Keywords:
All authors are members of the steering committee of the Japan Bioanalysis Forum (JBF SC) – who interviewed for the 10th anniversary issue of Bioanalysis– five members of the JBF SC to look back at the JBF's history and discuss their future expectations.
The Japan Bioanalysis Forum (JBF) was established in August 2011 to facilitate the discussion of regulated bioanalysis in Japan, interact with Japanese regulators in the field of bioanalysis, and represent Japan in the worldwide bioanalysis community. At the same time, the first JBF symposium, which was the first academic meeting focused on bioanalysis in Japan, was attended by over 200 dedicated individuals from industry, regulatory agencies and academia. The JBF has held the symposium once or twice per year and held its 10th anniversary symposium in February 2019. Throughout the symposiums, the JBF's activities such as preparation of the Bioanalytical Method Validation (BMV) guidelines were introduced and attendees discussed bioanalysis-related issues, shared information and fostered young researchers. To align with the 10th year anniversary issue of Bioanalysis, we would like to look back at JBF's history and discuss the future expectations based on interviews of five members of the JBF SC.
The interviewees are:
Noriko Katori (National Institute of Health Sciences [NIHS], Kanagawa, Japan; the founding member and has been the deputy representative of the JBF);
Masanari Mabuchi (Mitsubishi Tanabe Pharma Corp., Tokyo, Japan; the founding member and has been the deputy representative of the JBF);
Yoshiaki Ohtsu (Astellas Pharma, Inc., Ibaraki, Japan; the founding member and has been the deputy representative of the JBF);
Shinobu Kudoh (Yokogawa Electric Corp., Tokyo, Japan; member of the Global Bioanalysis Consortium [GBC] SC);
Jun Hosogi (Kyowa Hakko Kirin Co., Ltd, Shizuoka, Japan; has expertise in ligand binding assays).
Please tell us about the experience & challenges of the establishment of Japan Bioanalysis Forum & how organizations such as the European Bioanalysis Forum, Global Bioanalysis Consortium & American Association of Pharmaceutical Scientists contributed towards the set up?
Shinobu Kudoh
In 2008, I received an offer from my colleagues working overseas to participate in a global meeting with bioanalysts worldwide to reflect Japanese opinions. However, I turned down the proposal because my company had just decided to close the laboratory. Instead, I introduced several other researchers from Japanese pharmaceutical companies. However, I noticed that this approach was not effective. It was difficult to find an appropriate representative to participate in the global meeting from Japan when I received an offer to take over the roll just a few months after that Professor Kurokawa was taking a position as the 1st GBC-SC member of Japan. This is the prologue that was not recognized when the GBC was set up.
In those days, several meetings and workshops were held to discuss the necessity of the broader interpretation and revision of the US FDA guidance and to establish their own guidelines. The European Bioanalysis Forum (EBF) was setting up and they were preparing the first open symposium entitled ‘Burning Issues in Bioanalysis’. These events increased the momentum of the GBC to kick-off in the global community.
When the EBF invited me to the 4th EBF symposium in 2011, I presented the activities and future plans of the JBF. They welcomed our goals and aims but were cautious about our practical applications. My response to them was that “You can realize immediately the difference between Japanese stereotypical organizations and the JBF, which conducts its own activities under the commitment.”
Noriko Katori
In the latter half of 2010, the GBC invited the Pharmaceutical Society of Japan (PSJ) to join the GBC. Professor Haginaka (Mukogawa Woman's University) and Professor Masujima (Hiroshima University at that time) from the Department of Physical Sciences– who were responsible for analytical chemistry– addressed this. However, obtaining consent to participate in the GBC was difficult within the PSJ. At the same time, the GBC requested the regulatory authorities to give a lecture at the Asia–Pacific Bioanalytical Conference (1st APBC), Shanghai, China (sponsored by the Calibration and Validation Group [CVG]) in January 2011, and my participation was requested by Director Ono (NIHS). The conference also included over ten people from Japan, and it was strongly recognized that BMV guidelines are needed in Japan.
As a result, the JBF was launched as a place to have necessary collaborative discussions by people from industry–government–academia, centering on the participants of the first APBC. Before and after the JBF was launched, Fabio Garofolo, the organizer of the Calibration and Validation Group and founding member of the GBC, supported the JBF by giving a lecture in Japan. After the Great East Japan Earthquake (in March 2011), which was a difficult time, the first JBF symposium was finally held in August 2011. Professor Kurokawa, who was the first JBF representative and who became a GBC-SC member, was a former councillor of the Health, Labour and Welfare Ministry (MHLW). He reached out to the regulatory authorities such as the MHLW. However, discussing the GBC was difficult for him because he is not an expert in bioanalysis. After the first symposium, S Kudoh took over as the GBC-SC member.
