Abstract
The simulation and modeling of post-combustion CO2 capture systems are considered to be an important strategy to obtain process integrity and gain design confidence for the construction and commissioning of commercial post-combustion CO2 capture plants. It is therefore essential to obtain an understanding of the fundamental concepts of designing and modeling. This article reviews the concepts of designing a CO2 capture system with specific emphasis on the absorber for diameter and height. It covers several steps (i.e., empirical design method, theoretical design method, laboratory method and pilot plant techniques) used to design the absorber. A conceptual design of an overall CO2 capture process is also given in the article. Process validations of the modeling using ProMax with four existing pilot plants. (the International Test Centre of CO2 Capture pilot plant, the Esbjerg CASTOR pilot plant, the Institute of Thermodynamics and Thermal Process Engineering, Stuttgart pilot plant and the SINTEF/NTNU pilot plant) are presented. Moreover, a discussion of process integration of the CO2 capture plant into a fossil fuel-fired power plant is included in this paper.
(A) Emphirical design method, (B) Theoretical design method, (C) Laboratory method and (D) Pilot plant technique method.
Modified with permission from Citation[20].
![Figure 2. Design procedure of CO2 absorber. (A) Emphirical design method, (B) Theoretical design method, (C) Laboratory method and (D) Pilot plant technique method.Modified with permission from Citation[20].](/cms/asset/f5b6d836-6db6-426a-9b02-9b012432bd13/tcmt_a_10816195_f0002.gif)
CB: Concentration; G: Gas; L: Liquid; PA: Partial pressure.
Modified with permission from Citation[20].
![Figure 4. Small cell used for laboratory method.CB: Concentration; G: Gas; L: Liquid; PA: Partial pressure.Modified with permission from Citation[20].](/cms/asset/730fbbe9-a76f-4f23-928c-65e954cd2112/tcmt_a_10816195_f0004.gif)
γ: Unit volume of liquid; av: Interfacial area; CB: Concentration; G: Gas; KG; Overall gas mass transfer coeffiecient; KL; Overall liquid mass transfer coeffiecient; L: Liquid; PA: Partial pressure; Z: Height.
![Figure 5. String-of-sphere column Citation[45].γ: Unit volume of liquid; av: Interfacial area; CB: Concentration; G: Gas; KG; Overall gas mass transfer coeffiecient; KL; Overall liquid mass transfer coeffiecient; L: Liquid; PA: Partial pressure; Z: Height.](/cms/asset/3881253e-4dc1-4f20-891c-98826cced528/tcmt_a_10816195_f0005.gif)
Modified from with permission from Citation[20].
CB: Concentration; G: Gas; L: Liquid; PA: Partial pressure; Zm: Height.
![Figure 6. Pilot plant technique.Modified from with permission from Citation[20].CB: Concentration; G: Gas; L: Liquid; PA: Partial pressure; Zm: Height.](/cms/asset/bad5aa69-f32b-4d22-b996-9a156f8677eb/tcmt_a_10816195_f0006.gif)
HEX: Heat exchanger; L: Liquid; P: Pump; V: Vapour.
Reproduced with permission from Citation[15].
![Figure 7. Pilot plant schematic process flow diagram for (A) ITC, Esbjerg CASTOR and ITT (B) SINTEF/NTNU.HEX: Heat exchanger; L: Liquid; P: Pump; V: Vapour.Reproduced with permission from Citation[15].](/cms/asset/8dfd4f88-0384-4272-beb4-9567610ed241/tcmt_a_10816195_f0007.gif)
G: Generator; HX: Heat exchanger; PM: Particulate matter; RH: Reheater; SH: Superheater.
Modified with permission from Citation[16].
![Figure 20a. (A) An integration of CO2 capture into pulverized coal-fired power plant.G: Generator; HX: Heat exchanger; PM: Particulate matter; RH: Reheater; SH: Superheater.Modified with permission from Citation[16].](/cms/asset/7b290f64-23e7-4a7d-889e-b5845e259f69/tcmt_a_10816195_f0020a.gif)
Comp: Compressor; G: Generator; GT: Gar turbine; HP: High-pressure turbine; HX: Heat exchanger; IP: Intermediate-pressure turbine; LP: Low-pressure turbine.
Bolded numbers 1–4 indicate a connection of each stream line.
Modified with permission from Citation[50].
![Figure 20b. (B) An integration of CO2 capture into combined cycle gas turbine power plant.Comp: Compressor; G: Generator; GT: Gar turbine; HP: High-pressure turbine; HX: Heat exchanger; IP: Intermediate-pressure turbine; LP: Low-pressure turbine.Bolded numbers 1–4 indicate a connection of each stream line.Modified with permission from Citation[50].](/cms/asset/83164e02-1d98-471a-8c33-47eba105ca0b/tcmt_a_10816195_f0020b.gif)
G: Generator; IP: Intermediate-pressure turbine; LP: Low-pressure turbine.
G: Generator; IP: Intermediate-pressure turbine; LP: Low-pressure turbine.
Background
The capture of CO2 from industrial flue gases has become an important issue in recent years. The threat of climate change from increased levels of GHGs in the atmosphere is significant. Mean global temperatures are on the rise and various regions of the world have been experiencing adverse climate issues, for example, polar ice caps are melting at an alarming rate, heat waves and droughts are becoming commonplace, and the occurrence of violent weather has increased around the globe. Therefore, the need for research and development of cost-effective CO2 capture technologies is urgent.
The majority of industrial CO2 emissions come from fossil fuel- (i.e., coal, natural gas and oil) fired power stations, for example, electric utilities account for 29% of all CO2 emissions in Canada. In addition, according to the International Energy Agency (IEA) World Energy Outlook (2002) predictions, the world energy-related CO2 emissions will reach 38 billion tons by 2030, representing an increase of approximately 1.8% per year and 70% above 2000 levels Citation[1]. Since coal is relatively cheap, and exists in abundant quantities around the world, it is likely that it will remain the fuel of choice at thermal power stations for decades to come. If the CO2 emission rates at these facilities can be reduced in a cost-effective manner, the anthropogenic contribution to global warming will be minimized.
There are three process options for CO2 capture: pre-combustion, post-combustion and oxyfuel combustion. Depending on the process, various technologies for CO2 capture can be used including absorption, adsorption, membranes and hybrid applications of these three. In the immediate future, post-combustion capture via chemical absorption with amines appears to be the leading candidate for CO2 removal at fossil fuel fired power stations; however, several advances are being made with the other technology options. Two recent events, the IEA-sponsored 10th International Conference on Greenhouse Gas Control Technologies (GHGT-10, September 18–22, 2010, Amsterdam, The Netherlands) and the 7th IEA CO2 Capture Network (September 29–30, 2009, Regina, Canada) illustrate the demand for knowledge in this area. This underscores the need for technological breakthroughs to support as well as facilitate the CO2 emissions mitigation efforts.
