts of glucose and artificial sea salts to the wastewater. Under optimized culture conditions, the highest DHA and astaxanthin yields were 3.4 g/l and 7.7 mg/l, respectively, after 4 or 5 days of cultivation in a 3-l jar fermentor. The COD of the wastewater was reduced by 35%. Crude protein content and total free amino acid content were decreased by 67 and 85%, respectively, in the culture supernatant. Thus, it is possible to obtain high lipid productivity by selection of industrial wastewater suitable for algae.
Algal biomass production under outdoor conditions
One of the most important advantages of microalgal fuels compared with other oil crops is that algal culture requires far less land. For example, it has been estimated that the land needed to yield enough algal fuel to fulfill the annual US demand for oil is 4 Mha, which is approximately 750- and 250-fold less than the land needed for equivalent corn and soybean production, respectively Citation[6]. Unfortunately, algal mass cultures are far more difficult to control under field conditions than corn or soybean cultivation, and commercial application of algal fuels as alternatives to other biofuels relies on the feasibility of mass culturing of oil-producing algae on a very large scale. Several algae have been cultured outdoors on a large scale for commercial production of algae-derived foods, feeds and useful chemicals Citation[59]. These include Dunaliella and Spirulina in raceway ponds for carotenoids and food production, respectively, and Haematococcus pluvialis in closed solar-powered bioreactors for astaxanthin production. To date, however, efforts to cultivate oil-producing algae including B. braunii on a large scale outdoors have been virtually unsuccessful. Identifying a productive mass culture system for any oil-producing algae is one of the major challenges for algal biofuel production. For this reason, many researchers are currently addressing this issue.
▪ Open culture systems
Open culture systems are currently major cultivation schemes for commercial biomass production of algae. The simplest open culture system for algae is the utilization of natural ponds, currently used in the commercial production of Spirulina in Myanmar Citation[60]. This is, however, a rare successful case because the complex ecosystem of natural water environments is generally unfavorable for the large-scale culture of the desired algae. In contrast, artificial open ponds provide an environment in which contamination problems are more or less mitigated. The most commonly used open culture system for algae is the raceway pond, which is designed to fulfill a demand for cost-effective algal biomass production Citation[6]. In raceway ponds, the culture medium, which is generally up to 100 cm in depth, is continuously mixed by paddle wheels. As these systems work well in the cultivation of Dunaliella and Spirulina, commercial companies using this or similar systems are distributed worldwide from the USA, Israel and Ukraine to Asia. To date, however, few reports have addressed the cultivation of oil-producing algae in raceway ponds, and we consider most of the few reports on the cultivation of B. braunii in raceway ponds unreliable Citation[26]. The scarcity of raceway studies on oil-producing algae including B. braunii may be due to the unsuccessful efforts owing to several possible problems including contamination and culture instability (e.g., differing light intensity and differing evaporation speed of water) caused by changing weather conditions. Among them, contamination is one of the biggest drawbacks of open culture systems. However, this problem can be overcome by using extremophilic strains such as salt-tolerant ones. In this context, Dunaliella strains with high lipid contents are promising owing to their halophilic nature of this genus. Genetic manipulation of B. braunii or other oil-producing algae to strains showing extremophilic characteristics (e.g., high pH tolerance as used in the successful culture of Spirulina ) can also break through this barrier, although the open culture of genetically engineered strains is strictly prohibited by law in some countries (e.g., Japan). This kind of legal problems can be bypassed by using the chemically induced mutant. Indeed, we have developed a herbicide-resistant strain of B. brauniiCitation[45], which is highly promising, since it enables us to suppress algal contamination with herbicide treatment. Another reason for the rarity of the raceway culture of B. braunii may be the lower productivity of this system. In the case of other algae such as Dunaliella, the maximum concentration in raceway ponds is approximately 1 g dry weight/l, which is commercially not feasible given the lower economic value of biofuel than carotenoids or other useful chemicals Citation[61]. It is well known that algal biomass productivity in closed systems is much higher than in raceway ponds. This means that some of these problems can be solved using a closed culture system for the mass culture of algae, although the setup cost is higher.
▪ Closed culture systems
Closed culture systems for algae are more generally called ‘photobioreactors’. These include an array of variously shaped culture vessels such as transparent tanks, domes, tubes, thin layer plates and plastic bags, all of which are designed primarily to collect sunlight energy effectively Citation[62]. For example, the diameter of tubes in some photobioreactor is less than 10 cm. This configuration is based on the photochemical consideration that a high concentration of algal biomass reduces the penetration of light energy deep inside the photobioreactor, thereby limiting the growth of the algae. Importantly, apart from maximizing biomass productivity, photobioreactors need to be designed to minimize the cost of biomass production. Photobioreactors are also equipped with a gas-exchange apparatus (to supply CO2 and to remove O2 produced by photosynthesis) and a pump that generates flow to prevent algal sedimentation during culture as well as to enable the even distribution of CO2. Algal cultures are much more controllable in photobioreactors than in open systems, because contamination risks are greatly reduced and operational regulation of CO2 and other culture variables is feasible. By controlling the flow speed and shading some paths in tubular reactors, it is even possible to moderate the photoinhibition effect that can cause low productivity. In addition, the reduced loss of water and CO2 from the media and the high concentration of biomass generally obtained (up to 30-times those in open culture systems) can sharply reduce the cost of algal biomass production. Photobioreactors can also reduce the space required for algal cultivation, yielding high per-hectare biomass productivity. For these reasons, many researchers have been investigating the mass culture of algae in photobioreactors, and in fact some algae have been successfully cultured in photobioreactors in commercial companies Citation[202].