Yoshiaki Ohtsu
When I attended the 1st APBC, the GBC requested us that we nominate a GBC SC member from Japan by February 2011 and that he/she must be a representative from Japanese bioanalytical community. Hence, we had only 1 month to make an organization and decide a GBC-SC member.
Masanari Mabuchi
No associations focused on bioanalysis in Japan at that time, and so the approach from the GBC regarding whether Japan would participate as a member of the GBC SC did not reach target companies or departments.
We first searched and contacted an association regarding the GBC proposal, but did not receive positive responses from major organizations such as the Japan Pharmaceutical Manufacturers Association (JPMA) or PSJ. A longer time was needed to take action because the organization is large, but the time available to respond to the GBC was very short. However, the foundation members were highly motivated and decided to launch the JBF by forming a new organization, as suggested by N Katori. The JBF incorporated Japanese members in all the Harmonization Team (HT) and a SC of the GBC.
Preparations for the first symposium were wonderful and the executive members, including the founding members, were remarkably adaptable. Although no members had experience managing an academic society, all preparations such as the venue settings, program settings, speaker negotiations and opening and holding of all activities were conducted as if everyone had experience in this area. Numerous people were involved in preparing for the symposium after only hearing the JBF's activity policy. This marked the departure of the JBF's voyage.
The first JBF symposium was held soon after the JBF was formed. Please tell us about new features & changes that occurred thereafter?
Shinobu Kudoh
I remember the humid and poorly lit conference venue under electric power restriction after the Great East Japan Earthquake. The JBF was established as a gathering place for Japanese stakeholders engaged in work related to pharmacokinetics and bioanalysis. One large change was that the participants in the JBF rapidly became aware of the broader global network during discussions with the GBC HT, through the participation of EBF members and the presentation by the FDA. Being able to inform researchers overseas about the Japanese manner of thinking, behavior and organization building, went on to contribute to mutual understanding in various ways and have positive effects.
Noriko Katori
Although there was a limited awareness regarding BMV guidelines from the Japanese regulatory authorities until then, the large number of participants in the JBF symposium reflected the importance of bioanalysis and the interest in it. I also think that the topics of bioanalysis were covered by related societies such as the Biomedical–Analytical Sciences (BMAS) and Chromatography Workshop after the first JBF symposium and the importance of BMV guidelines were more familiar in academic fields.
Jun Hosogi
The JBF took a large and successful step in organizing the gathering of bioanalysts in Japan. Before JBF kicked off, no academic conferences focused on regulated bioanalysis and I think that the discussion was not sufficient even in other existing meetings. As opportunities were created to gather bioanalysts, information exchange among companies and regulatory authorities was greatly promoted.
Masanari Mabuchi
Japanese researchers involved in regulated bioanalysis had a wider vision after the first JBF symposium.
At the JBF kick-off meeting, I had difficulty understanding the JBF activities around me. But I gradually received good responses from colleagues by continuing this. After the BMV guidelines were issued in Japan, my activities related to the JBF generally became understood by my company. The JBF provides greater connections among people involved in bioanalysis, which was one of its purposes and a big step forward.
Discuss some of the significant events, turning points & influences on Japanese & global bioanalysis community, as well as achievements made by the JBF?
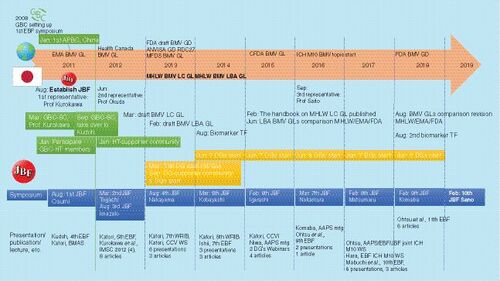
This question is in reference to , the JBF timeline.
Yoshiaki Ohtsu
The JBF encouraged Japanese scientists to participate the GBC HTs. As a result, the GBC successfully generated and spread out science-based recommendations globally and the JBF has been recognized as one of global bioanalytical communities. I would like to emphasize that efforts by Professor Kurokawa and S Kudoh – working as the GBC SCs– were also a keyfactor in its success.