The purpose of post-combustion capture is to capture CO2 from an industrial flue gas stream using a regenerable absorbent. Conversely, the specific objective of emerging technologies for the post-combustion CO2 capture process is to optimize the technology to capture CO2 with high efficiency, but with low capital and operating expenses (CAPEX and OPEX). These technologies include the use of CO2-capture absorbents, metal organic frameworks Citation[2], enzyme-based systems Citation[3] and ionic liquids Citation[4]. However, it has been reported that CO2 capture based on regenerable absorbents is the most mature and probably the most promising technology amongst all the aforementioned technologies Citation[5].
For absorbents-based CO2 capture, there are many types of absorbents that can be used, generally including physical, chemical or combined physical and chemical absorbents (or solvents). Chemical solvents using amines are the most likely to be used for post-combustion capture, as they have better effectiveness and efficiency to absorb CO2 at low partial pressures and total pressures than physical solvents. It is known that the partial pressure of CO2 in the flue gas is relatively low, typically in the range of 4–15% by volume, depending on the type of fossil fuel used. Thus, it is anticipated that amine-based absorbents (i.e., chemical solvents) will be used for the first-stage of post-combustion capture of CO2. This can be seen from existing facilities that do partial scrubbing of CO2 from the flue gases of commercial power plants (e.g., IMC Global Soda Ash Plant, Warrior Run Power Plant and Sumitomo Chemicals Plant Citation[6]) using amines. Based on these demonstration plants, it appears that up-scaling the technology to integrate into the full-scale power plant operation is promisingly feasible Citation[101,7].
The chemical absorbent (otherwise called chemical solvent) needs to be regenerated or stripped of the CO2 it absorbed before it is recycled back to the absorber. It is found that chemical solvents require more heat in order to break the strong chemical interaction between the CO2 and the solvent. Furthermore, there may be some operational issues, which can impede the process of CO2 capture from flue gas using amines. These include degradation of solvents and corrosion of equipment, both of which are caused by the presence of CO2, oxygen and some impurities in the gas stream such as oxides of sulfur, oxides of nitrogen and fly ash. Nevertheless, chemical solvent can efficiently remove CO2 from the flue gas in the presence of a large amount of non-condensable gas, and remedies, such as the use of inhibitors, can be provided to counteract some of the disadvantages, specifically solvent degradation and equipment corrosion Citation[8]
▪ Process simulators to model post-combustion CO2 capture using regenerable absorbent
Regardless of chemical reactions, the design of a conventional absorber is generally based on standard chemical engineering principles or techniques. However, these principles and techniques have not been previously applied in the design of a plant for the capture of CO2 from power plants. In addition, the necessary design parameters that are unique to this process are not available in commercial design packages and neither have they been validated in the literature. Therefore, it is worthwhile that an important step to confirming the feasibility of building a large CO2 capture plant is to set up a pilot plant or a demonstration plant. Another step requires performing process design and simulation. A front end engineering design (FEED) study that uses simulation techniques is also necessary to improve plant reliability and increase productivity before actually constructing the plant, especially for designs developed for new technologies. Process simulation can provide some of the results that would otherwise be gained through laboratory and/or pilot plant tests, and such simulation should be used as it would reduce the need for process revamping and improve start-up times. Although laboratory and especially pilot plant tests are important to get a good feel for the design, these tests could be expensive in regards to material and energy consumption and costs. Process simulation then becomes a tool that can be used successfully to compliment laboratory and pilot plant tests.
The development of process-design calculations from open literatures and also chemical process simulators from engineering developers has helped in the design of equipment and process. The early computer simulation tools could only be used for single operating unit, and it was difficult to get convergence for a complicated process integrated with many units. Up-to-date simulation software tools are reliable for completing a simulation of a power plant integrated with a CO2 capture plant. The heat efficiency of power generation is a critical factor. A power plant with a post-combustion, amine-based CO2 capture system will have reduced efficiency compared with one without CO2 capture because of the steam requirement for stripping CO2 from the amine solution (i.e., absorber regeneration). Reboiler heat duty for CO2 stripping clearly impacts the steam cost, hence process-configuration optimization becomes an efficient approach for system energy integration to minimize the heat duty. A number of process simulation tools, such as EBSILON®, GateCycle®, GT Pro®, PROATES®, Steam Pro® (for power plant simulation), Aspen Plus®, Aspen HYSYS®, CHEMASIM®, ProMax®, ProTreat® (for CO2 capture simulation), GT Pro/Master®, IECM®, Steam Pro/Master® and ThermoFlex® (for process integration) and other in-house software, have been developed for power plant and CO2 capture simulation Citation[102,9–17]. Even though such chemical simulators would help engineers to design and simulate the post-combustion capture system, the comprehension of the fundamental concepts and process validation is a must in order to verify whether the design is acceptable. Therfore, this article discusses these two issues. In addition, the concept to integrate the CO2 capture into fossil fuel fired power plant is discussed in the article.
CO2 capture plant design
▪ Description of process flow diagram of Co2 capture unit
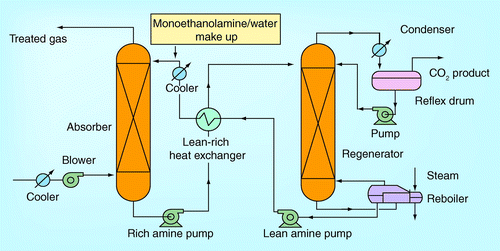
demonstrates the typical schematic diagram of the CO2 capture process using regenerable absorbent. For a general description of the process, the liquid solution containing the chemical absorbent is supplied to the top of the absorber while the flue gas stream from the industrial process flows counter-currently from the bottom to the top of the absorber by passing through the absorber column. The absorber column contains internals such as tray or random packing or structured packing, each of which provides the surface area of contact between the liquid and the gas. This contact allows the desired component to be transferred from the gas phase to the liquid phase. In the CO2 capture process, the liquid chemical absorbent preferentially removes CO2 from the gas stream while the other components of the gas stream that do not react with the chemical absorbent are vented to the atmosphere from the absorber top. The CO2-rich liquid solution exits at the bottom of the absorber. Then, the CO2-rich solution is pumped through the lean/rich heat exchanger before it enters the regenerator at the top for solvent regeneration and CO2 stripping. The carryover vapor stream of the solution is condensed back to the top of the regenerator, leaving mostly wet CO2 out of the stripping process as the CO2 product. The reboiler receiving the thermal heat primarily extracted from the steam-power cycle or other sources supplies the energy to the solution in the stripper. After regeneration, the lean solution carrying a lean amount of CO2 from the reboiler is passed through the rich-lean heat exchanger to transfer heat to the CO2-rich heat solution before the former is pumped through a cooler to cool the temperature down to 35–45°C before reintroducing into the absorber at the top. Provision is made for chemical absorbent makeup next to the CO2-lean liquid solution pump if necessary to maintain the solvent concentration at the specified operating level.
