Abstract
Welcome to volume 9 of Bioanalysis and Happy New Year to all of our readers. We would like to take the opportunity to look back 2016, which was another great year for us. We thank all our authors, readers and reviewers, as well as our Editorial Board members for their continued support. We very much look forward to working with everyone in 2017.
Keywords::
Content Highlights
Bioanalysis publishes a variety of articles covering key advances in this ever-evolving field and this foreword looks at some of the most-read content of 2016, at the time of writing this article (November 2016).
We saw several meeting reports featuring in our top-read content, with our most-read article being the Conference Report from the 9th GCC closed forum [Citation1], the objective of which was for CRO bioanalytical representatives to meet and discuss scientific and regulatory issues specific to bioanalysis. This was followed closely by the Conference Report on AAPS and US FDA Crystal City VI workshop on bioanalytical method validation for biomarkers [Citation2]. The meeting reports from the 7th Japan Bioanalysis Forum symposium [Citation3] and the Boston Society’s 11th Annual Applied Pharmaceutical Analysis conference [Citation4] were also popular with our readers.
Recommendation papers also proved to be popular articles in 2016. This included the updated recommendation from the European Bioanalysis Forum (EBF) on ‘Best practices for metabolite quantification in drug development’ [Citation5] as well as their recommendation paper on ‘Co-medication and interference test in bioanalysis’ [Citation6]. The first part of the 2016 White Paper published following the Workshop on Recent Issues in Bioanalysis with a focus on biomarker assay validation (BAV) [Citation7], which featured in our Special Focus Issue on ‘Bioanalysis of Biomarkers – Part I’, has also been highly accessed.
Several editorial-style articles providing a snapshot of issues of topical importance to the bioanalytical community featured in our most read content. Suma Ramagiri and Ian Moore discussed one of the hottest topics of the year in their editorial on ‘Hybridizing LBA with LC–MS/MS: the new norm for biologics quantification’ [Citation8], while Sam H Haidar and Kara A Scheibner from the US FDA provided a Commentary article on an important topic in ‘Bioanalytical inspections: organizational changes and regulatory perspectives’ [Citation9]. Research articles presenting novel work and representing important advancement in understanding of techniques [Citation10,Citation11] and review articles highlighting recent significant advances in research, ongoing challenges and unmet needs in which authors provide concise and critical appraisal of the subject matter [Citation12] continue to be well received by our readers.
To coincide with the sporting events in Rio, we featured some timely content on bioanalytical approaches to anti-doping, including two editorial articles on ‘Multianalyte LC–MS-based methods in doping control: what are the implications for doping athletes? [Citation13] and ‘Running ahead of doping: analytical advances and challenges faced by modern laboratories ahead of Rio 2016’ [Citation14], both featuring in our most-read list of 2016.
As we head into 2017, we will endeavor to continue capturing in the journal, the trends and evolution of the field of bioanalysis.
Special Focus Issues
The aim of our special focus issues is to highlight such current and happening themes in the field of bioanalysis. 2016 saw a range of topics for which we wanted to initiate a greater level of interest and awareness of the topics among the broader audience, and the details of these issues can be seen in .
We also have a number of exciting themed issues planned for 2017, which will include focus on the topics of Bioanalysis of Biopharmaceuticals, Outsourcing strategies in Bioanalysis and Emerging technologies in MS. The recently published first issue of 2017 was itself a Special Focus Issue on Methods and Techniques for Metabolic Phenotyping, Guest edited by Ian Wilson [Citation20].
Bioanalysis in the community
The key aim of the Bioanalysis editorial team is to ensure that the journal remains focused on the key themes and trends in the bioanalytical community, and we strive to achieve a strong presence in this community through reaching out to our readers and contributors.
We welcome unsolicited manuscript submissions through the online manuscript processing portal ScholarOne Manuscripts™ [Citation21] or contact our editorial team with feedback, suggestions, ideas, and of proposals for articles. It is also noteworthy that Future Science partners with Enago [Citation22] to provide pre-submission editing services for our authors.
We are on social media, where we continue to post about our articles and with a Bioanalysis LinkedIn group [Citation23], providing followers with the latest journal news and updates, and a Twitter account (@fsgbio) [Citation24] that provides updates on the latest and most interesting bioanalysis news and developments from the global community.
Higher readership is consistently seen by our articles that have been shared by the authors, so we are pleased to continue our partnership with Kudos [Citation25] in the New Year, to support our authors in sharing their work and tracking the outcomes of their activities. As social media evolves to become a powerful tool in disseminating scientific publications, we now also work with Altmetrics to measure the impact of our publications [Citation26].
Demographics of readers and contributors
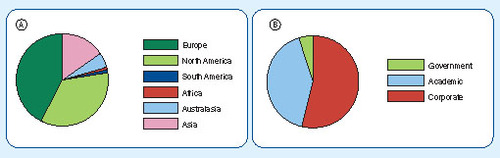
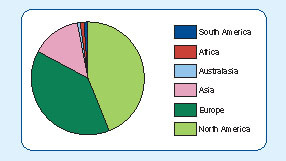
Bioanalysis has a wide reach, with both subscribers and authors based all around the globe. The majority of subscribers to the journal are from Europe and North America but we continue to see growth in the number of subscriptions from Asia and the rest of the world as shown in (A). The journal also attracts readers from a variety of working environments (B) as we strive to work with scientists from both corporate and academic surroundings. As can be seen from , Bioanalysis receives contributions from authors spread similarly across the globe.