Currently, few studies dealing with the cultivation of oil-producing algae in photobioreactors are available. For example, a recent study using a 480-l-scale tube bioreactor system comprising 40 independent tubes showed that the biomass productivity of B. braunii outdoors (under temperatures ranging from 14 to 37°C) was 0.125 g/l/day Citation[63]. Although this biomass productivity value is still not commercially cost-effective, we suggest that biotechnological innovation could overcome the difficulty of obtaining a large amount of B. braunii. Photobioreactors may also allow for the mixotrophic cultivation of oil-producing algae because several algae including B. braunii are known to grow much faster in the presence of organic carbon sources Citation[28]. However, for the practical application of mixotrophy, further refinement of bioreactors is necessary because the addition of carbon sources greatly increases the risk of bacterial contamination.
▪ Hybrid culture systems & combinatory systems
As noted above, open and closed culture systems have both advantages and disadvantages Citation[61]. To reconcile these issues, a hybrid system combining open ponds and photobioreactors has been developed Citation[61]. In this system, the initial small-scale culture is used as an inoculum for subsequent large-scale cultivation in an open pond. This idea has been already put into practical use in the cultivation of Haematococcus (Aquasearch, HI, USA).
Another promising cultivation scheme is to combine two or more cultures of different algae into one cultivation system. Because two phototrophic algae are mutually competitive for light, CO2 and nutrients, one of the algae species in this system should be a heterotroph. One possible idea is to allow heterotrophic algae to feed on the debris of phototrophic algae after the extraction of biofuel. To address this issue, we are now investigating a combinatory cultivation system using phototrophic B. braunii and heterotrophic Aurantiochytrium.
Downstream processing
Cultures in open ponds or photobioreactors contain low concentrations of algae (0.01–0.1%); therefore, recovering algal biomass from such dilute solutions requires processing steps such as harvesting, dewatering and lipid extraction. In recent years many of these processing technologies have been investigated for the conversion of algae to liquid transportation fuels.
Most of the tested technologies for harvesting and dewatering algae from cultures involve flocculation and sedimentation or dissolved air flotation, filtration and centrifugation. However, these are expensive, energy-consuming or unreliable in continuous large-scale operation Citation[64]. Other techniques such as acoustic focusing, manipulation of electric fields and bioharvesting have been proposed, but all remain in research and development stages Citation[15].
Lipid extraction includes the following approaches: solvent-based extraction using microwaves and/or sonication for cell disruption, use of solvents for milking algal cells without disrupting cellular functions and extraction bypass schemes such as engineered algal systems that secrete lipids directly into the growth medium. However, existing extraction techniques are mainly suitable for analytical- and laboratory-scale procedures or for the recovery or removal of high-value products Citation[15].
Thus, well-defined and demonstrated industrial-scale tests must be conducted before optimum downstream processing technologies can be selected.
Life cycle analysis of algal biodiesel for estimating process energy consumption & GHG emissions
Life cycle analysis (LCA) is a valuable tool for accounting for all energy use and GHG emissions incurred during biomass production and use of biofuels. Although relatively few studies have applied LCA to algae fuel pathways, LCA has become an integral part of assessing the energy and nature of energy products from microalgae in conjunction with the rapid expansion of research on algal biofuels Citation[43,65–69]. Some discrepancies have been recognized in several LCAs of algal biodiesel pathways with respect to energy balance and GHG emission, as described below.
A comparative LCA study on the production of biodiesel from the freshwater alga C. vulgaris cultivated in raceways has been performed to assess the energetic balance and the potential environmental impacts of the whole process chain, from biomass production to biodiesel combustion Citation[65]. Two different culture conditions, nominal fertilizing or nitrogen starvation, as well as two different extraction options, dry or wet extraction, were tested. Cultivation was carried out in one step, without using a specific facility dedicated to nitrogen deprivation or maintenance of the inoculum. Inventory analysis was performed without any allocation, but reflected the flows actively generated by the process chain. When the full energetic debt of the process chain was taken into account, it appeared that only a low nitrogen condition with a wet-extraction scenario has a positive balance. Moreover, this scenario always had lower impact on human health, ecosystem quality and resources. The study showed that any improvement of the oil extraction technique would have a direct impact on the sustainability of production. A similar LCA study investigated the global warming potential (GWP) and fossil-energy requirement of biodiesel production from C. vulgaris in paddlewheel-mixed raceway ponds Citation[66]. In this study, cultivation using a two-stage method was considered, whereby the cells were initially grown to a high concentration of biomass under nitrogen-sufficient conditions before the supply of nitrogen was discontinued, and the inventory analysis was done with allocation by converting the algal residue to CH4 by anaerobic digestion. Biodiesel produced from microalgae cultivated in raceway ponds would have approximately 80% lower GWP than fossil-derived diesel. In contrast to the former studies Citation[43,65], the electricity required during cultivation including circulation of the algae in the cultivation facility, recycling of both spent medium and nutrients, and the concentration of CO2 in the flue gas was found to contribute most to overall fossil energy requirement and GWP of the biodiesel production Citation[66].
