Abstract
Genome sequencing of cellulolytic bacteria combined with analyses using structural and sequence similarity software reveals potential cellulolytic enzymes not previously recovered by routine purification or shotgun cloning techniques. Comparison of the presence and absence of potential cellulases across the prokaryotes indicate that there are at least four distinct mechanisms of cellulose degradation; two distinct soluble systems and two separate cell-based systems can be postulated. None of the mechanisms appear completely homologous to the Trichoderma system of cellulose degradation. Minimum sets of bacterial cellulases can be predicted for the soluble sets based on comparison across genomes, leading to testable hypotheses on cellulose degradation.
Reproduced from Citation[106].
![Figure 1. Structural organization of cellulose from individual molecules to crystal, microfibrils, plant cell walls and finally whole plants.Reproduced from Citation[106].](/cms/asset/e3f5f855-72f6-4f72-bd87-17bf550658d2/tbfu_a_10816103_f0001.gif)
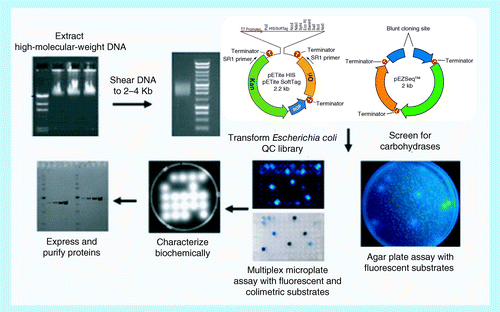
DNA is prepared, sheared, ligated into a vector, screened for positive colonies, and then the protein is expressed and characterized.
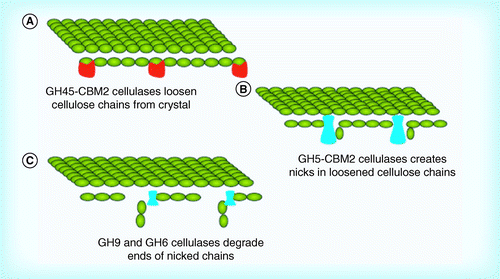
(A) A GH45 decrystallization of cellulose; (B) nicking of decrystallized cellulose; and (C) degradation of nicked chains.
CBM: Carbohydrate binding modules; GH: Glycosyl hydrolase.
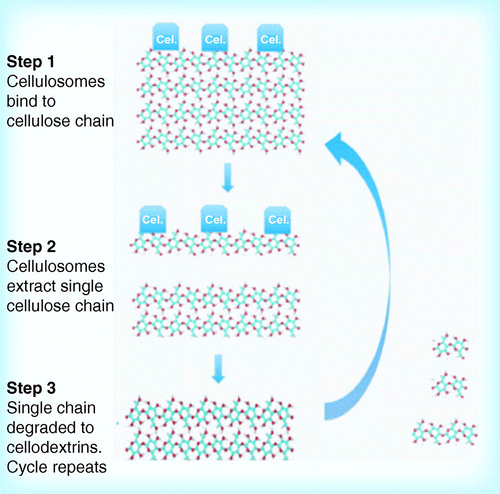
(A) Binding of cellulose to outer membrane; (B) abstraction of a single cellulose chain; (C) translocation of chain through outer membrane pore; and (D) cleavage of chain in periplasm and transport of oligosaccharides through inner membrane. Based on alginate transport and utilization model Citation[85].
![Figure 5. Potential mechanism for decrystallization and cellulose hydrolysis by Fibrobacter succinogenes, Cytophaga hutchinsonii and Sorangium cellulosum. (A) Binding of cellulose to outer membrane; (B) abstraction of a single cellulose chain; (C) translocation of chain through outer membrane pore; and (D) cleavage of chain in periplasm and transport of oligosaccharides through inner membrane. Based on alginate transport and utilization model Citation[85].](/cms/asset/f6a0cc3e-6a74-45ed-9dbd-a04b13de24f5/tbfu_a_10816103_f0005.gif)
Cellulose, a β-(1,4) homopolymer of glucose, is the most prevalent biopolymer on the planet. Cellulose is composed of very long, linear glucan chains, or microfibrils, with each individual chain containing between 3000 and 15,000 glucose units Citation[1]. Cellulose microfibrils, along with lignin and hemicellulose, comprise the plant cell wall . As a result of the uniform structure of the individual chains, cellulose is highly crystalline; this crystallinity gives structural rigidity to the plant cell wall. In spite of cellulose’s wide abundance and long history of study, the macromolecular structure of cellulose and its allomorphs remains the subject of considerable study Citation[2–6]. The effective and low cost enzymatic conversion of cellulose to glucose is regarded as essential for the production of biofuels from biomass; the status of research into enzymatic cellulose degradation and cellulases has been reviewed multiple times Citation[7–9].
In nature, cellulose is degraded enzymatically by complexes of multiple enzymes collectively called ‘cellulases’. The nomenclature of bacterial cellulases is plagued by the problem of attempting to identify and describe the activity of a single component enzyme of this multienzyme functional complex (this is somewhat akin to the problems encountered with assigning functions to individual ribosomal proteins). This difficulty has led to the proliferation of terms such as endocellulase, exocellulase, cellobiohydrolase and processive endocellulase to describe the activity of these individual components Citation[7]. These terms are informative under certain circumstances, but often suggest a greater understanding of cellulose degradation than actually exists.
In common use, three functional definitions of cellulase exist, each less restrictive than the previous:
▪ The most stringent definition is an enzyme that releases a physiologically relevant amount of sugar from the crystalline cellulose present in biomass, a purified crystalline cellulose or a crystalline product of cellulose degradation (Avicel®). These enzymes are a subset of the next definition. By this definition, β-glucosidase, while important in cellulose degradation, is not a cellulase. Unfortunately, few enzymes characterized to date can meet these exacting criteria; | |||||
▪ A more commonly used, less stringent, definition is an enzyme that hydrolyzes any of a variety of nonphysiological cellulose surrogates, including carboxymethyl cellulose, phosphoric acid swollen cellulose or 4-methylumbelliferyl-β-D-cellobioside. These enzymes may or may not release carbohydrate from crystalline celluloses; many xylanases and β-glucanases hydrolyze nonphysiological substrates but are inactive on cellulose. It is assumed that eventually these enzymes will be subjected to analysis by the criteria in the previous definition to confirm their classification; | |||||
▪ The least stringent definition is an enzyme or putative enzyme that possesses a high level of sequence homology to an enzyme identified in the previous two definitions. This is a tentative classification and assumes that sufficient effort will be applied to move the candidate to meeting the previous two definitions. These enzymes will be referred here as ‘potential cellulases’. | |||||
Approaches to cellulase discovery
▪ Conventional approaches to cellulase discovery before cloning
Purification of individual enzymes of interest from the culture of a known organism or a novel isolate is a long-standing technique of classical biochemistry. In the era before DNA sequencing and cloning, this method was the sole route to obtaining and characterizing novel cellulases Citation[10–19]. In this early work, protein sequence data was either not reported or a limited N-terminal sequence was published; making matching of purified enzymes to gene sequences extremely challenging. This method has also been used to isolate whole cellulosomes for analysis Citation[20–22]. The data from early work is often difficult to reconcile with sequence data; for example, Fibrobacter succinogenes is annotated as containing four GH45 family members, but which of these four has been described is nearly impossible to ascertain Citation[23].