At the JBF kick-off, majority of bioanalysts in Japan were LC–MS experts for small-molecule drugs, and only a small number of bioanalysts had expertise in other molecules or other techniques. Then, ligand binding assay (LBA)specialists increased gradually with growing number of biopharmaceuticals and biomarker analyses. Analyses of antidrug antibody (ADA) and antibody–drug conjugate (ADC) needed discussions not only their analytical methods but also fit-for-purpose approach; what should be analyzed and which sensitivity should be required through drug development. Analysis of large-molecule pharmaceuticals and biomarkers by LC–MS provided us with an opportunity to re-consider the analytical performance requirement. Introducing new technologies, such as dried-blood spots, flow cytometry and quantitative polymerase chain reaction (qPCR) – among others, was also a good opportunity to stimulate the JBF activity.
The JBF contributed to the preparation of Japanese BMV guidelines (BMV guideline for chromatographic assay [LC-GL] and BMV guideline for LBA [LBA-GL]) and published the handbook for LC-GL. From our viewpoint, the JBF facilitated regulatory science on bioanalysis in Japan.
Globally, we see value of human network cultured through the GBC activity. Recently, we provided input to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) M10 by collaboration with the American Association of Pharmaceutical Scientists (AAPS) and the EBF. I would like to keep the relationship with them.
Shinobu Kudoh
The major contributions of the JBF have been described in my response to Question 2 before. The Japanese BMV guidelines did not have a great impact on the global bioanalytical community except for the fellowship. This was because they did not include the original requirements in Japan and only reflected the previous guidelines by the FDA and European Medicines Agency (EMA).
Jun Hosogi
I believe that issuing the Japanese BMV guidelines and publishing its handbook has been a great achievement. Before the Japanese guidelines were issued, we had to refer to the FDA and EMA guidelines. The domestic guidelines clarified the MHLW requirements.
Looking back at JBF's history, the kick-off of the discussion group (DG) was a turning point. Since roughly 2015, the number of publications or external presentations based on the DG activities have increased. When the JBF was established, such external activities were mainly based on the personal efforts of Japanese bioanalysts. The JBF DGs organized such activities and increased the contribution of Japan to the international bioanalysis community.
Noriko Katori
Until the JBF was established, there were nearly no BMV-related announcements from Japan, but the JBF enabled us to announce Japan's situation overseas. In Japan, the JBF plays a central role in creating BMV guidelines. The regulatory party at the time stated that it was too early to complete the guideline made by Japan. Guideline preparation at that time was generally a common method of preparing a draft while coordinating the views of various companies with the help of related organizations such as the Japan Pharmaceutical Manufacturers Association, but a long time, even years, was required to aggregate opinions during reconciliation. I think that the early guidelines issues were related to the efforts of JBF members who are experts. Finally, it was decided that Japan (Dr Ishii, NIHS) would act as a rapporteur of the ICH-M10. Originally, if there were no involvement of the LBA task force and Dr Ishii, and no involvement of the biomarker task force and Dr Saito in the Japan Agency for Medical Research and Development (AMED) study group, this Japanese-led ICH topic would not have been realized.
Masanari Mabuchi
Executive members struggled with co-hosting the 3rd and 4th JBF symposia, but it allowed them to gain experience. We determined how to hold a symposium including setting the meeting venue and co-hosting the symposium spread the recognition of the JBF, developing relationships with the sponsors (the benefit of support).
The idea that LBA guidelines were distinguished from chromatography (divided by methodology) was also evaluated overseas. The JBF task force for preparing the preliminary draft LBA guideline was necessary to expand the LBA analytical knowledge, as this was very difficult for members who had only chromatographic experience.
The DG is one of the key activities of the JBF. Over 30 DGs have been created & discuss their own activities in the JBF symposium. The first trial DGs were organized in 2012 & have continued into this fiscal year. Please tell us the future directions & expectations of the DGs?
Yoshiaki Ohtsu
The outcomes of DG, which included the survey results from Japanese researchers, were extensively used in Japanese companies. It meets a Japanese feature who likes to know other's opinions. I greatly appreciate the DG promotion members about the success of DG activities in the JBF. I think the DG starting timing was good. In the past, Japanese pharmaceutical companies did not like to have open communication with other company scientists. However, their mindset changed recently owing to the DG activities.
As I mentioned above, bioanalysis is changing. I think the DG is sustainable because new topics in this field are abundant. We will also discuss some matters on which other countries have not reached conclusion yet. Although it is likely for us to have many difficulties we have not faced yet, we also can deliver our novel conclusions to a global setting. That must be pretty interesting for us.