The LCA associated with algae production was compared with those of switchgrass, canola and corn farming Citation[43]. These conventional crops had lower environmental impact than algae in energy use, GHG emissions and water regardless of cultivation location. Algae performed favorably only with respect to total land use and eutrophication potential. It was suggested that the large environmental footprint of algae cultivation was driven predominantly by upstream impacts, such as the demand for CO2 and fertilizer. A comparable LCA study of a notional production system designed for Australian conditions was conducted to compare biodiesel production from this alga with those from canola and ultra-low-sulfur diesel, with three different scenarios for CO2 supplementation, as well as two different production rates Citation[67]. The inventory analysis was performed with allocation by substituting the algal residue for CH4 produced by its anaerobic digestion. Algae GHG emissions were very favorable compared with those from canola and ultra-low-sulfur diesel, in contrast to the former study Citation[43].
An LCA of the stage from algal culture to fuel production, distribution and storage, called a well-to-pump (WTP) system, was investigated using two types of harvesting processes (filter press and centrifugation) Citation[68]. The analysis was performed with allocation by converting the algal residue to fuel ethanol and replacing distiller’s dried grains with solubles with algal residue. Energy demands were negative for the both processes and CO2 emission was negative for the filter press process. Thermal algal dewatering required the largest energy input (89%). An LCA of GHG emissions and energy use was performed for the whole algae-based pathway for producing biofuels such as biodiesel, renewable diesel and renewable gasoline Citation[69]. The portion of the whole pathway, called the well-to-wheels portion, includes two steps: the WTP portion and the pump-to-wheels portion for the vehicle use. In this study, coproducts were treated with a hybrid approach that combined energy allocation and displacement methods. Fossil energy consumption for algal fuel at the WTP stage was 2.6-times higher than that for low-sulfur petroleum diesel production, as opposed to the former study Citation[68]. The well-to-wheels fossil energy consumption for algal biodiesel was 45% of low-sulfur petroleum diesel. The largest contribution to fossil energy use for algal biodiesel was from the growth and first dewatering stage, which mostly involved fertilizer manufacturing, mixing and pumping.
Thus, as pointed out by Liu et al.Citation[70], Grierson and Strezov Citation[71] and Murthy Citation[64], there are fundamental differences in approaches to system boundary definition and there is general confusion regarding system boundaries, goals, functional units, impact reporting categories and/or methods of allocation. In the case of algal biomass, it seems that allocation is a key methodological issue that needs to be strictly consistent for the assessment of all technologies and pathways.
Economic aspects of future algal fuels
Early work by Benemann and Oswald estimated photosynthetic algal oil costs to be US$0.25–0.43/l, assuming that the targeted alga produced 20 or 60 g/m2/day with a high lipid content (40% of dry weight), equivalent to 10% total solar conversion efficiency Citation[72]. However, this overall analysis derived from studies carried out between 1978 and 1994, and additional R&D breakthroughs were required to attain such high productivity Citation[10]. Recently, algal oil costs were estimated for three oil production scenarios, all of which used water and nutrients supplied by municipal wastewater and additional CO2 supplied by flue gas from a natural gas-fired power plant Citation[73]. The scenarios differed in their primary process objective (wastewater treatment or oil production) and farm size (100 or 400 ha). The major technical assumptions were algal biomass with 25% TAG and 22 g/m2/day average annual total biomass productivity, of which 20 g/m2/day were harvested. Considering the recent progress in algal biomass productivity in open pond cultures Citation[59], these assumptions are quite reasonable. The costs, without credit for wastewater treatment, were $1.9–2.6/l for all scenarios. However, when wastewater treatment revenue from biological oxygen demand removal was included, the total cost of oil production decreased drastically to $0.18/l, indicating that wastewater treatment revenue was more than that for the capital and operation costs of an algal oil production facility. R&D with a large-scale demonstration plant is needed to verify the effects of wastewater treatment revenue on the economics of algal fuel production. Another economic study showed that biomass production costs, which did not include extraction and refining of oils, were $6.13/kg, $5.14/kg and $7.39/kg for open ponds, tubular photobioreactors and flat-panel photobioreactors, respectively, and these are now operating on a commercial scale. Optimizing production for several important cost factors (irradiation conditions, mixing, photosynthetic efficiency of the system, and media and CO2 costs), yielded a price of $0.84/kg of biomass Citation[74]. In this case, if the algal biomass has the same TAG content (25%) as assumed by Lundquist et al. and if extraction costs account for 16% of the total cost, oil cost can be calculated to be approximately $3.23/l. This figure is approximately similar to the costs without credit for wastewater treatment Citation[73].