▪ Library screening of genomic & metagenomic libraries
The conventional method described above was rapidly supplanted by the introduction of cloning technology. With the advent of this technology, the need to purify enzymes individually from complex mixtures of proteins was reduced, but not eliminated. In this technique, DNA is isolated and purified from a cellulolytic organism such as Clostridium thermocellum. The purified DNA is then either enzymatically or mechanically fragmented and the fragments are ligated into a suitable expression vector. The vector is transformed into a competent Escherichia coli strain, and the resulting clones screened for cellulase activity .
Using these shotgun cloning techniques, cellulases were cloned from Cellulomonas fimiCitation[24,25], Clostridium cellulolyticumCitation[26] and F. succinogenesCitation[27,28], as well as many of the individual components of the C. thermocellum cellulosome Citation[29–33]. A similar approach was utilized to clone cellulases from samples of metagenomic DNA prepared from an environmental sample of microbes. A variety of glycosyl hydrolases (GH) have been recovered from screening of metagenomic libraries Citation[34–38]; the majority of the GH recovered were not cellulases Citation[39].
▪ Bioinformatic approaches to cellulase discovery
The combination of whole genome sequencing and activity prediction by sequence comparison has led to a revolution in understanding cellulose degradation and cellulases. A detailed description of whole genome sequencing is beyond the scope of this review. Genomic DNA is prepared, broken into large and small fragments, the individual fragments are sequenced, and the sequences of the large fragments are used to assemble the sequences into a closed, circular genome. Complete genome sequences are available from a number of sources including the Integrated Microbial Genomes (IMG) Citation[40,101]. Often, the fragments cannot be assembled into a single unit, and anywhere from 20 to 200 fragments remain unassembled. This situation results in a ‘draft genome’. Often there is no desire to continue assembly of these draft genomes and they are considered completed.
Once a genome sequence is determined, a variety of bioinformatics tools are used to identify and annotate the individual genes in the genome. All genes in the genome are typically identified using software such as Prodigal Citation[41] or Rapid Annotation using Subsystem Technology (RAST) Citation[42]. The predicted genes are translated and used to search resources including the National Center for Biotechnology Information nonredundant database, Universal Protein Resource (UniProt), TIGRFam, Pfam, PRofils pour l’Identification Automatique du Métabolisme (PRIAM), Kyoto Encyclopedia of Genes and Genomes (KEGG), Clusters of Orthologous Groups (COG) and InterPro databases. A proposed description of each predicted protein is obtained from the combination of the results obtained. Identification of potential cellulases can then be derived from the results of direct sequence homology comparisons to known proteins (blastp) Citation[43]. Another method of identifying cellulases is by comparison to the sequences of conserved structural elements in known cellulases (Carbohydrate Active Enzyme Database [CAZy]) Citation[44,102] and Pfam Citation[45–47,103]. Due to the differences in search methodologies and databases, the results obtained using these two methods may yield slightly different results:
▪ While InterPro can be used to identify the presence of dockerin domains, CAZy is not equipped to do this analysis; | |||||
▪ InterPro can identify more structural domains in carbohydrases than CAZy. For example, InterPro identifies fibronectin-like domains (fn3) and immunoglobulin-like domains in many carbohydrases. These domains are not identified in the CAZy database; CAZy only identifies GH, carbohydrate esterase and carbohydrate binding modules (CBM); | |||||
▪ InterPro identifies some domains as mixed CBM (e.g., CBM5/12); CAZy identifies the same domain as two separate CBMs, a CBM5 and CBM12; | |||||
▪ CAZy provides links to both characterized proteins and protein structures for each domain type; these are more difficult to access from InterPro. | |||||
For genomes not annotated by one of the large pipelines, individuals can submit gene sequences or whole genomes for either CAZy sequence- or Pfam-based annotation Citation[41,104]. The site also is able to do family associations between, for example, GH family members and CBM family members. Whole genomes or individual gene sequences can be also be analyzed for CAZy family members by using dbCAN Citation[48,105]. The majority of published reports now utilize the CAZy conventions to describe the domain structure of carbohydrases including cellulases; this review will follow the CAZy conventions in discussing protein structures. Within CAZy only, a limited number of families contain cellulolytic enzymes, currently these families include GH5, GH6, GH7, GH8, GH9, GH12, GH45, GH48, GH51 and GH61 (GH61 family members have been reclassified by CAZy into a new class, AA9, containing proteins that are copper-dependent lytic polysaccharide monooxygenases). Similarly, a limited number of CBM families have members that can bind to crystalline cellulose; these families include CBM1, CBM2, CBM3 and CBM4. It is of utmost importance to realize that family membership does not imply cellulolytic activity. For example, the GH5 family contains both mannanases and cellulases; construction of a family tree for each individual protein (using Basic Local Alignment Search Tool [BLAST] or other resources) will assist in prediction of cellulolytic potential. While bioinformatics can predict cellulolytic activity, cloning of the predicted gene and expression and characterization of the protein is the true proof of cellulolytic activity.
A major application of bioinformatics is to understand the number of cellulases needed to degrade cellulose and the mechanism these enzymes use to degrade cellulose. Significant progress has been made with fungal enzymes in identifying a minimum set of enzymes capable of degrading cellulose and biomass Citation[49]. In the Trichoderma system, CBHI (GH7), CBHII, (GH6) and EG1 (GH6) are the three cellulases to produce >80% conversion of crystalline cellulose in the presence of β-glucosidase; all three contain CBM1 domains. In the proposed mechanism, CBHI produces cellobiose processively from the reducing end of a cellulose chain, while CBHII produces cellobiose processively from the nonreducing end of the cellulose chain. EG1 functions to internally nick the cellulose chain and generate new cellulose chain ends for attack by CBHI and CBHII. All three enzymes utilize acidic residues to catalyze the addition of water across the glycosidic bond. The CBM1 domains bind to cellulose and extract a single chain from the fibril, feeding it into the active sites and causing motion of the enzymes along the cellulose chain Citation[50]. Homologues of this set of enzymes can be found in a number of fungi, including many Trichoderma and Aspergillus species. Recently, AA9 enzymes have been postulated to assist in cellulose degradation by TrichodermaCitation[51]. These enzymes do not catalyze a hydrolytic cleavage of the cellulose chain; these enzymes oxidatively cleave the chain, generating cellobionic acid as one product. The role of these AA9 and their bacterial homologues (CAZy family AA10), in cellulose hydrolysis is still unclear. Among certain fungi the role of these enzymes may take on increased importance; homologues of the Trichoderma set of cellulases are not present in all cellulolytic fungi.