▪ Design methods for CO2 absorber
One of the important units in the absorbent-based post combustion CO2 capture unit is the absorber. The design of the absorber involves determining or specifying the proper absorber size required for a given CO2 capture operation. There are several steps to design an absorber size. These are given in Citation[18–20]. The specific operating designs are defined in terms of the independent parameters (e.g., flow rate of gas and liquid-phases and flue-gas compositions and gas–liquid contacting system). Physical properties data on both phases must then be obtained to determine the superficial velocities and absorber diameter based on flooding conditions.
▪ Absorber diameter
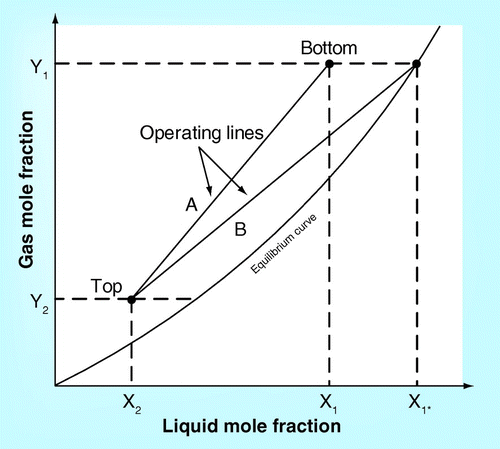
In order to calculate the diameter of CO2 absorber, the solubility of CO2 in chemical absorbent under a specified temperature and equilibrium should be conducted Citation[21]. A solubility plot from experiment or open literature between mole fraction of CO2 in gas and liquid phases provides the operating line of (L/G)operating and minimum operating line of (L/G)min. An example of the solubility plot and operating line is demonstrated in .
With the specified gas flow rate inlet, the liquid flow rate would be obtained from (L/G)operating. The calculation of the flow parameter (FLG) should later be performed by using the following equation.
Equation 1
Where L, G, rG and ri denote liquid flow rate (kg/min), gas flow rate (kg/min), gas density (kg/m3) and liquid density (kg/m3), respectively. The physical properties such as density can be obtained from experiment, open literature or chemical process simulation software (e.g., Aspen and ProMax) Citation[22–25]. Following this step is to determine ordinate (e) from the flooding curve Citation[26]. The mass flow rate of gas per unit cross sectional area of column is calculated from .
Equation 2
Where G´, F, φ, gc and µ are mass flow rate of gas per unit cross sectional area of column (kg/ m2 s), packing factor, ratio of specific gravity of the liquid and water, gravitational constant (m2/s) and viscosity of liquid (cP), respectively. Diameter of the absorber can be computed by:
Equation 3
Equation 4
Where A and f represent cross sectional area (m2) and flooding factor, respectively.
▪ Absorber height
Empirical design method
There are several studies that use the empirical design approach to design the column height Citation[21,27]. The calculation step of this method is given in at Route #1. To calculate the packing height of the tower, a material balance of the transferred component A should be performed as:
Equation 5
by:
Equation 6a
Equation 6b
Where av denotes interfacial area per unit volume of packing (m2/m3); CA,L is concentration of A on the liquid bulk (kmol/m3); C*A is concentration of component A in equilibrium with gas phase (kmol/m3); GI; is is inert gas molar flow rate (kmol/m2 s); KG is overall gas mass transfer coefficient (kmol/m2 s kPa); KL is overall liquid mass transfer coefficient (m/s); NA is mass transfer flux of the absorbed component A (kmol/m2 s); P is total pressure (kPa); yA,G is mole fraction of component A in gas bulk; and y*A is mole fraction of component A which is in equilibrium with liquid bulk.
By simplifying , the column height can be expressed as:
Equation 7
The mass-transfer coefficient can be assumed as an independent parameter of the absorbed component concentration. Therefore, the column height is presumably simplified to:
Equation 8
Where, GI/KGaVP represents the height of a transfer unit (HTU), while the following equation represents the number of transfer units (NTU) Citation[28].
The mass transfer coefficient can be obtained from experimental measurement at the bottom and top of the absorber. This is called the average overall volumetric mass transfer coefficient, (KGav)ave, which can be given as:
Equation 9
Where, the log-mean driving force, (YA.G – Y*A)lm, can be identified by:
Equation 10
The volumetric mass transfer coefficient is assumed to be constant along the column height and it is basically correlated as a function of the operating parameters such as flow rates, pressure and temperature. Although the empirical design method requires experimental data, it is unreliable when chemical reactions get involved and where the enhancement factor significantly changes, unless the data are obtained under conditions equivalent to a full-scale absorber.
Theoretical design method
The theoretical design method procedure is given in Route #2 in . The theoretical design for absorption with chemical reaction was first proposed by Astarita (1967) and Danckwerts (1970) Citation[29,30]. Their efforts were primarily directed towards developing expressions for the local mass transfer coefficients. A set of design equations for isothermal gas absorbers with chemical reaction was later developed by Joshi et al. (1981) Citation[31]. However, assuming adiabatic conditions would have been more realistic because heat losses are generally small in industrial absorbers Citation[32,33]. A rigorous design procedure for adiabatic gas absorption with chemical reaction was subsequently described by Pandya (1983) Citation[33]. The procedure was based on Treybal’s concepts (1969) Citation[32] for adiabatic, physical gas absorption and Danckwert’s work (1970) Citation[30] on isothermal gas absorption with chemical reaction. Pandya’s procedure accounted for the heats of absorption and reaction, solvent evaporation and condensation, chemical reactions in the liquid phase and heat and mass transfer resistances in both phases Citation[33].