Since 2007, Solazyme, Inc. has been producing algal oil from heterotrophic microalgae grown in commercially sized standard industrial fermentation equipment (75,000-l scale). The company is projecting oil production cost to be $0.91/l Citation[203], much cheaper than the cost with photosynthetic algae.
Thus, the integration of water treatment and algal biomass production, coupled with a hybrid production system, which comprised phototrophic and heterotrophic algae, are the most important issues for improving the economics of future algal fuels.
Conclusion
Most oleaginous microalgae accumulate TAGs as their main lipid constituents. TAGs must be converted into FAMEs by transesterification. However, the low oxidative stability and cold-weather performance problems of FAMEs preclude their use as jet fuel. Thus, the major challenge in this context is the development of the isomerization chemistry. In contrast, triterpenic hydrocarbons produced by B. braunii and Aurantiochytrium are the most suitable algal oils for replacing conventional petroleum-derived transportation fuels such as diesel, jet fuels and gasoline. However, other than these two species, no algae with high intracellular hydrocarbon content have been identified. Approximately 40,000 algal species have been described, whereas the number of undescribed or unknown algal species is estimated to be 300,000–10,000,000 Citation[75]. Therefore, screening for hydrocarbon-producing algae is important for meeting drop-in fuel requirements.
It is likely that the energy innovation potential of oleaginous microalgae is higher than that of terrestrial oil plants, but to date the large-scale commercial production of biodiesel or renewable diesel, kerosene or gasoline from oleaginous microalgae has not been reported. In particular, as pointed out in this article, existing techniques of algal production, harvesting and dewatering, and extraction and refinement, are mainly suitable for small-scale procedures or for the recovery or removal of high-value products. Also, newly proposed techniques are still in the R&D stage. Well-defined and well-demonstrated industrial-scale tests must be conducted before any decision regarding the optimum technologies can be made. This situation strongly influences LCA studies for algal fuel production. It is certain that LCA is a valuable tool for accounting for all energy use and GHG emissions incurred during the mass cultivation of algae and the subsequent downstream processing, but there are differences and discrepancies in the results from many of the published LCAs. It is important not only to improve the LCA methodology but also to obtain reliable data on inputs and outputs from industrial-scale experiments. The estimated cost remains high compared with that of fossil crude oils. Integration of algal fuel production and wastewater treatment processes, coupled with a hybrid production system, which comprised photosynthetic and heterotrophic microalgae, is probably the most effective means of decreasing the cost of algal fuel and making it competitive with fossil crude oil.
Future perspective
There have been few evaluations of the economics of future possible commercialization of biofuel production by microalgae. We analyzed the commercial feasibility of B. braunii fuel production using a conceptually designed thin plastic membrane bag system on a 19-ha scale Citation[76]. Utilizing corporate financial analyses methods Citation[77,78], the total discounted cash flow and net present value were estimated to be $7 million and $4.66 million, respectively, when oil price was assumed to be $1.53/l. This indicates that such an oil production plant offers promising prospects for investors, at least as long as fossil oil prices are above $1.53/l. Coupling algal oil productivity (100 tons/ha/year) with energy demand data for fossil fuels from the International Energy Agency Citation[79–83], we suggest that even at an algal fuel production of $1.53/l, the algal oil production business would become strongly competitive in the fuel market by the mid-21st century . Land-use limitations are one of the hurdles for the algal oil production business. International Energy Agency statistics indicate that total demand for fossil fuels is projected to reach approximately 2 × 104 million tons of oil equivalent by the mid-21st century. Substituting terrestrial plant fuels for fossil fuels would require approximately 12.3 Gha of land. However, algal oil productivity of 100 tons/ha/year would require only approximately 123 Mha to produce the same amount of oil to meet demand. Although the theoretical maximum oil production by photosynthetic microalgae was calculated to be 354 tons/ha/year, and the best case to be 40–53 tons/ha/year Citation[84], these estimates apply to an algal production system that relies only on solar energy input for driving growth and oil production. Additional energy inputs, such as sugars for mixotrophic or heterotrophic growth, were not considered. Thus, establishing a hybrid production system using phototrophic and heterotrophic algae in combination with wastewater treatment processes could open up new avenues over the next decade that would dramatically exceed both the theoretical maximum and the best case calculated for photosynthetic algae and would make algal fuels competitive with fossil fuels on the commercial market.
Table 1. Oil-producing algae.
Table 2. Biomass and lipid productivities of microalgae cultured in wastewaters.
Type of photosynthesis that harnesses water as an electron donor and releases oxygen; the most common type of photosynthesis in land plants and autotrophic algae.
Algae without chloroplasts, some of which have lost their chloroplasts secondarily, and others that belong to the basal autotrophic lineage that forked prior to the occurrence of endosymbiosis.
Open pond for the mass culturing of microalgae that is equipped with a paddle wheel to generate circular water flow; the pond is often oval shaped, looking like a circuit from above.
Specialized culture vessel designed for microalgae to acquire optimal amounts of light energy for photosynthesis.
Value of the anticipated revenue stream from an investment, as of today or on any given date. Since money multiplies on its own, a dollar received today is less valuable than a dollar received in the future. The sum of all discounted cash flows for an explicit value period is the total discounted cash flow.