Since GH7 and CBM1 family members do not exist in the bacterial world, it is apparent that differences must exist in the way cellulose is degraded in the bacterial and fungal realms. Bioinformatics allows the identification of all potential cellulases in sequenced bacterial genomes and to identify potential combinations of GH family members required for cellulose degradation by bacterial systems. For this review, twelve closed genomes of prokaryotes with demonstrated ability to degrade native crystalline cellulose were analyzed for potential cellulases (Box 1). This is not meant to be a comprehensive list of all cellulolytic organisms or sequenced organisms, but reflects a sampling of the cellulolytic organisms with closed genomes for which annotations in the National Center for Biotechnology Information and CAZy were available to all researchers. For example, Teredinibacter, Saccharophagus and Thermobifida species all have characterized cellulolytic systems. The supplementary material presented contains details on each of the potential cellulases identified by annotation as well as references to the purified protein if extant. It is instructive to compare the list of potential cellulases to the known and characterized cellulases; all the potential cellulases have not been characterized in any of the genomes.
Cellulose degradation by soluble enzymes of the Gram-positive bacteria
There are a significant number of cellulose degraders among the Gram-positive organisms; cellulolytic organisms are found in the Streptomycetaceae, Cellulomonadaceae, Acidothermaceae, Thermoanaerobacterales and Clostridiaceae families. The cellulolytic members of the Streptomycetaceae, Cellulomonadaceae, Acidothermaceae and Thermoanaerobacterales typically produce small (8–12 enzymes) sets of only secreted, soluble GH5, GH6, GH9, GH12 and GH48 family members; many of the members have one or more CBM domains attached, typically CBM2 or CBM3 .
▪ Streptomyces & Acidothermus
A number of Streptomyces species are known to be efficient degraders of cellulose. Two of the cellulose degraders, Streptomyces flavogriseus ATCC 33331™ and Streptomyces sp. SirexAA-E (Sacte) currently have finished genomes that have been analyzed by CAZy. The two genomes show a great deal of similarity in cellulase family members and CBM distribution. The S. flavogriseus genome shows a set of ten potential cellulolytic enzymes, four GH5, two GH6, two GH12, and one each of GH9 and GH48 (Supplementary Table A). Of these ten genes, five have CBM2 domains, four have no CBM domain, and one has both a CBM2 and CBM4 domain. The Sacte genome has fewer potential cellulases, three GH5 and one each of GH6, GH9, GH12 and GH48 (Supplementary Table B). All except the GH12 have CBM2 domains; the GH9 possesses a CBM4 domain in addition to the CBM2. None of the potential cellulases of these two organisms have been characterized (Supplementary Tables A & B).
Acidothermus cellulolyticus 11B ATCC 43068™, an aerobic thermophile isolated from a hot spring at Yellowstone National Park (WY, USA) is currently the only sequenced Acidothermus species Citation[52]. In spite of the great difference in habitat, the cellulolytic enzyme set of A. cellulolyticus is similar to that of the Streptomyces. All three enzyme sets are a mixture of GH5, GH6, GH9, GH12 and GH48 cellulases. The cellulolytic enzyme set of A. cellulolyticus is similar in size to that of S. flavogriseus, containing one fewer GH module than the Streptomyces (nine vs ten; Supplementary Table C). The cellulase set of A. cellulolyticus has two distinct features; one is the presence of a multifunctional enzyme (A. cellulolyticus_0615, GH6 and GH12) and the second is the presence of multiple CBM modules per enzyme (CBM2 and CBM3 modules). These two features are reminiscent of the cellulases found in Caldicellulosiruptor species and may reflect an adaptation to cellulase degradation at higher temperature. Of the eight potential cellulases in the organism, four have been characterized; three appear to be cellulases and the fourth is a GH5 mannanase (Supplementary Table C).
▪ Cellulomonas
The cellulomonads are Gram-positive organisms that possess the ability to grow both aerobically and anaerobically by fermentation. A large number of Cellulomonas species are known for their ability to degrade cellulose, including Cellulomonas flavigena, C. fimi, Cellulomonas uda and Cellulomonas gelidaCitation[53]. In spite of their well-known ability to degrade cellulose, only two of these species have completed genomes, C. flavigenaCitation[54] and C. fimiCitation[55]. Surprisingly, both strains possess the exact same combination of 12 potential cellulases. Six cellulases, CenA through CenE and CbhA, have been purified and characterized from C. fimi. The genome sequencing of C. fimi revealed an additional six potential cellulases in the genome, two GH5, one GH6 and three GH9 (Supplementary Table D). Cfla has 11 potential cellulases with CBM domains, seven with CBM2, two with CBM2 and CBM3, and two with two CBM4 domains; one potential GH5 cellulase has no CBM. C. flavigena has 11 potential cellulases with CBM domains, seven with CBM2, two with CBM2 and CBM3, and two with two CBM4 domains. The genome sequencing of Cellvibrio gilvus revealed the organism was actually a member of the cellulomonads Citation[55]. C. gilvus, another prolific cellulose degrader, possesses a smaller set of cellulases than the other two Cellulomonas species; its genome contains nine potential cellulases: two GH5, four GH6, two GH9 and one GH48 (Supplementary Table E). Five of these enzymes possess CBM2 domains, one possesses both a CBM3 and CBM2 domains, and three do not have CBM domains. In the case of C. fimi, almost all the potential cellulases have been characterized; C. flavigena has only a single characterized enzyme and C. gilvus has none (Supplementary Tables D, E & F).
▪ Caldicellulosiruptor
Caldicellulosiruptor species are anaerobic, thermophilic, Gram-positive cellulolytic organisms isolated from hot springs Citation[56]. A number of genomes have been sequenced Citation[57], and gene expression data was obtained during growth on cellulose Citation[58]. The cellulase sets of Caldicellulosiruptor species are similar to the A. cellulolyticus set of cellulases. Caldicellulosiruptor saccharolyticus DSM 8903 (Supplementary Table G) and Caldicellulosiruptor kronotskyensis 2002 (Calkro) (Supplementary Table H) each possesses six GH5, two GH9 and three GH48 soluble, secreted enzymes, here is no evidence of cohesion-dockerin or other cell wall attachments in C. saccharolyticus and Calkro. In both C. saccharolyticus and Calkro, one or more CBM3 domains are attached to many of the GH family members; a number of enzymes containing two separate GH domains are also present in both C. saccharolyticus and Calkro. Unlike A. cellulolyticus, CBM2 domains are totally absent from these two organisms.