A mathematical model based on Pandya’s algorithm (1983) Citation[33] is used to illustrate the theoretical design approach. The assumptions that are typically invoked are: the reaction is fast and takes place only in the liquid film; heat and mass transfer resistances for the liquid phase are negligible; the interfacial area is the same for heat and mass transfer; axial dispersion is negligible. The principal model equations are presented as follows.
Overall differential material balance:
Equation 11
Liquid-side mass transfer:
Equation 12
Mass transfer for component A and S:
Equation 13
Equation 14
Interfacial equilibrium:
Equation 15
Heat balance for liquid phase:
Equation 16
Heat balance for gas phase:
Equation 17
Where vAB denotes stoichiometry; CP,L is heat capacity of solution (kJ/m3 K); CP,j is heat capacity of component j in the gas (kJ/kmol K); hG is heat transfer coefficient (kJ/m2 s K); H is Henry’s constant (kPa m3/kmol); HR is heat of absorption and reaction (kJ/kmol); HS is heat of vaporization of solvent S (kJ/kmol); I is enhancement factor; kG is gas mass transfer coefficient (kmol/m2 s kPa); kLO is physical liquid mass transfer coefficient (m/s); T is temperature (K); and Y is mole ratio. Five components are presented in a subscript which is comprised of an inert carrier gas (I), an acid gas (A) (i.e. CO2), an inert liquid solvent (S) (i.e., water vapor), a reactant (B) in the liquid and a reaction product (C).
To determine the packing height for a given absorption duty, the above equations must be solved concurrently. Since the equations are nonlinear, numerical methods need to be employed. For designing an absorber operating in countercurrent mode, the temperatures and compositions of the lean absorbent and raw feed gas are usually known while the properties of the off gas and rich solution are partially specified. As a result, the conditions at either end of the absorber are known and a two-point boundary value problem can be defined. Before the model equations can be evaluated, various parameters (e.g., mass transfer coefficients, solubility, reaction rate constant and so on) are required. They may be obtained from experimental measurements or correlations. However, considerable discrepancies between reported data must be taken into account and they can strongly effect the results Citation[34,35].
For physical gas absorption data, correlations are available for some standard systems and conditions. When correlations are unavailable, the data have to be determined experimentally. The physicochemical data such as solubility, diffusivity and rate constants are also required for the case of absorption with chemical reaction. These data have to be obtained for each system at the specific conditions. Although the reaction kinetics and the equilibrium solubility may be obtained experimentally, performing these experiments is time consuming.
Even though the CO2-amine systems have been studied for many years and are common in gas-sweetening industries, the understanding of the reaction kinetics is still required. Some researchers agree on the reaction mechanism but even then, they find that the rate constants may differ by orders of magnitude Citation[36]. High discrepancies can also appear in the equilibrium solubility data. Austgen et al. (1989) reported that the differences among the data reported in the open literature for the CO2 amine system can be as high as 50% Citation[37]. It is also difficult to measure the diffusivity and solubility for CO2 absorption with chemical reaction owing to the fact that diffusion and reaction take place simultaneously in the liquid phase. Besides the experiments, solubilities and diffusitivities of gases in liquids can be estimated by using the method developed by Morsi and Charpentier (1981) Citation[38]. The most important step in the theoretical design method is the determination of the enhancement factor, I, which is a function of the reaction mechanism and the properties of all components in the liquid film. To calculate the enhancement factor, a set of partial differential equations have to be solved with the simultaneous diffusional mass transfer and chemical reactions in the liquid film. The 1D diffusion equation for species j can be defined by:
Equation 18
Where Dj, rj, x and t represent diffusitivity (m2/s), rate of reaction of the solute gas in the liquid film (kmol/m3 s), distance from gas-liquid interface (m) and contact time (s), respectively.
The simplified enhancement factor can be obtained from the empirical models proposed by Rase (1977) and Astarita (1983) (for instantaneous irreversible reaction) Citation[39,40], DeCoursey and Thring (1989) (for irreversible reaction of finite rate) Citation[41], Perry and Green (1984) (for first-order and pseudo first-order irreversible reactions) Citation[27] and Decoursey (1982), Decoursey and Thring (1989), and Winkelman et al. (1992) (for reversible reaction) Citation[41–43].
Laboratory Method
According to the previous discussion, physicochemical data and enhancement factors are difficult to identify and, consequently, laboratory models for designing gas absorbers with chemical reaction have been proposed Citation[44–47]. The step of this method is demonstrated in in Route #3. The rate of absorption per unit interfacial volume or mass transfer rate (Rv, kmol/m3 s) is assumed on the basis of the absorber hydrodynamics and the concentrations of reactants in gas and liquid phases. illustrates a small cell that is a gas–liquid contactor at steady state, and two stirrers that keep the concentrations in the bulk of the gas and liquid phases to be uniform.
Equation 19
The advantage of this method eliminates having to determine parameters such as reaction rate, solubility and diffusivity. It is noted that such a model can be used when the reaction rate is fast enough to take place in the liquid film near the interface, which can imply no reaction in the bulk of the liquid. Alper and Danckwerts (1976) developed a new model, the so called complete model Citation[45]. This model was developed from string-of-sphere column as given in Citation[45]. The absorber height by using this model can be calculated by:
Equation 20
Where subscript m(m) respectively represents the model column.
Pilot plant technique method
This method was proposed by Tontiwachwuthikul et al. (1989–1994) Citation[18–20]. The method is intended to scale up a pilot plant or bench-scale plant to an industrial scale plant as illustrated in Citation[18–20]. However, such a method needs to assume that hydrodynamic characteristics of the pilot plant or bench-scale plant are the same as those of the industrial scale plant. The material balance around the absorber is given by:
Equation 21
There are several methods to determine Rv. However, most of them cannot be used when certain parameters are unknown. Using this method can help to determine Rv by assuming it as a function of the fluid concentration as given by .
Equation 22
Rv can also be obtained from the absorption rate from the liquid composition profile as follows:
Equation 23
The absorber height can then be calculated by . This method does not require the hydrodynamic and physicochemical parameters. The step of this method is demonstrated in in Route #4. Another advantage of this method is that it is applicable to all cases regardless of whether the rate of absorption is obtained by the reaction in the liquid film or in the bulk of the liquid or both, and no matter how fast or slow the reaction rate is.
The PPT approach has been successfully applied to a packed tower in which CO2 is absorbed into NaOH solutions and aqueous solutions of 2-amino-2-methyl-1-propanol (AMP), the most commonly used sterically hindered amine. The validation results were reported in Citation[18–20], which show a good agreement within 12%.
