Welcome to Volume 14 of Bioanalysis. We would like to take this opportunity to reflect on the editorial highlights seen over the past year and look ahead to 2022. We want to thank all our authors, peer reviewers and readers, as well as our dedicated Editorial Board members for their continued support.
Bioanalysis publishes a variety of articles covering key advances in this ever-evolving field and this section looks at some of the most-read content of the year, at the time of writing this article (November 2021).
Content highlights
COVID-19
If in 2020 the COVID-19 pandemic challenged the scientific community, putting research and education on hold and placing lives at risk, 2021 offered some hope despite the world still very much in the grips of the pandemic. As we head into 2022, many nations across the globe are in the process of rolling out their COVID-19 vaccination programs while simultaneously tackling new emerging variants of the virus through regular updated diagnostics (there have of course been disparities in the global response and although it is a topic beyond this discussion, one we must keep in mind).
These are landmark events that go on to highlight the importance of the scientific and bioanalytical community, with timely work and discussions shifting towards safe and patient-centric sampling procedures, development of diagnostic assays, and supporting various stages of the vaccine-development process.
The journal continued to publish articles concerning COVID-19 over the course of 2021, which have all been made available free to read. Published in an early 2021 issue and one of our most accessed articles of the year was the Methodology paper ‘Validation of dried blood spot sample modifications to two commercially available COVID-19 IgG antibody immunoassays.’ The purpose of this article was to determine if two commercially available anti- SARS-CoV-2 enzyme-linked immunosorbent assays for IgG antibodies against spike S1 subunit and nucleocapsid proteins could be validated for use with dried blood spots [Citation1]. A proof-of-concept Research article on adaptation of Elecsys® anti- SARS-CoV-2 immunoassay to dried blood spots was also among out most-viewed content [Citation2].
We also published a two-part Special Focus Issue exploring the key applications, developments and challenges in the industry as a result of the COVID -19 pandemic, guest edited by Chad J Briscoe (BioAgilytix, Hamburg, Germany) and Matthew Barfield (F Hoffmann–La Roche Ltd, Basel, Switzerland) [Citation3,Citation4]. With perspectives from bioanalysts, regulators as well as focused reviews and further research papers on SARS-CoV-2 serology assays and immunoassay methods for drug quantification.
General content
One of our top-accessed paper was the Review article on ‘Flow cytometry: principles, applications and recent advances’ by Sonal M Manohar and colleagues [Citation5], which explained the general principles, main applications and recent advances in the field of flow cytometry. Another was the paper by Sivan Cohen and Shan Chung on ‘In vitro immunogenicity prediction: bridging between innate and adaptive immunity’, which highlighted the role of innate and adaptive immune cells as the drivers of immunogenicity, summarizing the use of these cells in assays to predict clinical antidrug antibodies [Citation6].
Among our research-style papers, the Research paper by Chang Liu and colleagues on a novel cell-based FcRn-dependent recycling assay for predictive pharmacokinetic (PK) assessment of therapeutic antibodies [Citation7] and the Methodology by Noritaka Hashii and colleagues on their easy, low-cost and versatile mass spectrometric method for the bioanalysis of a therapeutic monoclonal antibody in human serum (which employed peptide adsorption-controlled LC/MS using selected reaction monitoring mode) [Citation8].
Editorials are short articles that provide an insight into, or snapshot of issues of topical importance to the journal's target audience and the intention is that they offer an expert perspective on a topic of interest. These often prove quite popular reads and this year, we had a few interesting pieces which made our most read list. As such Boris Gorovits explored the ‘Complexity and diversity of bioanalytical support for gene therapy modalities’ in his well-received Editorial piece [Citation9], while Enaksha R Wickremsinhe and Christopher A James explored how the revolution in oncology has also brought about new bioanalytical challenges including the development of methods for novel modalities, the need to analyze competitors' molecules in combination studies and designing specificity and combination stability experiments in a complex polypharmacy environment [Citation10].