▪ Cellulose degradation mechanism
Based on the potential cellulases conserved in the nine organisms analyzed, the following predictions can be made and tested: there may exist minimum sets of soluble Gram-positive cellulases that are capable of degrading crystalline cellulose; the minimum set of enzymes would be a GH5, GH9 and GH48, each with a suitable CBM (CBM2 at mesophilic temperatures, CBM3 or multiple CBM3 modules at elevated temperatures). The mechanism of action of these minimum sets may be similar to that proposed for the Trichoderma reesei minimum set of CBHI, CBHII and EG1, or may be completely different. Members of GH6 and GH12 may function to increase accessibility of cellulose to the minimum set or increase the apparent rate of cellulose hydrolysis by attacking sites not recognized by the GH5, GH9 and GH48.
Cellulose degradation by soluble enzymes of Cellvibrio
There are fewer isolated cellulose degraders among the Gram-negative bacteria than among the Gram-positive bacteria. This may be result of a bias in isolation or sequencing of cellulolytic organisms, or by a lower number of Gram-negative cellulolytic organisms in the environment. No Gram-negative organism possesses a cellulolytic set of enzymes comparable to either the Gram-positive sets of cellulases described above or the fungal set described for T. reeseiCitation[7,59]. Of the Gram-negatives, Cellvibrio japonicus appears to have the most ‘normal’ collection of cellulases (Supplementary Table M). The C. japonicus genome codes for 15 GH5, three GH9 and one GH6. The organism produces no GH8, GH12 or GH48 cellulases; C. japonicus does produce one GH45 cellulase; the function of GH45 cellulases is poorly understood. While GH45 is an enzyme group normally associated with eukaryotic organisms, the genome of F. succinogenes codes for four GH45 cellulases. CBM2, CBM6 and CBM10 domains are present in many of C. japonicus cellulases; CBM6 and CBM10 are rarely seen with Gram-positive cellulases.
▪ Cellulose degradation mechanism
The lack of GH48 family members and the presence of a GH45 family member suggests that C. japonicus most likely does not utilize the mechanism of cellulose degradation utilized by the Gram-positives. A potential mechanism is that the GH45 first decrystallizes the cellulose structure. The decrystallized cellulase then undergoes endo-attack via the numerous GH5 enzymes. GH6, GH9 and other GH5 members would degrade the cleavage sites generated by the GH5 enzymes to cellobiose.
Cellulosome producers: Clostridium & Ruminococcus
▪ Clostridium
The cellulolytic members of the Clostridiaceae typically produce a mixture of soluble and cell-bound (cellulosomal) cellulases; these organisms can produce greater than 20 potential cellulolytic enzymes .
When one thinks of bacterial cellulases, the first system to come to mind is the cellulosomal system of C. thermocellum, possibly the most studied bacterial cellulase system Citation[60,61]. Cellulosomal structures are also found in several other clostridia, including Clostridium cellulovoransCitation[62]. This does not mean that all clostridia rely on cellulosomes for cellulose degradation; Clostridium phytofermentans ISDg has a set of cellulases (three GH5, one each GH8, GH9, GH12 and GH48) similar to that of the Streptomyces and Cellulomonas(Supplementary Table I)Citation[63]. This assortment of potential cellulases adds support to the hypothesis of a bacterial minimum set of a GH5, GH9 and GH48 described above. C. thermocellum has the largest set of characterized cellulases of any organism: CelA, CelC, CelD, CelE, CelG, CelH, CelI, CelK, CelN, CelO, CelR, CelS and CbhA have all been cloned, expressed and characterized from C. thermocellum(Supplementary Table J). Whole genome sequencing has revealed that this is only a fraction of the cellulolytic enzymes present in C. thermocellum; 28 potential cellulases are present in the genome. The cellulases of C. thermocellum are almost entirely cellulosomal; of the 28, 25 have dockerin domains, while only two (celI and CelY) are secreted as soluble enzymes and one (celC) is intracellular. C. thermocellum possesses a skewed distribution of GH family members, 16 GH9 and 9 GH5; C. thermocellum possesses only one GH8, two GH48 and no GH12 cellulases. CBM domains, when present, are mostly GH3 and GH4; no CBM2 domains are found in the genome, making it significantly different from the other Gram-positives. The distribution of C. cellulovorans cellulases is similar to C. thermocellum, 12 GH9 and 14 GH5; C. cellulovorans possesses only one GH8, one GH48 and no GH12 cellulases. C. cellulovorans possesses a higher number of noncellulosomal enzymes than C. thermocellum; five enzymes in C. cellulovorans are secreted, soluble enzymes, while two are LPXTG domain proteins covalently linked directly to the cell wall Citation[64]. Unlike C. thermocellum, only four GH5 enzymes from C. cellulovorans have been characterized (Supplementary Table K).
▪ Ruminococcus
Ruminococcus albus 7 is a mesophilic rumen organism that possesses a wide range of metabolic capacities Citation[65]. The genome revealed that R. albus possesses cellulosomal structures with both cohesion and dockerin modules present. R. albus has predominantly GH5 (13) and GH9 (eight) cellulases; it also possesses one GH8 and GH48 (Supplementary Table L). 15 of 23 cellulases possess attached CBM domains; CBM3, CBM4 and CBM37 predominate. In contrast to C. thermocellum and C. cellulovorans, where most of the cellulases are part of the cellulosome, only six of the 23 cellulases have dockerin domains in R. albus. Almost all of the potential cellulases in R. albus have not been characterized.
▪ Cellulose degradation mechanism
Cellulosomal systems may function analogously to the Cellvibrio system described in . The multiple CBM3 domains may act as multiple anchors to decrystallize the cellulose structure. The decrystallized cellulase would then undergo predominantly endo-attack, again, via the numerous GH5 enzymes. GH5, GH8, GH9 and GH48 family members would degrade the cleavage sites generated to cellobiose. The process would be repeated, generating the pits seen in micrographs Citation[66]. Soluble cellulases would degrade cellulose that was inaccessible to the cellulosomal enzymes to complete the hydrolysis . The function of the soluble cellulolytic enzymes remains a mystery; the presence of both a soluble GH48 and a GH9 in C. thermocellum suggests a linkage to the soluble cellulase set of the other Gram-positives.