The difference between the present value of cash inflows and the present value of cash outflows. Net present value is used in capital budgeting to analyze the profitability of an investment or project. A positive net present value indicates a desirable investment project.
Background
▪ Algae have been closely associated with the global environment throughout the history of the Earth. | |||||
▪ Algae have high biomass productivity compared with land plants and produce various economically valuable products. Furthermore, algal industries do not compete directly with food production. | |||||
▪ Algal refining has the potential to improve the world’s energy situation. | |||||
Oleaginous algae & their products
▪ Many oleaginous algae have been reported, and most of them synthesize triacylglycerol (TAG) as a storage lipid. | |||||
▪ TAG is converted into fatty acid methyl esters for utilization as biodiesel. | |||||
▪ Only a very few species of algae, namely Botryococcus braunii and Aurantiochytrium sp., accumulate high amounts of hydrocarbons. | |||||
▪ Algal hydrocarbons are the most suitable oils for replacing existing transportation fuels and are compatible with the existing petroleum infrastructure. | |||||
Culture, growth & oil productivity of oleaginous algae under laboratory conditions
▪ B. braunii accumulates large amounts of hydrocarbons typified by botryococcenes. B. braunii is classified into several chemical races based on its hydrocarbon products. | |||||
▪ A novel strain of Aurantiochytrium, strain 18W-13a, accumulates high amounts of squalene. | |||||
▪ Chlorella, Dunaliella, Neochloris, Nannochloropsis and Phaeodactylum are also major oil-producing algae that produce primarily TAGs. | |||||
Algae & wastewater
▪ Wastewater has the potential to provide nutrients or to replace the culture medium for algal growth and oil production. | |||||
▪ Most of the studies using wastewater have been conducted on a small laboratory scale. | |||||
▪ Pilot- or demonstration-scale investigations are required to address the most appropriate ways to integrate algal biofuel production and wastewater treatment processes. | |||||
Algal biomass production under outdoor conditions
▪ Open-pond systems do not appear to be cost-effective means for producing biofuel from microalgae, unless oil productivity can be greatly enhanced or high-value byproducts can be produced. | |||||
▪ Photobioreactors of various shapes have been developed, which enable higher production of algal fuels. | |||||
▪ Combination systems that couple open and closed cultures are highly promising for the production of cost-effective biofuels from microalgae. | |||||
Downstream processing
▪ Existing technologies for harvesting, dewatering and lipid extraction are expensive, energy-consuming or unreliable for continuous large-scale operations. | |||||
▪ Well-defined industrial-scale demonstrations are required for the selection of optimum downstream processing technologies. | |||||
Life cycle analysis of algal biodiesel for estimating process energy consumption & GHG emissions
▪ Life cycle analysis has become essential for assessing the energy consumed and the nature of energy products obtained from microalgae, in conjunction with the rapid expansion of research on algal biofuels. | |||||
▪ Some discrepancies have been recognized in several life cycle analyses of algal biodiesel pathways with respect to energy balance and GHG emissions. | |||||
▪ Allocation is a key methodological issue that must be kept strictly consistent for the assessment of all technologies and pathways. | |||||
Economic aspects of future algal fuels
▪ The estimated cost remains expensive compared with the cost of fossil crude oils. | |||||
▪ Integration of algal fuel production and wastewater treatment processes is likely to be the most effective way of lowering the cost of algal fuel and making it competitive with fossil crude oil. | |||||
▪ R&D using a large-scale demonstration plant is needed to verify the effect of wastewater treatment revenue on the economy of algal fuel production. | |||||
Financial & competing interests disclosure
The authors would like to thank University of Tsukuba, Ministry of Education, Culture, Sports, Science and Technology, Japan Society for Promoting Science, Japan Science and Technology Agency and New Energy and Industrial Technology Development Organization for funding to support our research activities. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Wijffels RH, Barbosa MJ. An outlook on microalgal biofuels. Science329(5993),796–799 (2010).
- Bosak T, Liang B, Sim MS, Petroff AP. Morphological record of oxygenic photosynthesis in conical stromatolites. Proc. Natl Acad. Sci. USA106(27),10939–10943 (2009).
- Kump LR. The rise of atmospheric oxygen. Nature451(7176),277–278 (2008).
- Payne JL, Boyer AG, Brown JH et al. Two-phase increase in the maximum size of life over 3.5 billion years reflects biological innovation and environmental opportunity. Proc. Natl Acad. Sci. USA106(1),24–27 (2009).
- Stasiuk LD. Confocal laser scanning fluorescence microscopy of Botryococcus alginite from boghead oil shale, Boltysk, Ukraine: selective preservation of various micro-algal components. Org. Geochem.30,1021–1026 (1999).
- Chisti Y. Biodiesel from microalgae. Biotech. Adv.25(3),294–306 (2007).
- Chisti Y. Fuels from microalgae. Biofuels1(2),233–235 (2010).
- Vijayaragh K, Karthik R, Kamala Nalini SP. Hydrogen generation from algae: a review. J. Plant. Sci.5(1),1–19 (2010).