Industry-led recommendation papers and perspectives continue to be popular among the readers in 2021. This included all three parts of our 2020 White Paper on Recent Issues in Bioanalysis (which were published in early 2021) [Citation11–13] as well as the recommendation from the European Bioanalysis Forum on a strategic approach to nonclinical immunogenicity assessment [Citation14]. The recommendations from the 13th Global CRO Council (GCC) closed forum for bioanalysis, for the content and management of Certificates of Analysis for reference standards was another of highly accessed White Papers [Citation15].
Special focus issues
The aim of our special focus issues is to highlight happening themes in the field of bioanalysis. 2021 saw a handful of topics where we wanted to initiate a greater level of interest and awareness of among the broader audience, the details of which these issues can be seen in .
In addition to our two-part Special Focus Issue on COVID-19: bioanalytical considerations, contributions and lessons [Citation3,Citation4] discussed above, we focused on ‘Critical Reagents: Advanced Applications’ in a themed issue, which explored current and effective strategies for critical reagent characterization, storage, stability, retesting and life cycle management for ligand-binding assays and flow cytometry. Articles in this issue were inspired by the interactive discussion during the 13th WRIB (2019) and discussed specific case studies and industry standards in critical reagents characterization and management for PK, biomarker and ADA assays by ligand-binding and flow cytometry [Citation16].
We had another issue dedicated to ‘Flow cytometry’, which has become a widely applied bioanalytical technology in clinical trials [Citation17]. The multiplex capabilities of flow cytometry support a broad spectrum of biomarker analyses, focused on (not limited to) cellular analyses. There has been increasing demand in flow cytometry assays to support assays that provide PK-type data, typically in cell therapy studies. The issue covered the possibilities, new developments and challenges of performing flow cytometry.
Bioanalysis in the community
The key aim of the Bioanalysis editorial team is to ensure that the journal remains focused on the key themes and trends in the bioanalytical community, and we strive to achieve a strong presence in this community through reaching out to our readers and contributors.
We welcome unsolicited manuscript submissions through the online manuscript processing portal ScholarOne Manuscripts™ [Citation18], and are happy for authors to contact our editorial team with feedback, suggestions, ideas and proposals for articles. We are also on social media, where we continue to post about our articles through a Bioanalysis LinkedIn group [Citation19], providing followers with the latest journal news and updates.
Demographics
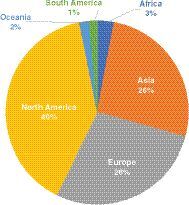
Bioanalysis has a wide reach around the globe and has seen continued increase in readership over the past year with the continental ratio similar to last year – with the majority of readers based in North America, Europe and Asia ().
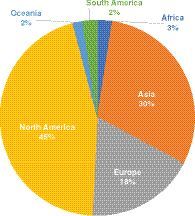
The authorship demographics largely reflect that of our readership – with the majority of authors submitting from North America and Europe, with a noticeable increase in contributors from Asia over the last 12 months ().
Bioanalysis Zone
We highly recommend you visit our sister digital hub – Bioanalysis Zone [Citation20]. Launched in 2011, it has attracted over 17,000 active members from the global bioanalytical community. Each month, thousands of members visit the site to read industry news, original research exclusive interviews and commentaries. It provides a forum for the community to discuss recent developments and pose any questions related to this fast-moving field, and membership is completely free of charge.
The Journal and the Zone work in collaboration on several projects, with the vision of ensuring research and advancements in the field are presented in the most appropriate and accessible manner to the bioanalytical community.
Concluding remarks
We appreciate the support and engagement that the journal has received over the past year especially considering the circumstances and look forward to what is to come in 2022. We would like to thank everyone for their continued feedback and support – we look forward to working with you all in 2022.
Table 1. Special focus issues published in 2021.
Financial & competing interests disclosure
S Nadarajah is the Managing Editor of Bioanalysis, and an employee of Newlands Press Ltd. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Zava TT , ZavaDT. Validation of dried blood spot sample modifications to two commercially available COVID-19 IgG antibody immunoassays. Bioanalysis13(1), 13–28 (2020).