Cellulose degradation by other Gram-negative bacteria
Three Gram-negative organisms, Cytophaga hutchinsonii(Supplementary Table P), Sorangium cellulosum(Supplementary Table O) and F. succinogenes(Supplementary Table N) share a number of common features in spite of their differences in classification, habitats and metabolisms:
▪ All three organisms possess the ability to degrade crystalline cellulose; | |||||
▪ All three organisms attach to cellulose surfaces and appear able to move along the surface of the cellulose; | |||||
▪ All three organisms possess a combination of GH5, GH8 and GH9 cellulases . In addition to these three families, S. cellulosum contains one GH12 cellulase and F. succinogenes contains four GH45 and one GH51 cellulase; | |||||
▪ None of the three possess either CBM2 or CBM3 domains, and only a rare CBM4 domain. The majority of cellulases possess no CBM domains at all; | |||||
▪ None of the three possess cohesins or dockerin domains on the cellulases. | |||||
▪ Cellulose degradation mechanism
The mechanism of cellulose hydrolysis by the ‘other’ organisms is most problematic. With no evidence of cellulose-binding CBM domains, these organisms do not degrade cellulose via any of the above-described mechanisms. A potential route to cellulose degradation may involve a non-CBM mediated binding to the cellulose surface; binding to solid carbohydrate surfaces is a common feature of many Gram-negative organisms Citation[67]. The binding would act to decrystallize one or more cellulose chains, which would then be fed through the outer membrane for degradation by cellulases secreted into the periplasm. Because the chain is no longer crystalline, no CBM modules would be required for its rapid degradation. The translocation of the cellulose chain through the membrane would also result in motion of the organism along the cellulose fibril. Once this chain is generated, it appears to be transported to the periplasm where cellulases degrade the chain into cellooligosaccharides Citation[68]. Cellobiose and other cellooligosaccharides would then be transported through the inner membrane for metabolism . Such a mechanism would be in agreement with observation of motility of all three organisms when adsorbed on cellulose surfaces, as well as requirements for attachment to the surface for cellulose degradation.
Final summary
One should consider any published reports of isolated bacterial cellulases or cellulolytic organisms with skepticism. In the author’s personal experience, C. cellulovorans EngD showed excellent activity on small substrates, but none on cellulose Citation[69]. Similarly, the appealingly named Bacillus cellulosilyticus was shown by a combination of genomic, microbiological and enzymatic techniques to be incapable of degrading cellulose Citation[70]. Claims for cellulolytic enzymes and organisms should be judged on whether or not significant hydrolysis of a crystalline cellulose substrate occurs. To be considered a true cellulase, enzymes should function at physiologically relevant (not almost stoichiometric) concentrations and in a physiologically relevant time frame. Claims for ‘improved’ bacterial cellulases should also be judged on whether the parent molecule is, in fact, cellulolytic. Caveat emptor!
Future perspective
Bioinformatics is rapidly changing the study of cellulases and their activity. In the past, individual cellulases from selected organisms were purified and characterized in isolation. Often the characterization involved unnatural large- and small-molecule substrates. Four trends will predominate over the next 5–10 years:
▪ Advanced systems such as genome-wide transcriptomic and proteomic analyses of cellulolytic organisms will be utilized to identify subsets of GH family members intimately involved in cellulose degradation Citation[71–73]; | |||||
▪ Activity studies will no longer involve single enzymes, but mixtures of enzymes from a single organism. With the reduced cost and effort needed for cloning, complete sets of cellulases from a single enzyme will be expressed, purified and recombined to test hypotheses. There may exist minimum sets of soluble Gram-positive cellulases that are capable of degrading crystalline cellulose. These minimum sets would include one member each of GH5, GH9 and GH48. Each would also possess a CBM module, most likely a CBM2. The genes coding for these three enzymes can be individually cloned via PCR from genomic DNA or synthesized chemically, and then expressed in a suitable host such as E. coli. The enzymes can then be purified, combined with a β-glucosidase and evaluated for extent of cellulose hydrolysis using a Trichoderma product as a benchmark. A minimum of 80% conversion of cellulose to glucose would mark a successful minimum bacterial set; the hypothesis of a minimum set of Cellvibrio soluble enzymes could be tested in an analogous manner. The genes coding for GH45, GH6, GH9 and GH5 cellulases can be individually cloned via PCR from genomic DNA or synthesized chemically, and then expressed in a suitable host such as E. coli. The enzymes can then be purified, combined with a β-glucosidase and evaluated for extent of cellulose hydrolysis using a Trichoderma product as a benchmark. Furthermore, they can be tested with individual components of the Gram-positive set to understand differences in mechanism between the two soluble paradigms; the hypothesis that the mechanism of action of Gram-positive cellulase minimum sets may be similar to that of the T. reesei minimum set of CBHI, CBHII and EG1 can be tested. Purified T. reesei enzymes in combination with, for example, A. cellulolyticus or C. saccharolyticus enzymes, with a similar pH and temperature range, can be evaluated for effectiveness of cellulose degradation; hypotheses concerning the mechanism of action of cellulosomes can be evaluated by comparing using soluble C. thermocellum enzymes, cellulosomes isolated from C. thermocellum, and artificially generated ‘minicellulosomes’ Citation[74–79]; | |||||
▪ Genetic systems will be developed for multiple cellulolytic organisms, and gene deletion experiments will be conducted to examine the effect of targeted gene deletions on cellulose degradation. These results will help clarify the role of individual enzymes in cellulose hydrolysis Citation[78,79]; | |||||
▪ Once minimum sets of functional, soluble cellulases are determined, construction of novel cellulases will accelerate, sparked by the confluence of bioinformatics and gene synthesis. Large-scale domain mixing experiments will be conducted to determine best combinations of CBM, linker and GH family members Citation[80–84]. These experiments will not only provide important information on the relationship between structure and activity, but also provide new, high activity cellulases to reduce the cost of producing biofuels. | |||||
Table 1. Potential cellulases of Gram-positive organisms.
Table 2. Cellulases of Clostridiaceae.
Table 3. Cellulases of Gram-negative organisms.
Organism
Streptomyces flavogriseus ATCC 33331™
Streptomyces sp. SirexAA-E
Acidothermus cellulolyticus 11B ATCC 43068™
Cellulomonas fimi ATCC 484™
Cellulomonas flavigena DSM 20109™
Cellulomonas gilvus ATCC 13127™
Caldicellulosiruptor saccharolyticus DSM 8903™
Caldicellulosiruptor kronotskyensis 2002
Clostridium phytofermentans ISDg
Clostridium thermocellum ATCC 27405
Clostridium cellulovorans 743B
Ruminococcus albus 7
Cellvibrio japonicus Ueda107
Cytophaga hutchinsonii ATCC 33406™
Fibrobacter succinogenes subspeciessuccinogenes S85
Sorangium cellulosum ‘So ce 56
Enzymes or domains within enzymes that catalyze the hydrolysis of the glycosidic linkage of glycosides, generating an increase in the number of reducing ends in the products. Also referred to as glycosidases and glycosyl hydrolases.
Contiguous protein domains that lack any catalytic activity and are able to bind to carbohydrate chains.
Genome sequences from a single organism reduced to a single, circular chromosome.
Bacteria that are stained pink or red by Gram staining. These bacteria are surrounded by an inner cell membrane, a cell wall and an outer membrane outside the cell wall.
Bacteria that are stained dark blue or violet by Gram staining. These bacteria have a single cell membrane surrounded by a thick peptidoglycan cell wall.