In conclusion, for designing absorbers using reactive solvents, the empirical design method is not recommended for design of the absorber for gas absorption with chemical reaction. This implies that it would carry significant error when the method is used in the CO2 capture process in which there is a chemical reaction between the absorbent and CO2. Using this method potentially leads to over design of the absorber height. The theoretical design method is accurate for the CO2 absorber design since it requires all the involved parameters as discussed previously. However, the calculation step is complicated since it is difficult to obtain all the parameters, especially the hydrodynamic and the physicochemical properties. Lack of knowledge of some of these parameters may cause significant error. Meanwhile, the laboratory method is applicable when the reaction mechanism and kinetics may not be acquired. Nevertheless, a limitation of this method is how laboratory experimental setup (e.g., a stirred cell) can be represented as a full-scale absorber.
However, for many practical situations, very little is known about the system (e.g., CO2 absorption into mixed reactive solvents using structured packed towers). Under such conditions, the use of the PPT is advantageous and practical in real industrial environments Citation[18–20].
Process design
A comprehension of experimental pilot plants becomes essential to develop the process flow diagram of CO2 capture for use in commercial or large scale plants. Therefore, this section gives some examples of the model validation results obtained from four different pilot plants: the International Test Centre for CO2 Capture Citation[103], the Esbjerg CASTOR Pilot Plant (DONG), Institute of Thermodynamics and Thermal Process Engineering (ITT), Stuttgart pilot plant, and the SINTEF/NTNU pilot plant. This review discusses the validation of temperature, CO2 concentration profile, CO2 mass balance and reboiler heat duty. The experimental results are validated with simulation results by means of the coefficient of correlation (i.e., R2 coefficient). The average absolute deviation (percentage AAD) of each parameter output is discussed. The process validation provides the necessary confidence to scale up the pilot plants to large scale plants. It should be noted that the full details of the four pilot plant simulations using Aspen, ProMax, CHEMASIM, ProTreat and CO2SIM are available in Luo et al. (2009) Citation[15] under the consortium of International Cooperation and Exchange CO2 Capture using Amine Processes (CAPRICE) Citation[104]. Finally, a discussion of the integration of large-scale CO2 plant to coal-fired and natural gas-fired power plant is discussed in next subsection.
▪ Brief description of each pilot plant
The schematic diagrams for the four pilot plants are given in Citation[15]. demonstrate the schematic diagrams of the International Test Centre of CO2 Capture (ITC), Esbjerg CASTOR and ITT pilot plants. Those three pilot plants have in similar design concept. They have an absorber composed of 2 sections (i.e., wash water and absorber sections) packed with packing material. A wash water section, on top of the top section, is use to cool down the solution to minimize monoethanolamine (MEA) loss. Treated gas (V3) is released at the top of the absorber while the liquid solution flows downwards to the bottom of the absorber. A rich amine pump (P1) is used to increase the liquid pressure before flowing through the lean-rich heat exchanger (HEX1) and then the regenerator. The regenerator is packed with packing material. At the top section of the regenerator, there is a condenser that condenses the regenerator overhead, in order to aid the separation of the condensed stream into the reflux stream (mainly containing water [L10]) and the CO2 product (V4). The reflux stream is subsequently recirculated back to the regenerator. A reboiler at the bottom of the regenerator is used to heat the liquid solution. The lean amine solution (L2) is cooled down by a cooler (HEX2) and in turn is introduced back to the absorber. shows the schematic diagram of SINTEF/NTNU pilot plant. The conceptualized design of this plant is similar to ITC, Esbjerg CASTOR and ITT pilot plants as previously mentioned but this plant has no wash water section on the top section of the absorber. In addition, the process uses recycled gas from the condenser (HEX3) outlet to return to the top section of the absorber. This condenser also works as a separator that separates liquid (L11) to the bottom tank. The liquid directly flows to the reboiler. It should be noted that these four different pilot plants used 30% wt. MEA as the chemical absorbent to capture CO2. A brief summary of each plant configuration is given in .