- Marchand A , RoullandI, SemenceF, EricssonM. Adaptation of Elecsys® anti-severe acute respiratory syndrome coronavirus-2 immunoassay to dried blood spots: proof of concept. Bioanalysis13(3), 161–167 (2021).
- Bioanalysis Special Focus Issue. COVID-19: bioanalytical considerations, contributions and lessons – part 1. https://www.tandfonline.com/toc/bio/13/15
- Bioanalysis Special Focus Issue. COVID-19: bioanalytical considerations, contributions and lessons – part 2. https://www.tandfonline.com/toc/bio/13/22
- Manohar SM , ShahP, NairA. Flow cytometry: principles, applications and recent advances. Bioanalysis13(3), 181–198 (2021).
- Cohen S , ChungS. In vitro immunogenicity prediction: bridging between innate and adaptive immunity. Bioanalysis13(13), 1071–1081 (2021).
- A cell-based FcRn-dependent recycling assay for predictive pharmacokinetic assessment of therapeutic antibodies. Bioanalysis13(14), 1135–1144 (2021).
- Hashii N , TousakaY, AraiKet al. Bioanalysis of therapeutic monoclonal antibody by peptide adsorption-controlled LC–MS. Bioanalysis13(4), 265–276 (2021).
- Gorovits B . Complexity and diversity of bioanalytical support for gene therapy modalities. Bioanalysis13(2), 65–68 (2021).
- Wickremsinhe ER , JamesCA. Bioanalysis and the oncology revolution. Bioanalysis13(5), 291–294 (2021).
- Neubert H , AlleySC, LeeAet al. 2020 White Paper on Recent Issues in Bioanalysis: BMV of Hybrid Assays, Acoustic MS, HRMS, Data Integrity, Endogenous Compounds, Microsampling and Microbiome (Part 1 – Recommendations on Industry/Regulators Consensus on BMV of Biotherapeutics by LCMS, Advanced Application in Hybrid Assays, Regulatory Challenges in Mass Spec, Innovation in Small Molecules, Peptides and Oligos). Bioanalysis13(4), 203–238 (2021).
- Spitz S , ZhangY, FischerSet al. 2020 White Paper on Recent Issues in Bioanalysis: BAV Guidance, CLSI H62, Biotherapeutics Stability, Parallelism Testing, CyTOF and Regulatory Feedback (Part 2A – Recommendations on Biotherapeutics Stability, PK LBA Regulated Bioanalysis, Biomarkers Assays, Cytometry Validation & Innovation Part 2B – Regulatory Agencies' Inputs on Bioanalysis, Biomarkers, Immunogenicity, Gene & Cell Therapy and Vaccine). Bioanalysis13(5), 295–361 (2021).
- Corsaro B , YangT-Y, MurphyRet al. 2020 White Paper on Recent Issues in Bioanalysis: Vaccine Assay Validation, qPCR Assay Validation, QC for CAR-T Flow Cytometry, NAb Assay Harmonization and ELISpot Validation (Part 3 – Recommendations on Immunogenicity Assay Strategies, NAb Assays, Biosimilars and FDA/EMA Immunogenicity Guidance/Guideline, Gene & Cell Therapy and Vaccine Assays). Bioanalysis13(6), 415–463 (2021).
- Laurén A , GoodmanJ, BlaesJet al. A strategic approach to nonclinical immunogenicity assessment: a recommendation from the European Bioanalysis Forum. Bioanalysis13(7), 537–549 (2021).
- Bower J , ZimmerJ, McCownSet al. Recommendations for the content and management of Certificates of Analysis for reference standards from the GCC for bioanalysis. Bioanalysis13(8), 609–619 (2021).
- Bioanalysis Special Focus Issue. Critical Reagents: Advanced Applications. https://www.tandfonline.com/toc/bio/13/10
- Bioanalysis Special Focus Issue. Flow Cytometry. https://www.tandfonline.com/toc/bio/13/21
- ScholarOne Manuscripts™. Bioanalysis. https://mc04.manuscriptcentral.com/fs-bio
- Bioanalysis LinkedIn Group . http://www.linkedin.com/groups/2819540
- Bioanalysis Zone . http://www.bioanalysis-zone.com