Approaches to cellulase discovery
▪ Sequencing of a bacterial genome yields the sequences of all potential proteins produced by the organism. With a sequenced genome, use of bioinformatics tools allows prediction of the number of potential cellulases in the genome and the sequences of the potential cellulases. | |||||
Cellulose degradation by soluble enzymes of the Gram-positive bacteria
▪ Bacterial genomes predict at least four distinct mechanisms for cellulose degradation; none map directly to the characterized fungal mechanism. Some Gram-positive bacteria secrete soluble sets of 8–12 potential cellulases from six structural families; a set of three cellulases may be the minimum requirement for effective cellulose degradation. | |||||
Cellulose degradation by soluble enzymes of Cellvibrio
▪ One Gram-negative bacterium secretes soluble cellulases; the potential set of cellulases is much larger than the Gram-positive secreted set, and contains different structural families, suggesting a different mechanism of degradation. | |||||
Cellulosome producers: Clostridium & Ruminococcus
▪ Some anaerobic Gram-positive bacteria contain cell-linked cellulases; these bacteria have >20 cellulases in their genomes. The mechanism of degradation may involve separate cellulose decrystallization and hydrolysis steps. | |||||
Cellulose degradation by other Gram-negatives
▪ A number of Gram-negative organisms appear to degrade crystalline cellulose via an unknown mechanism. This mechanism appears to involve the organism binding to cellulose surfaces and removing cellulose from the surface of the fibril. | |||||
Future perspective
▪ Bioinformatics will enable the study of the function of individual cellulases in vivo by guiding the design of gene deletion experiments. Guided by bioinformatics, novel cellulases will be generated from existing molecules by gene synthesis. | |||||
tbfu_a_10816103_sm0001.doc
Download MS Word (547.5 KB)Acknowledgements
The author would like to thank numerous researchers at the US Department of Energy Great Lakes Bioenergy Research Center (WI, USA), BioEnergy Science Center (TN, USA), and the National Renewable Energy Laboratory (CO, USA) for useful discussions over the past 5 years.
Financial & competing interests disclosure
The author is an employee and shareholder of C5-6 Technologies (WI, USA), a company that creates bio-based solutions to efficiently convert biomass into five and six carbon sugars. This work was funded by the US Department of Energy Great Lakes Bioenergy Research Center (WI, USA; US Department of Energy Biological and Environmental Research Office of Science DE-FC02–07ER64494). AMDG. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Yoshiharu N. Structure and properties of the cellulose microfibril. J. Wood Sci.55,241–249 (2009).
- O’Sullivan AC. Cellulose: the structure slowly unravels. Cellulose4,173–207 (1997).
- Zhang YH, Cui J, Lynd LR, Kuang LR. A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: evidence from enzymatic hydrolysis and supramolecular structure. Biomacromolecules7,644–648 (2006).
- Bucko T, Tunega D, Angyan JG, Hafner J. Ab initio study of structure and interconversion of native cellulose phases. J. Phys. Chem. A.115,10097–10105 (2011).
- Hall M, Bansal P, Lee JH, Realff MJ, Bommarius AS. Cellulose crystallinity – a key predictor of the enzymatic hydrolysis rate. FEBS J.277,1571–1582 (2010).
- Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK. Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels3,10 (2010).
- Himmel ME, Xu Q, Luo Y et al. Microbial enzyme systems for biomass conversion: emerging paradigms. Biofuels1,323–341 (2010).
- Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev.66,506–577 (2002).
- Weimer PJ, Odt CL. Cellulose degradation by ruminal microbes: physiological and hydrolytic diversity among ruminal cellulolytic bacteria. In: Enzymatic Degradation of Insoluble Carbohydrates. Saddler JN, Penner M (Eds). American Chemical Society, Washington, DC, USA, 291–304 (1996).
- Yamane K, Suzuki H, Nisizawa K. Purification and properties of extracellular and cell-bound cellulase components of Pseudomonas fluorescens var. cellulosa. J. Biochem.67,19–35 (1970).
- Sami AJ, Akhter MW. Purification and characterization of three extracellular carboxymethylcellulases of Cellulomonas flavigena. Biochem. Soc. Trans.18,651 (1990).
- Van Sumere CF, Van Cappellen E. Purification and properties of carboxymethyl cellulase from Aspergillus niger. Arch. Int. Physiol. Biochim.73,377–378 (1965).
- Wood TM, McCrae SI. The purification and properties of the C 1 component of Trichoderma koningii cellulase. Biochem. J.128,1183–1192 (1972).
- Shikata S, Nsizawa K. Purification and properties of an exo-cellulase component of novel type from Trichoderma miride. J. Biochem.78,499–512 (1975).
- Hurst PL, Nielsen J, Sullivan PA, Shepherd MG. Purification and properties of a cellulase from Aspergillus niger. Biochem. J.165,33–41 (1977).
- Kanda T, Nakakubo S, Wakabayashi K, Nisizawa K. Purification and properties of an exo-cellulase of Avicelase type from a wood-rotting fungus, Irpex lacteus (Polyporus tulipiferae). J. Biochem.84,1217–1226 (1978).
- Tian X, Wang X. Purification and properties of alkaline cellulase from alkalophilic Bacillus N6–27. Wei Sheng Wu Xue Bao38,310–312 (1998).
- Wood TM, McCrae SI, Macfarlane CC. The isolation, purification and properties of the cellobiohydrolase component of Penicillium funiculosum cellulase. Biochem. J.189,51–65 (1980).
- Kanda T, Wakabayashi K, Nisizawa K. Purification and properties of a lower-molecular-weight endo-cellulase from Irpex lacteus (Polyporus tulipiferae). J. Biochem.87,1625–1634 (1980).
- Bhat S, Goodenough PW, Bhat MK, Owen E. Isolation of four major subunits from Clostridium thermocellum cellulosome and their synergism in the hydrolysis of crystalline cellulose. Int. J. Biol. Macromol.16,335–342 (1994).
- Ponpium P, Ratanakhanokchai K, Kyu KL. Isolation and properties of a cellulosome-type multienzyme complex of the thermophilic Bacteroides sp. strain P-1. Enzyme Microb. Technol.26,459–465 (2000).
- Waeonukul R, Kyu KL, Sakka K, Ratanakhanokchai K. Isolation and characterization of a multienzyme complex (cellulosome) of the Paenibacillus curdlanolyticus B-6 grown on Avicel under aerobic conditions. J. Biosci. Bioeng.107,610–614 (2009).
- Seon Park J, Russell JB, Wilson DB. Characterization of a family 45 glycosyl hydrolase from Fibrobacter succinogenes S85. Anaerobe13,83–88 (2007).
- Whittle DJ, Kilburn DG, Warren RA, Miller Jr. RC. Molecular cloning of a Cellulomonas fimi cellulose gene in Escherichia coli. Gene17,139–145 (1982).
- Moser B, Gilkes NR, Kilburn DG, Warren RA, Miller Jr. RC. Purification and characterization of endoglucanase C of Cellulomonas fimi, cloning of the gene, and analysis of in vivo transcripts of the gene. Appl. Environ. Microbiol.55,2480–2487 (1989).