Bioanalysis Zone
If you have not yet visited Bioanalysis Zone [Citation27], we highly recommend you take a look around the site, which is an interactive online resource for the bioanalytical community. Since launching in 2011, Bioanalysis Zone has attracted over 7,500 active members from the global bioanalytical community, representing the pharmaceutical, biotech and CRO industries, along with academia and healthcare. Each month, thousands of members visit the site to read industry news, original research exclusive interviews and commentaries. It provides a forum for the community to discuss recent developments and pose any questions related to this fast-moving field, and membership is completely free of charge.
Table 1. Special focus/themed issues published in 2016.
Financial & competing interests disclosure
S Nadarajah is an employee of Future Science Ltd. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Hayes R , LeLacheurR, DumontIet al. 9th GCC Closed Forum: CAPA in regulated bioanalysis; method robustness, biosimilars, preclinical method validation, endogenous biomarkers, whole blood stability, regulatory audit experiences and electronic laboratory notebooks. Bioanalysis8 (6), 487–495 (2016).
- Lowes S , AckermannBL. AAPS and US FDA Crystal City VI workshop on bioanalytical method validation for biomarkers. Bioanalysis8 (3), 163–167 (2016).
- Takahiro Nakamura . 7th Japan Bioanalysis Forum symposium: regulated bioanalysis, to a new stage. Bioanalysis8 (20), 2097–2102 (2016).
- Lee V , LiuA, GroeberEet al. Boston Society’s 11th Annual Applied Pharmaceutical Analysis conference. Bioanalysis8 (4), 259–264 (2016).
- Timmerman P , BlechS, WhiteSet al. Best practices for metabolite quantification in drug development: updated recommendation from the European Bioanalysis Forum. Bioanalysis8 (12), 1297–1305 (2016).
- de Zwart M , LauseckerB, GlobigSet al. Co-medication and interference testing in bioanalysis: a European Bioanalysis Forum recommendation. Bioanalysis8 (19), 2065–2070 (2016).
- Yang E , WelinkJ, CapeSet al. 2016 White Paper on recent issues in bioanalysis: focus on biomarker assay validation (BAV) (Part 1 – small molecules, peptides and small molecule biomarkers by LCMS). Bioanalysis8 (22), 2363–2378 (2016).
- Ramagiri S , MooreI. Hybridizing LBA with LC–MS/MS: the new norm for biologics quantification. Bioanalysis8 (6), 483–486 (2016).
- Haidar SH , ScheibnerKA. Bioanalytical inspections: organizational changes and regulatory perspectives. Bioanalysis8 (10), 999–1002 (2016).
- Kellie JF , KehlerJR, SzapacsME. Application of high-resolution MS for development of peptide and large-molecule drug candidates. Bioanalysis8 (3), 169–177 (2016).
- Hammond TG , MoesS, YouhannaSet al. Development and characterization of a pseudo multiple reaction monitoring method for the quantification of human uromodulin in urine. Bioanalysis8 (12), 1279–1296 (2016).
- Ayoglu B , SchwenkJM, NilssonP. Antigen arrays for profiling autoantibody repertoires. Bioanalysis8 (10), 1105–1126 (2016).
- Botrè F , de la TorreX, MazzarinoM. Multianalyte LC–MS-based methods in doping control: what are the implications for doping athletes?Bioanalysis8 (11), 1129–1132 (2016).
- de Aquino Neto FR , SarderlaVF, MirottiL, PizzattiL. Running ahead of doping: analytical advances and challenges faced by modern laboratories ahead of Rio 2016. Bioanalysis8 (17), 1753–1756 (2016).
- Microscale Bioanalysis. www.tandfonline.com/toc/bio/8/9.
- Immunoaffinity MS. www.tandfonline.com/toc/bio/8/15.
- HRMS in DMPK. www.tandfonline.com/toc/bio/8/16.
- Bioanalysis of Biomarkers – Part 1. www.tandfonline.com/toc/bio/8/22.
- Bioanalysis of Biomarkers – Part 2. www.tandfonline.com/toc/bio/8/23.
- Methods and Techniques for Metabolic Phenotyping. www.tandfonline.com/toc/bio/9/1.
- ScholarOne Mnauscripts™ – Bioanalysis. https://mc04.manuscriptcentral.com/fs-bio.
- Future Science – Enago. http://futurescience.enago.com/.
- Bioanalysis LinkedIn Group . www.linkedin.com/groups/2819540.
- Bioanalysis Twitter Page. https://twitter.com/fsgbio.
- Future Science – Kudos. www.tandfonline.com/page/kudos.
- Altmetrics. www.altmetric.com.
- Bioanalysis Zone. www.bioanalysis-zone.com.