- Yen H-W, Brune DE. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol.98(1),130–134 (2007).
- Sheehan J, Dunahay TG, Benemann JR, Roessler PG, Weissman JC. A Look Back at the US Department of Energy’s Aquatic Species Program: Biodiesel from Algae. National Renewable Energy Laboratory, Golden, CO, USA (1998).
- Haag AL. Algae bloom again. Nature447(31),520–521 (2007).
- Wada H, Murata N. The essential role of phosphatidylglycerol in photosynthesis. Photosynth. Res.92(2),205–215 (2007).
- Atadashi IM, Aroua MK, Abdul-Aziz A. High quality biodiesel and its diesel engine application. Renew Sustain Energ Rev.14(7),1999–2008 (2010).
- Aatola H, Larmi M, Sarjovaara T, Mikkonen S. Hydrotreatedd vegetable oil (HVO) as a renewable diesel fuel: trade-off between NOx particulate emission and fuel consumption of a heavy duty engine. SAE Int. J. Engines1,1251–1262 (2008).
- Fishman D, Majumdar R, Morello J, Pate R, Yang J. National Algal Biofuels Technology Roadmap. US Department of Energy, Office of Energy Efficiency and Renewable Energy, Biomass Program, Washington, DC, USA (2010).
- Rezanka T, Zahradník J, Podojil M. Hydrocarbons in green and blue-green algae. Folia Microbiologica27(6),450–454 (1982).
- Metzger P, Largeau C. Botryococcus braunii: a rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol.66(5),486–496 (2005).
- Kawachi M, Tanoi T, Demura M, Kaya K, Watanabe MM. Relationship between hydrocarbons and molecular phylogeny of Botryococcus braunii. Algal Res. doi:10.1016/j.algal.05.003 (2012) (In press).
- Niehaus TD, Okada S, Devarenne TP et al. Identification of unique mechanisms for triterpene biosynthesis in Botryococcus braunii. Proc. Natl Acad. Sci. USA108(30),12260–12265 (2011).
- Nagano S, Yamamoto S, Nagakubo M, Atsumi K, Watanabe MM. Physical properties of hydrocarbon oils produced by Botryococcus braunii with special reference to the density, kinematic viscosity, surface tension, and distillation properties. Procedia Environ. Sci.15,73–79 (2012).
- Peyronel D, Artaud J, Iatrides MC, Rancurel P, Chevalier JL. Fatty acid and squalene compositions of Mediterranean Centrophorus SPP egg and liver oils in relation to age. Lipids19(9),643–648 (1984).
- Chang M-H, Kim H-J, Jahng K-Y, Hong S-C. The isolation and characterization of Pseudozyma sp. JCC 207, a novel producer of squalene. Appl. Microbiol. Biotechnol.78(6),963–972 (2008).
- Kimura T. Liu C. Li X. Maekawa T. Asaoka S. Conversion of isoprenoid oil by catalytic cracking and hydrocracking over nanoporous hybrid catalysts. J. Biomed. Biotechnol.637125 (2012).
- Chaiwong K, Kiatsiriroat T, Vorayos N, Thararax C. Biochar production from freshwater algae by slow pyrolysis. J. Sci. Technol.6(2),186–195 (2012).
- Demura M, Kawachi M, Koshikawa H, Nakayama T, Mayuzumi Y, Watanabe MM. Succession of genetic diversity of Botryococcus braunii (Trebouxiophyceae) in two Japanese reservoirs. Procedia Environ. Sci.1(5),3–11 (2012).
- Watanabe MM, Tanabe Y. Biology and industrial potential of Botryococcus braunii. In: Handbook of Microalgal Culture (2nd Edition). Wiley-Blackwell, Oxford, UK (2012) (In press).
- Ge Y, Lu J, Tian G. Growth characteristics of Botryococcus braunii 765 under high CO2 concentration in photobioreactor. Bioresour. Technol.102,130–134 (2011).
- Tanoi T, Kawachi M, Watanabe MM. Effects of carbon source on growth and morphology of Botryococcus braunii. J. Appl. Phycol.23(1),25–33 (2011).
- Ioki M, Baba M, Bidadi H et al. Modes of hydrocarbon oil biosynthesis revealed by comparative gene expression analysis for race A and race B strains of Botryococcus braunii. Bioresour. Technol.109,271–276 (2012).
- Yokoyama R, Honda D. Taxonomic rearrangement of the genus Schizochytrium sensu lato based on morphology, chemotaxonomic characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium gen. nov. Mycoscience48(4),199–211 (2007).
- Honda D, Yokochi T, Nakahara T, Erata M, Higashihara T. Schizochytrium limacinum sp. nov., a new thraustochytrid from a mangrove area in the west Pacific Ocean. Mycol. Res.102(4),439–448 (1998).
- Raghu-Kumar S. Schizochytrium mangrovei sp. nov., a thraustochytrid from mangroves in India. Transact. British Mycol. Soc.90(4),627–631 (1988).
- Rosa SM, Soria MA, Vélez CG, Galvagno MA. Improvement of a two-stage fermentation process for docosahexaenoic acid production by Aurantiochytrium limacinum SR21 applying statistical experimental designs and data analysis. Bioresour. Technol.101(7),2367–2374 (2010).