- Faure E, Bagnara C, Belaich A, Belaich JP. Cloning and expression of two cellulase genes of Clostridium cellulolyticum in Escherichia coli. Gene65,51–58 (1988).
- Cavicchioli R, Watson K. Molecular cloning, expression, and characterization of endoglucanase genes from Fibrobacter succinogenes AR1. Appl. Environ. Microbiol.57,359–365 (1991).
- Ozcan N, Cunningham C, Harris WJ. Cloning of a cellulase gene from the rumen anaerobe Fibrobacter succinogenes SD35 and partial characterization of the gene product. Lett. Appl. Microbiol.22,85–89 (1996).
- Aminov RI, Gribanova LK, Kataeva IA et al. Cloning and expression of Clostridium thermocellum F7 endoglucanase gene in gram-negative bacteria. Genetika26,1391–1398 (1990).
- Bumazkin BK, Velikodvorskaya GA, Tuka K, Mogutov MA, Strongin A. Cloning of Clostridium thermocellum endoglucanase genes in Escherichia coli. Biochem. Biophys. Res. Commun.167,1057–1064 (1990).
- Tsoi TV, Bukhtiiarova MG, Aminov RI et al. Cloning and expression of Clostridium thermocellum F7 cellulase genes in Escherichia coli and Bacillus subtilis cells. Genetika26,1349–1360 (1990).
- Tuka K, Zverlov VV, Bumazkin BK, Velikodvorskaya GA, Strongin A. Cloning and expression of Clostridium thermocellum genes coding for thermostable exoglucanases (cellobiohydrolases) in Escherichia coli cells. Biochem. Biophys. Res. Commun.169,1055–1060 (1990).
- Romaniec MP, Kobayashi T, Fauth U, Gerngross UT, Demain AL. Cloning and expression of a Clostridium thermocellum DNA fragment that encodes a protein related to cellulosome component SL. Appl. Biochem. Biotechnol.31,119–134 (1991).
- Li LL, Taghavi S, McCorkle SM et al. Bioprospecting metagenomics of decaying wood: mining for new glycoside hydrolases. Biotechnol. Biofuels4,23 (2011).
- Ye M, Li G, Liang WQ, Liu YH. Molecular cloning and characterization of a novel metagenome-derived multicopper oxidase with alkaline laccase activity and highly soluble expression. Appl. Microbiol. Biotechnol.87,1023–1031 (2010).
- Shedova EN, Lunina NA, Berezina OV et al. Expression of the genes CelA and XylA isolated from a fragment of metagenomic DNA in Escherichia coli. Mol. Gen. Mikrobiol. Virusol.2,28–32 (2009).
- Shedova EN, Berezina OV, Lunina NA et al. Cloning and characterization of a large metagenomic DNA fragment containing glycosyl-hydrolase genes. Mol. Gen. Mikrobiol. Virusol.1,11–15 (2009).
- Xu YQ, Duan CJ, Zhou QN, Tang JL, Feng JX. Cloning and identification of cellulase genes from uncultured microorganisms in pulp sediments from paper mill effluent. Wei Sheng Wu Xue Bao46,783–788 (2006).
- Li LL, McCorkle SR, Monchy S, Taghavi S, van der Lelie D. Bioprospecting metagenomes: glycosyl hydrolases for converting biomass. Biotechnol. Biofuels2,10 (2009).
- Markowitz VM, Chen IM, Palaniappan K et al. IMG: the Integrated Microbial Genomes database and comparative analysis system. Nucleic Acids Res.40,D115–D122 (2012).
- Park BH, Karpinets TV, Syed MH, Leuze MR, Uberbacher EC. CAZymes analysis toolkit (CAT): web service for searching and analyzing carbohydrate-active enzymes in a newly sequenced organism using CAZy database. Glycobiology20,1574–1584 (2010).
- Aziz RK, Bartels D, Best AA et al. The RAST server: rapid annotations using subsystems technology. BMC Genomics9,75 (2008).
- Altschul SF, Madden TL, Schaffer AA et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res.25,3389–3402 (1997).
- Cantarel BL, Coutinho PM, Rancurel C et al. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res.37,D233–D238 (2009).
- Finn RD, Mistry J, Tate J et al. The Pfam protein families database. Nucleic Acids Res.38,D211–D222 (2010).
- Baxevanis AD. Current Protocols in Bioinformatics. John Wiley & Sons, NJ, USA (2002).
- Mistry J, Finn R. Pfam: a domain-centric method for analyzing proteins and proteomes. Methods Mol. Biol.396,43–58 (2007).
- Yin Y, Mao X, Yang J et al. dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res.40,W445–W451 (2012).
- Gao D, Chundawat SP, Krishnan C, Balan V, Dale BE. Mixture optimization of six core glycosyl hydrolases for maximizing saccharification of ammonia fiber expansion (AFEX) pretreated corn stover. Bioresour. Technol.101,2770–2781 (2010).
- Zhong L, Matthews JF, Hansen PI et al. Computational simulations of the Trichoderma reesei cellobiohydrolase I acting on microcrystalline cellulose I-beta: the enzyme-substrate complex. Carbohydr. Res.344,1984–1992 (2009).
- Harris PV, Welner D, McFarland KC et al. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: structure and function of a large, enigmatic family. Biochemistry49,3305–3316 (2010).
- Mohagheghi A, Grohmann K, Himmel M, Leighton L, Updegraff DM. Isolation and characterization of Acidothermus cellulolyticus gen. nov., sp. nov., a new genus of thermophilic, acidophilic, cellulolytic bacteria. Int. J. Syst. Bacteriol.36,435–443 (1986).
- Thayer DW, Lowther SV, Phillips JG. Cellulolytic activities of strains of the genus Cellulomonas. Int. J. Syst. Bacteriol.34(4),432–438 (1984).
- Abt B, Foster B, Lapidus A et al. Complete genome sequence of Cellulomonas flavigena type strain (134). Stand. Genomic Sci.3,15–25 (2010).
- Christopherson MR, Suen G, Bramhacharya S et al. The genome sequences of Cellulomonas fimi and “Cellvibrio gilvus” reveal the cellulolytic strategies of two facultative anaerobes, transfer of “Cellvibrio gilvus” to the genus Cellulomonas, and proposal of Cellulomonas gilvus sp. nov. PLoS ONE8,e53954 (2013).
- Miroshnichenko ML, Kublanov IV, Kostrikina NA et al.Caldicellulosiruptor kronotskyensis sp. nov. and Caldicellulosiruptor hydrothermalis sp. nov., two extremely thermophilic, cellulolytic, anaerobic bacteria from Kamchatka thermal springs. Int. J. Syst. Evol. Microbiol.58,1492–1496 (2008).
- van de Werken HJ, Verhaart MR, VanFossen AL et al. Hydrogenomics of the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Appl. Environ. Microbiol.74,6720–6729 (2008).