Modeling approach
This review article shows only the ProMax simulation for four different pilot plants. The use of other software, which includes Aspen Rad Frac, Protreat, Promax, Aspen Rate Sep, CHEMASIM and CO2SIM is not discussed in the paper. However, its detail can be found in our collaborative work Citation[15]. All simulations appearing in this article were performed at the ITC in the University of Regina, Canada. Four datasets from each of the ITC and SINTEF/NTNU pilot plants, and one data set from each of the other two plants (Esbjerg CASTOR, and ITT Stuttgart pilot plants) were used to validate the simulation results.
The parameter inputs used in the simulation are summarized as follows:
▪ Configurations of absorber and regenerator (e.g., packing type, flooding factor, system factor, residence time of liquid holdup and pressure drop), and configurations of pumps, intercoolers and heat exchanger (e.g., pressure and efficiency). These parameters are assigned by each individual pilot plant design. | |||||
▪ Temperature approach between rich amine inlet and lean amine outlet in lean-rich heat exchanger (LRX). It is to be noted that the simulation does not fix or assign any lean and rich solution temperature inlet and outlet in LRX, absorber and regenerator. These temperatures are calculated on the basis of thermodynamics and chemical reactions. | |||||
▪ Reflux temperature at the top section of the regenerator. The temperature is set by the process parameters obtained from each four different pilot plants. | |||||
▪ Capture performance. This is assigned to control the percentage of CO2 capture capability between absorber inlet and outlet. It should be noted that the liquid flow rate and CO2 loading of lean and rich solutions are not assigned as process inputs. These parameters are optimized by the capture performance. | |||||
▪ Simulation results
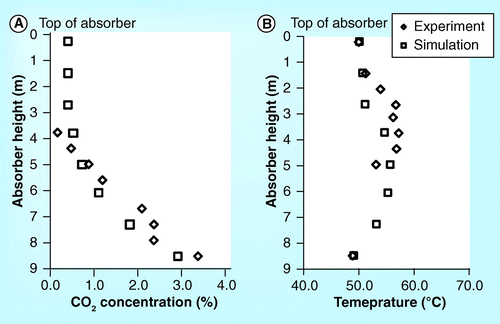
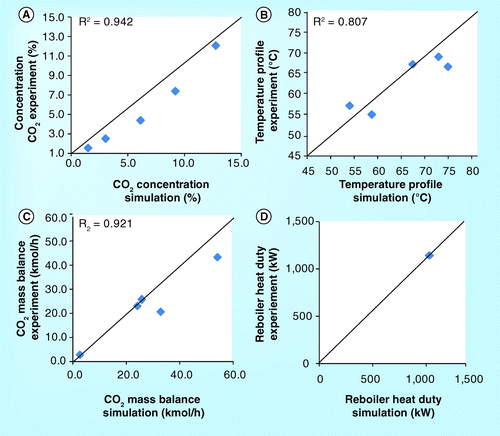
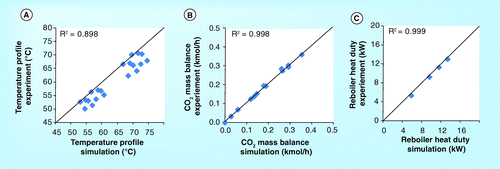
ITC pilot plant
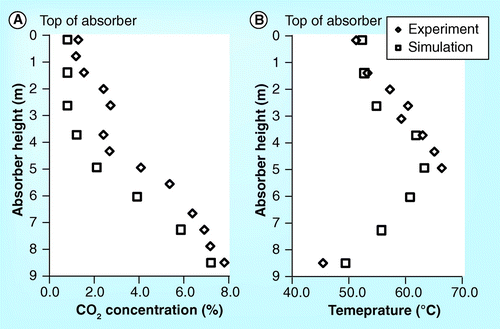
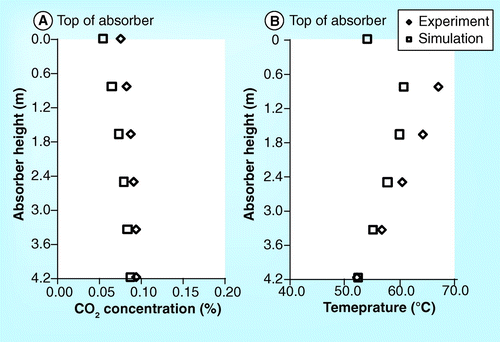
demonstrate the CO2 concentration profile of the 10-m absorber height of the ITC pilot plant. It should be noted that all figures set the top section of the absorber as the beginning level (z = 0). The figures show that the CO2 concentration obtained from simulation is lower than the experiment. In addition, the simulation roughly predicts the CO2 concentration profile in the washing water section but predicts the profile in the absorber section well. Both simulation and experimental results are in very good agreement. demonstrate a validation of temperature profile between simulation and experimental results. It was found that both results are in a good correlation.
shows the parity plot of CO2 concentration, temperature profile, CO2 mass balance and reboiler heat duty between simulation and experimental results. All parity plots have R2 greater than 0.8. Particularly, the correlation of CO2 mass balance and reboiler heat duty has an R2 higher than 0.95. This implies that the simulation can predict the experimental process outputs obtained from the ITC pilot plant.
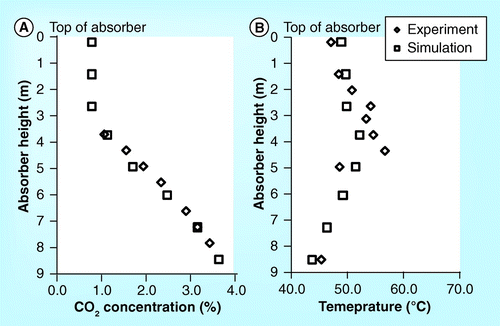
Esbjerg CASTOR pilot plant (DONG)
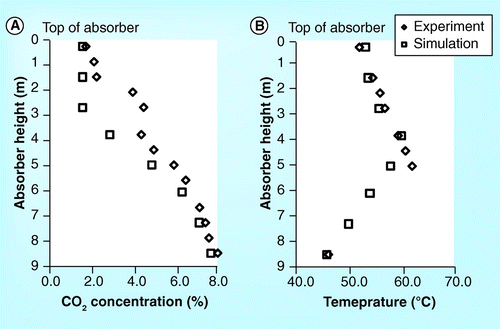
demonstrate the validation of the CO2 concentration and temperature profile of the 20 meter absorber height of the Esbjerg CASTOR pilot plant Citation[15]. The simulation results are slightly lower than the experimental results but their trends are consistent.
show the parity plot of CO2 concentration, temperature profile, CO2 mass balance and reboiler heat duty. The R2 of all plots is relatively high. This indicates that the process simulator gives results that are able to predict the actual experimental process parameters in the Esbjerg CASTOR pilot plant.
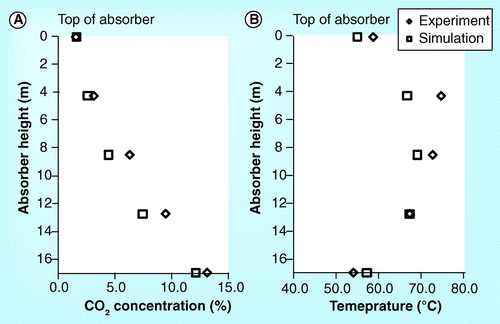
ITT Stuttgart pilot plant
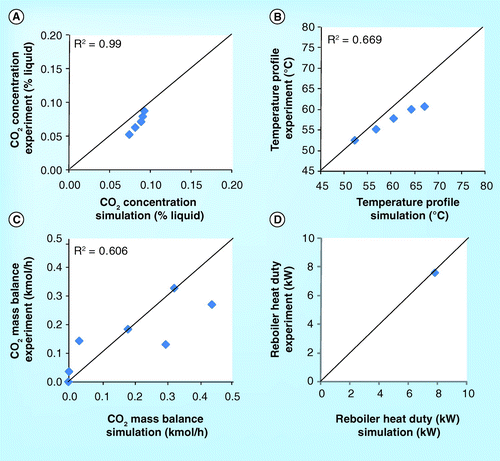
show the validation of CO2 concentration and temperature profile for the 4.6 meter absorber height of the ITT pilot plant Citation[15]. The simulation gives a slightly lower CO2 concentration and temperature profile than the experimental results. At the top of the absorber, the temperature is slightly inconsistent . The temperature outlet of the experiment is 40°C while that of the simulation is approximately above 50°C.