- Liang Y, Sarkany N, Cui Y et al. Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresour. Technol.101(10),3623–3627 (2010).
- Chen G, Fan KW, Lu FP et al. Optimization of nitrogen source for enhanced production of squalene from thraustochytrid Aurantiochytrium sp. New Biotechnol.27(4),382–389 (2010).
- Kaya K, Nakazawa A, Matsuura H et al. Thraustochytrid Aurantiochytrium sp. 18W-13a accumulates high amounts of squalene. Biosci. Biotechnol. Biochem.75(11),2246–2248 (2011).
- Nakazawa A, Matsuura H, Kose R et al. Optimization of culture conditions of the thraustochytrid Aurantiochytrium sp. strain 18W-13a for squalene production. Bioresour. Technol.109,287–291 (2012).
- Oswald WJ, Gotaas HB. Golueke CG, Kellen WR, Gloyna EF, Hermann ER. Algae in waste treatment. Sewage Ind. Wastes29,437–457 (1957).
- Pittman JK. Dean AP, Osundeko O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol.102,17–25 (2011).
- Lam MK, Lee KT. Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol. Adv.30,673–690 (2012).
- Canakci M. Sanli H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol.35,431–441 (2008).
- Xiong W, Li XF, Xiang JY, Wu QY. High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl. Microbiol. Biotechnol.78,29–36 (2008).
- Clarens AF, Resurreccion EP, White MA, Colosi LM. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ. Sci. Technol.44,1813–1819 (2010).
- Yonezawa N, Matsuura H, Shiho M, Kaya K, Watanabe MM. Effects of soybean curd wastewater on the growth and hydrocarbon production of Botryococcus braunii strain BOT-22. Bioresour Technol.109,304–307 (2012).
- Ioki M, Ohkoshi M, Nakahira-Yanaka Y, Nakajima N, Watanabe MM. Isolation of herbicide-resistant mutants of Botryococcus braunii. Bioresour. Technol.109,300–303 (2012).
- Li Y, Zhou W, Hu B, Min M, Chen P, Ruan RR. Integration of algae cultivation as biodiesel production feedstock with municipal wastewater treatment: strains screening and significance evaluation of environmental factors. Bioresour. Technol.102,10861–10867 (2011).
- Órpez R, Martínez ME, Hodaifa G, Yousfi FE, Jbari N, Sánchez S. Growth of the microalga Botryococcus braunii in secondarily treated sewage. Desalination246,625–630 (2009).
- Zhou W, Li Y, Min M, Hu B, Chen P, Ruan R. Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresour. Technol.102,6909–6919 (2011).
- Sydney EB, da Silva TE, Tokarski A et al. Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl. Energy88,3291–3294 (2011).
- Zhou W, Min M, Li Y et al. A hetero-photoautotrophic two-stage cultivation process to improve wastewater nutrient removal and enhance algal lipid accumulation. Bioresour. Technol.110,448–455 (2012).
- An J-Y, Sim S-J, Lee JS, Kim BW. Hydrocarbon production from secondarily treated piggery wastewater by the green alga Botryococcus braunii. J. Appl. Phycol.15,185–191 (2003).
- Ji M-K, Kim H-C, Sapireddy VR et al. Simultaneous nutrient removal and lipid production from pretreated piggery wastewater by Chlorella vulgaris YSW-04. Appl. Microbiol. Biothechnol. doi:10.1007/s00253–012–4097-x (2012) (Epub ahead of print).
- Wang L, Li Y, Chen P et al. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour. Technol.101,2623–2628 (2010).
- Wang H, Xiong H, Hui Z, Zeng X. Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids. Bioresour. Technol.104,215–220 (2012).
- Kushwaha JP. Srivastava VC. Mall ID. An overview of various technologies for the treatment of dairy wastewaters. Crit. Rev. Food Sci. Nutr.51,442–452 (2011).
- Chinnasamy S, Bhatnagar A, Hunt RW, Das KC. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol.101,3097–3105 (2010).
- Hongyang S, Yalei Z, Chunmin Z, Xuefei Z, Jinpeng L. Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour. Technol.102,9884–9890 (2011).
- Yamasaki T, Aki T, Shinozaki M, Taguchi M, Kawamoto S, Ono K. Utilization of Shochu distillery wastewater for production of polyunsaturated fatty acids and xanthophylls using thraustochytrid. J. Biosci. Bioeng.102,323–327 (2006).
- Brennan L, Owende P. Biofuels from microalgae – a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sust. Energy Rev.14,557–577 (2010).
- Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng.101(2),87–96 (2006).
- Schenk PM, Thomas-Hall SR, Stephens E et al. Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res1,20–43 (2008).
- Posten C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci.9,165–177 (2009).
- Shimamura R, Watanabe S, Sakakura Y, Shiho M, Kaya K, Watanabe MM. Development of Botryococcus seed culture system for future mass culture. Procedia Environ. Sci.15,80–89 (2012).