- Dam P, Kataeva I, Yang S-J et al. Insights into plant biomass conversion from the genome of the anaerobic thermophilic bacterium Caldicellulosiruptor bescii DSM 6725. Nucleic Acids Res.39(8),3240–3254 (2011).
- Peterson R, Nevalainen H. Trichoderma reesei RUT-C30 – thirty years of strain improvement. Microbiology158,58–68 (2012).
- Demain AL, Newcomb M, Wu JH. Cellulase, clostridia, and ethanol. Microbiol. Mol. Biol. Rev.69,124–154 (2005).
- Fontes CMGA, Gilbert HJ. Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu. Rev. Biochem.79,655–681 (2010).
- Tamaru Y, Miyake H, Kuroda K, Ueda M, Doi RH. Comparative genomics of the mesophilic cellulosome-producing Clostridium cellulovorans and its application to biofuel production via consolidated bioprocessing. Environ. Technol.31,889–903 (2010).
- Warnick TA, Methe BA, Leschine SB. Clostridium phytofermentans sp. nov., a cellulolytic mesophile from forest soil. Int. J. Syst. Evol. Microbiol.52,1155–1160 (2002).
- Boekhorst J, de Been MW, Kleerebezem M, Siezen RJ. Genome-wide detection and analysis of cell wall-bound proteins with LPxTG-like sorting motifs. J. Bacteriol.187,4928–4934 (2005).
- Suen G, Stevenson DM, Bruce DC et al. Complete genome of the cellulolytic ruminal bacterium Ruminococcus albus 7. J. Bacteriol.193,5574–5575 (2011).
- Wang ZW, Lee SH, Elkins JG, Morrell-Falvey JL. Spatial and temporal dynamics of cellulose degradation and biofilm formation by Caldicellulosiruptor obsidiansis and Clostridium thermocellum. AMB Express1,30 (2011).
- Maruyama Y, Momma M, Mikami B, Hashimoto W, Murata K. Crystal structure of a novel bacterial cell-surface flagellin binding to a polysaccharide. Biochemistry47,1393–1402 (2008).
- Suen G, Weimer PJ, Stevenson DM et al. The complete genome sequence of Fibrobacter succinogenes S85 reveals a cellulolytic and metabolic specialist. PLoS ONE6,e18814 (2011).
- Bianchetti CM, Brumm P, Smith RW et al. Structure, dynamics, and specificity of endoglucanase D from Clostridium cellulovorans. J. Mol. Biol. (2013).
- Mead D, Drinkwater C, Brumm PJ. Genomic and enzymatic results show Bacillus cellulosilyticus uses a novel set of LPXTA carbohydrases to hydrolyze polysaccharides. PLoS ONE8,e61131 (2013).
- Takasuka TE, Book AJ, Lewin GR, Currie CR, Fox BG. Aerobic deconstruction of cellulosic biomass by an insect-associated Streptomyces. Sci. Rep.3,1030 (2013).
- VanFossen AL, Ozdemir I, Zelin SL, Kelly RM. Glycoside hydrolase inventory drives plant polysaccharide deconstruction by the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Biotechnol. Bioeng.108,1559–1569 (2011).
- Stevenson DM, Weimer PJ. Expression of 17 genes in Clostridium thermocellum ATCC 27405 during fermentation of cellulose or cellobiose in continuous culture. Appl. Environ. Microbiol.71,4672–4678 (2005).
- Hyeon JE, Jeon WJ, Whang SY, Han SO. Production of minicellulosomes for the enhanced hydrolysis of cellulosic substrates by recombinant Corynebacterium glutamicum. Enzyme Microb. Technol.48,371–377 (2011).
- Hyeon JE, Yu KO, Suh DJ et al. Production of minicellulosomes from Clostridium cellulovorans for the fermentation of cellulosic ethanol using engineered recombinant Saccharomyces cerevisiae. FEMS Microbiol. Lett.310,39–47 (2010).
- Mingardon F, Chanal A, Lopez-Contreras AM et al. Incorporation of fungal cellulases in bacterial minicellulosomes yields viable, synergistically acting cellulolytic complexes. Appl. Environ. Microbiol.73,3822–3832 (2007).
- Cho HY, Yukawa H, Inui M, Doi RH, Wong SL. Production of minicellulosomes from Clostridium cellulovorans in Bacillus subtilis WB800. Appl. Environ. Microbiol.70,5704–5707 (2004).
- Olson DG, Tripathi SA, Giannone RJ et al. Deletion of the Cel48S cellulase from Clostridium thermocellum. Proc. Natl Acad. Sci. USA107,17727–17732 (2010).
- Tolonen AC, Chilaka AC, Church GM. Targeted gene inactivation in Clostridium phytofermentans shows that cellulose degradation requires the family 9 hydrolase Cphy3367. Mol. Microbiol.74,1300–1313 (2009).
- Bras JL, Cartmell A, Carvalho AL et al. Structural insights into a unique cellulase fold and mechanism of cellulose hydrolysis. Proc. Natl Acad. Sci. USA108,5237–5242 (2011).
- Vazana Y, Morais S, Barak Y, Lamed R, Bayer EA. Interplay between Clostridium thermocellum family 48 and family 9 cellulases in cellulosomal versus noncellulosomal states. Appl. Environ. Microbiol.76,3236–3243 (2010).
- Ito Y, Ikeuchi A, Imamura C. Advanced evolutionary molecular engineering to produce thermostable cellulase by using a small but efficient library. Protein Eng. Des. Sel.26,73–79 (2013).
- Thongekkaew J, Ikeda H, Masaki K, Iefuji H. Fusion of cellulose binding domain from Trichoderma reesei CBHI to Cryptococcus sp. S-2 cellulase enhances its binding affinity and its cellulolytic activity to insoluble cellulosic substrates. Enzyme Microb. Technol.52,241–246 (2013).
- Mahadevan SA, Wi SG, Lee DS, Bae HJ. Site-directed mutagenesis and CBM engineering of Cel5A (Thermotoga maritima). FEMS Microbiol. Lett.287,205–211 (2008).
- Hashimoto W, He J, Wada Y et al. Proteomics-based identification of outer-membrane proteins responsible for import of macromolecules in Sphingomonas sp. A1: alginate-binding flagellin on the cell surface. Biochemistry44,13783–13794 (2005).
▪ Websites
- US Department of Energy Joint Genome Institue. http://img.jgi.doe.gov/cgi-bin/w/main.cgi
- Carbohydrate-Active enZYmes Database. www.cazy.org
- Wellcome Tust, Sanger Institute. Pfam database. http://pfam.sanger.ac.uk
- BioEnergy Science Center Knowledge Base. http://mothra.ornl.gov/cgi-bin/cat.cgi
- dbCAN. Webserver and database for Carbohydrate-active enzyme ANnotation. http://csbl.bmb.uga.edu/dbCAN/annotate.php
- US Department of Energy. Genomic science program. http://genomicscience.energy.gov