show the parity plot of CO2 concentration, temperature profile, CO2 mass balance, and reboiler heat duty. The CO2 concentration, reboiler heat duty obtained from the simulation are in an excellent correlation (R2 = 0.99) with the experimental result whereas the temperature profile and CO2 mass balance do not properly correlate with the experimental result (R2 < 0.67). The poor simulation results rom the ITT pilot plant might be attributed to the following:
▪ Over simplification of the schematic diagram of the model used for simulation as compared with the actual pilot plant, which is more complicated (e.g., extra hidden equipment); | |||||
▪ The positions of the temperature probe attached along the absorber height in the pilot plant may not be the same as in the model. | |||||
SINTEF/NTNU Pilot Plant
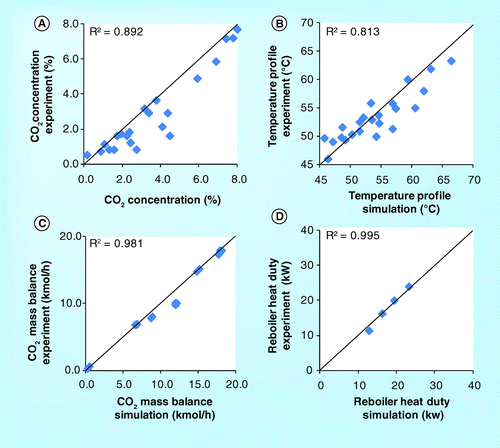
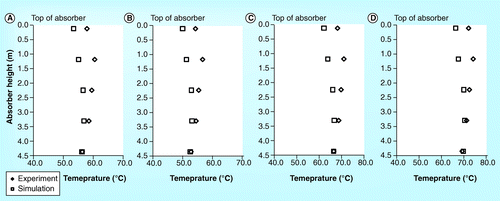
demonstrate the temperature profile for the 4.4 meter absorber height of the SINTEF/NTNU pilot plant. All four data sets have been selected to further validate the simulation results with the experimental results since it has no CO2 concentration profile recorded by SINTEF/NTNU. The figures show that temperature profile obtained from the simulation is obviously lower than the experiment. However, the temperature at the bottom of the absorber for both simulation and experiment are comparable. In general, both simulation and experiment are consistent with R2 of 0.90 .
demonstrate the parity plot of CO2 mass balance and reboiler heat duty. It was found that both simulation and experiment are excellently correlated with an R2 above 0.99.
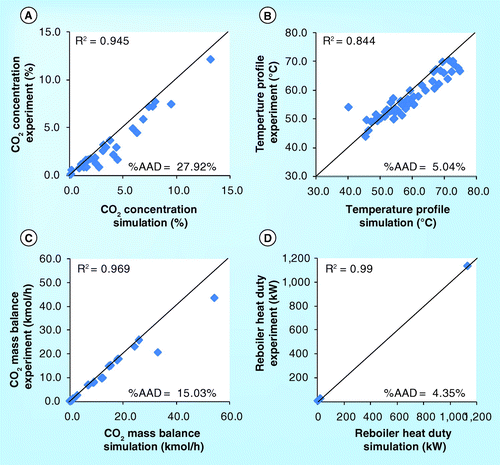
The simulation using ProMax gave satisfactory results. The deviation between simulation and experimental outputs for all parameters from the ITC, Esbjerg Dong and SINTEF/NTNU pilots were small (ADD = 10.9%) whereas the deviation between simulation and experimental outputs for ITT were high for CO2 mass balance and temperature (ADD = 43.7%) but small for other parameters (ADD = 9.35%).
A comparison of the simulation and experimental results from all the four pilot plants are plotted in the same parity plot in . The average R2 and %AAD for the four pilot plants were measured for various parameters. The average R2 and%AAD for CO2 concentration were 0.95 and 27.9%, respectively ; for temperature profile, they were 0.84 and 5.0%, respectively ; for CO2 mass balance, they were 0.97 and 15.0%, respectively ; and for the reboiler heat duty, they were 0.99 and 4.4%, respectively . These imply that this process simulator could be used to simulate the pilot plants.
Process integration
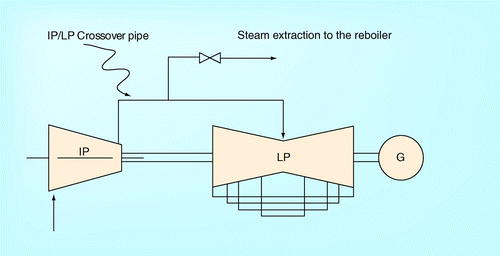
The post-combustion CO2 capture using amines is commonly integrated into large point CO2 sources of which the process captures CO2 after the combustion has taken place in fossil fuel-fired power plant (e.g., coal-fired power plant or natural gas-fired combined cycle gas turbine [CCGT]) Citation[5,6]. The steam is extracted from a series of steam turbines in the power plant to the reboiler in the CO2 capture process. show an example of steam extraction from coal-fired and natural gas-fired power plants, respectively Citation[16,48]. According to the figures, superheated steam is extracted from the crossover pipe between the intermediate pressure (IP) and low pressure (LP) ports. The steam as a thermal energy source is fed to the reboiler located at the bottom of the regenerator. Such energy is used to obtain a lean-CO2 loaded amine solution from a rich-CO2 loaded amine solution. Then, the used steam (as condensate) is routed back to the deaerator in the power plant.
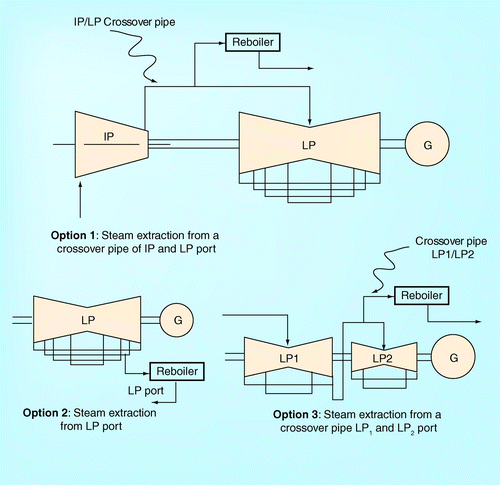
Apart from extracting the steam from the crossover pipe between the IP and the LP ports as shown in the figures, another option is to extract superheated steam from the LP port. It is found from Romeo et al. and Sanpasertparnich et al. that extracting the steam from the LP port offers a lower heat duty for regenerating the amine by stripping the CO2Citation[13,16]. A high pressure steam that can still be used as a working fluid to drive the turbine, resulting in a significant reduction of the energy penalty to regenerate the rich-CO2 solution. However, Alie proposed that a suitable and realistic extracting location should be in the crossover pipe between the IP and LP ports Citation[9]. Extracting the steam from LP port would be inaccessible since the port is located on the underside of the turbine Citation[9]. Even though, currently, there is a physical limitation in extracting the steam from LP port, the authors realize that there are two possibilities to extract the steam from the LP turbine as illustrated in :
▪ A new design of low pressure steam turbine, which offers an accessible steam extraction location; | |||||
▪ A split of the low pressure steam turbine into more than one, such that the steam can be extracted from LP/LP port. | |||||
The first option requires a turbine manufacturer to redesign the LP turbine casing and space of turbine blades to allow an insert pipe for steam extraction. In the case that the first option is not feasible, instead of extracting superheated steam from the crossover pipe in between the IP and LP ports, the splitting of one LP turbine into two turbines and extracting the superheated steam from the crossover pipe between the two LP ports would be practical.