Shinobu Kudoh
It has been 6 years since the DG kicked-off. I think that the DG style consisting of small groups based on interest in similar topics promotes detailed discussions.However, I have heard that some DGs lack direction or only engage in conversation, with one group becoming ceremonial and unable to open discussion or conduct activities. Therefore, the DG strategic approach should be amended by initiatives from the JBF SC.
Jun Hosogi
Although some DGs focusing on LBA have published their outcomes, the contribution of LBA DGs to the international bioanalysis community remains limited compared with the contributions of small molecular DGs. I hope that the LBA DGs publish their opinions more frequently and have lively discussions with related communities such as the EBF.
Noriko Katori
The advantage of DGs is that the practical issues are discussed across the company and knowledge can be shared. These groups are also helpful for networking. I hope that these activities will become more energized in the future.
Masanari Mabuchi
The JBF provided discussion opportunities in a short time, which was a great achievement. Additionally, the trial DG discussion by teleconference was based on the experience of GBC-HT participation.
The next JBF symposium is a milestone as the 10th anniversary. Please tell us the future directions & expectations of the JBF?
Noriko Katori
At a stage when harmonization of the guidelines is progressing at the ICH M10, it would be natural that the significance of the JBF will become focused on DGs, which deals with practical issues rather than involvement in the guidelines themselves. In addition to advances in analytical technology, systems such as information and communication technology (ICT) and artificial inteligence (AI) will change. We think that these situations will require progressive discussions.
Yoshiaki Ohtsu
Many analytical instruments and computers are utilized for the regulated bioanalysis; however, manual procedures still remain. We have to keep up a sufficient number of researchers with GLP adherence and adequate bioanalytical skills. Generation turnover is inevitable in the long term because current researchers cannot be at the same age in future.
Recently, we consider the development of next generation by the JBF. We provided the educational program and poster session for young generation at the 10th symposium. The number of births is declining in Japan and government policy and public opinion does not appear to accept significant increase of new immigrants from overseas. This means decrease of young workers. Bioanalysis industry must compete with the other industries for acquiring the young workers. Therefore, regulated bioanalysis field should be more attractive.
Innovation outside our research field, such as AI, may affect regulated bioanalysis. Although Japan had lagged western countries in popularization of LC–MS, we would like to do our best for the future innovation.
The other one is working together beyond the countries. At present, we have frequent communication with western countries. However, collaboration with other Asian countries may also become considerable in future although the JBF has communication with China and India. In other industries, collaboration with Asian counterparts is already important for Japanese organizations.
Shinobu Kudoh
The JBF should not transition into a traditional Japanese academic style society, where eminent scholars are presenters and the audience listens to them attentively and are quiet. I hope the JBF becomes a precious forum that provides a place for open discussion on daily issues associated with technical and specific practices for all participants.
Jun Hosogi
I think it is important to maintain the benefits of corporate researchers, as most JBF participants are from the industry.
Thus, the topics in the symposium should be close to the daily practices of pharmaceutical companies rather than cutting-edge analytical technologies.
Although some bioanalysis methods using LC–MS and LBA are well-developed technologies, many points remain to be discussed. I hope that the JBF will address these points in subsequent symposiums after the release of the ICH M10 guidance.
Masanari Mabuchi
I have worked to provide discussion opportunities, but presentation to public was conducted by limited members. I also feel that there are few opportunities to comment on the consensus opinion as the JBF and so I would like to reach to conclusions through the DGs. The JBF is not only related to the authorities, but also may propose its own consolidated opinions to the authorities.
We are currently searching for connections with other areas through bioanalysis. This action may result in forward and backward progress, but will hopefully reach the desired direction if the JBF-SC continues its discussions with enthusiasm.
Do you have any further comments?
Noriko Katori
In 2008, discussions of the incurred samples reanalysis (ISR) in the BMV guidelines were organized and held by Hisanori Hara (Novartis Pharma AG) at the 56th Annual Conference on Mass Spectrometry. At that time, Dr Kawanishi (Chief of Pharmaceutical Department, NIHS) and I attended this meeting, and Dr Viswanathan from the FDA delivered a lecture. I think that the participation to this symposium largely contributed to the fact that NIHS was involved in dispatching the speaker to the 1st APBC, which resulted in the establishment of the JBF. I would like to thank you again for the related persons.
Disclaimer
The opinions expressed in this interview are those of the interviewees and do not necessarily reflect the views of the Japan Bioanalysis Forum (JBF) or Newlands Press, Ltd.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.