- Murthy GS. Overview and assessment of algal biofuels production technologies. In: Biofuels – Alternative Feedstocks and Conversion Processes. Academic Press, Waltham, MA, USA, 415–437 (2011).
- Larden L, Hèlias A, Sialve B, Steyer JP, Bernard O. Life-cycle assessment of biodiesel production from microalgae. Environ. Sci. Technol.43,6475–6481 (2009).
- Stephenson AL, Kazamia E, Dennis JS, Howe CJ, Scott SA, Smith AG. Life-cycle assessment of potential algal biodiesel production in the United Kingdom: a comparison of raceways and air-lift tubular bioreactors. Energy Fuels24,4062–4077 (2010).
- Campbell PK, Beer T, Batten D. Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour. Technol.102,50–56 (2011).
- Sander K, Murthy GS. Life cycle analysis of algae biodiesel. Int. J. Life Cycle Assess.15,704–714 (2010).
- Frank ED, Han J, Palou-Rivera I, Elgowainy A, Wang MQ. Life-Cycle Analysis of Algal Lipid Fuels with the GREET Model. ANL/ESD/11–15. Energy System Division, Argonne National Laboratory, US Department of Energy, Lemont, IL, USA (2011).
- Liu X, Clarens AF, Colosi LM. Algae biodiesel has potential despite inconclusive results to date. Bioresour. Technol.104,803–806 (2012).
- Grierson S, Strezov V. Life cycle assessment of the microalgae biofuel value chain. Presented at: Binature 2012: The Third International Conference of Bioenvironment, Biodiversity and Renewable Energies. St Maarten, The Netherlands, 25–30 March 2012.
- Benemann JR, Oswald WJ. Systems and Economic Analysis of Microalgae Ponds for Conversion of CO2 to Biomass. Final Report to the Department of Energy. Pittsburg Energy Technology Center, Pittsburg, PA, USA (1996).
- Lundquist TJ, Woertz, IC, Quinn NWT, Benemann JR. A Realistic Technology and Engineering Assessment of Algae Biofuel Production. Energy Biosciences Institute, University of California, Berkeley, CA, USA (2010).
- Norsker NH, Barbosa MJ, Vermue MH, Wijffels R. Microalgal production – close look at the economics. Biotechnol. Adv.29,24–27 (2011).
- Norton TA, Michael Melkonian M, Andersen RA. Algal biodiversity. Phycologia35(4),308–326 (1996).
- Shiho M, Kawachi M, Horioka K et al. Business evaluation of an oil production system with green micro-algae Botryococcus braunii. Procedia Environ. Sci.15,90–109 (2012).
- Brealey RA, Myers ST, Allen F. Principles of Corporate Finance. McGraw-Hill International, NY, USA (2008).
- Hiller D, Ross S, Westerfield R, Jaffe J, Jordan B. Corporate Finance. McGraw-Hill Education, Maidenhead, UK (2010).
- International Energy Agency. World Energy Outlook 2006. International Energy Agency/OECD, Paris, France (2006).
- International Energy Agency. World Energy Outlook 2007. International Energy Agency/OECD, Paris, France (2007).
- International Energy Agency. World Energy Outlook 2008. International Energy Agency/OECD, Paris, France (2008).
- International Energy Agency. World Energy Outlook 2009. International Energy Agency/OECD, Paris, France (2009).
- International Energy Agency. World Energy Outlook 2010. International Energy Agency/OECD, Paris, France (2010).
- Weyer K, Bush DR, Darzins A, Wilson BD. Theoretical maximum algal oil production. Bioenergy Res.3,204–213 (2010).
- Eroglu E, Okada S, Melis A. Hydrocarbon productivities in different Botryococcus strains: comparative methods in product quantification. J. Appl. Phycol.23,763–775 (2011).
- Rodolfi L, Chini Zittelli G, Bassi N et al. Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng.102,100–112 (2009).
- Gouveia L, Oliveira AC. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol.36,269–274. (2009).
- Chen Y, Wang J, Liu T, Gao L. Effects of initial population density (IPD) on growth and lipid composition of Nannochloropsis sp. J. Appl. Phycol. (2012) (Epub ahead of print).
- Li Y, Horsman M, Wang B, Wu N, Lan CQ. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol.81,629–636 (2008).
- Satoh A, Kato M, Yamato K et al. Characterization of the lipid accumulation in a new microalgal species, Pseudochoricystis ellipsoidea (Trebouxiophyceae). J. Jpn Inst. Energy89,909–913 (2010).
- Tucci S, Vacula R, Krajcovic J, Proksch P, Martin W. Variability of wax ester fermentation in natural and bleached Euglena gracilis strains in response to oxygen and the elongase inhibitor flufenacet. J. Eukaryot. Microbiol.57,63–69 (2010).
▪ Patent
- Kurano N, Sekiguchi H, Sato A, Matsuda S, Adachi K, Atsumi M: US7981648 B2 (2011).
▪ Websites
- Solazyme. http://solazyme.com
- Algenol Biofuels. www.algenolbiofuels.com
- Biofuels Digest. Solazyme files $100M IPO. www.biofuelsdigest.com/bdigest/2011/03/15/solazyme-files-100m-ipo