In addition to the location of the steam extraction, the quality and quantity of the steam from the turbine are important parameters one should be aware of. The precautions include:
▪ The extracted steam temperature from the turbine should not be above the temperature that can cause CO2 absorbent to undergo thermal degradation and/or corrosion Citation[9,49]. If the temperature is over the limit, an extra unit such as de-superheater unit must be integrated before the reboiler Citation[10,50]; | |||||
▪ Extracting possibly the lowest quality steam is advantageous, since high quality steam offers higher power generation Citation[9,16]; | |||||
▪ The steam flow rate supplied to the reboiler must be sufficient to regenerate CO2 absorbent and to reach the percentage of CO2 capture performance design. | |||||
Future perspective
It is essential to have a comprehensive knowledge of how to design, model and simulate the post combustion CO2 capture system based on regenerable amines. Unless the equipment and process are systematically designed, the heat consumption as well as the CAPEX and OPEX for the CO2 capture plant will be very high. There are many companies and organizations that focus only on modifying the process and techniques used in gas purification processes to fit the CO2 capture process. Examples are split flow, combined concurrent-counter current and intercooler configurations Citation[21,51]. The development of compression ratio to reduce multiple stages of CO2 compressors and utilizing heat from the compression for amine regeneration has been proposed. Advanced design of packed column for the absorber and regenerator to enhance gas–liquid contacting time and mass transfer coefficient is also competitively developed. In addition to these issues, improvements in CO2 capture efficiency and amine-regeneration efficiency, as well as the developments of effective inhibitors to reduce solvent degradation, corrosion and foaming should be pursued in order to actualize the CO2 capture process using absorbents.
Commercial process simulators as well as in-house softwares are continuously being developed by embedding the improvements in certain aspects of the process technologies and providing a user-friendly interface. The technology would then support any user who may not have the background on the CO2 capture system design. This would increase risk, uncertainty and unreliability when such designs are implemented. As such, it is important that all simulations must be capable of representing and validating any operating condition and equipment dimensions before users scale up the process to integrate into fossil fuel-fired power plants. Some simulation software companies have not programmed their software based on theoretical designs and existing pilot plant data, making such software unreliable. For instance, the programmer may put incorrect thermodynamics calculation algorithm in the software, or obsolete thermodynamics databases. These can lead to poor engineering design integrity. Therefore, any engineering consulting company must have specialists in this particular field. Therefore, more pilot plants duplicating up-scale system would be built within this decade. Collaborations to share the pilot-plant data would be seen soon. The integration of regenerable solvent based CO2 capture system into existing or new fossil fuel-fired power plants will possibly occur in this decade, but it would be only for partial capture defined as the capture of CO2 from less than 100% of the total flue gas stream from the power plant. A full integration would be implemented when regulatory policy is in place and will be enforced, and the government confirms that it will compensate power producers for the cost of capturing CO2. Full integration will also be helped by the development of cost-effective, process-efficient, environmentally safe and operation-reliable CO2 capture technologies.
Table 1. Brief description of process parameters in four different pilot plants.
Organic compounds containing the amino functional group with one or more alkyl or aryl groups attached to the nitrogen. They can be classified as primary, secondary and tertiary amines depending on the numbers of alkyl or aryl groups attached to the nitrogen of the amino functional group.
A conceptual process design for industry and engineering projects. It includes material and energy balances, 2D/3D models, equipment designs and lists, process layout, schedule/plan, estimate, purchase-ready equipment specification and so on. Essentially, engineering project undergoes with these steps: pre-FEED; FEED; detailed design; procurement; construction; testing; and commissioning.
Prime mover used to convert dynamic and pressured steam into energy by turning blades of a series of turbines; for example, high pressure turbine (HP), intermediate pressure turbine (IP) and low pressure turbine (LP).
▪ Of post-combustion CO2 capture technologies, CO2 capture-based regenerable solvent is the most mature and promising technology. In addition, post-combustion CO2 capture based on regenerable solvent is best suited to capture CO2 at a low partial pressure of approximately 4–15% by volume of CO2. Consequently, the technology can be integrated into pulverized coal-fired, natural gas-fired and oil-fired power plants. | |||||
▪ It is clear that regenerating CO2-rich amines to CO2-lean amines consumes significant heat. This can be reduced considerably by proper process configuration optimization. | |||||
▪ There are many constraints on the modeling and simulation strategy to designing a CO2 capture plant. These include the difficulty to solve numerical modeling equations, establishing mechanisms of chemical reactions, the reliability of properties (e.g., thermodynamic, chemical and physical) of the system components, amongst other things. These limitations make it necessary to further validate innovative process configurations that are only confirmed by simulation using commercial simulators. Several commercial and non-commercial programs are available to model the post-combustion capture of CO2. This includes EBSILON®, GateCycle®, GT Pro®, PROATES®, Steam Pro® (for power plant simulation), Aspen Plus®, Aspen HYSYS®, CHEMASIM®, ProMax®, ProTreat® (for CO2 capture simulation) and GT Pro/Master®, IECM®, Steam Pro/Master®, and ThermoFlex® (for process integration). | |||||
▪ In the design of the absorber, the empirical design method is not applicable when a system has chemical reaction, while the theoretical design method requires a number of involved parameters to calculate the absorber height. The laboratory method is based on a small-scale experimental setup, such as stirred cell and string-of-sphere column, whereas the pilot plant technique method is based on equivalent geometry to a full-scale CO2 absorber but a smaller dimensions. The disadvantage of the laboratory method, therefore, is that it does not represent a full-scale CO2 absorber. | |||||
▪ Process validation using existing CO2 capture pilot plants is necessary to obtain process integrity and confidence for up-scaling the CO2 capture process. ProMax as one of the process simulators is able to validate the experimental data obtaining from: - the International Test Centre of CO2 Capture (ITC) pilot plant; - the Esbjerg CASTOR pilot plant; - the ITT Stuttgart pilot plant; - the SINTEF/NTNU pilot plant. | |||||
▪ Upon the integration of the CO2 capture plant with a fossil fuel-fired electric power plant, it is clear that there are two points for steam extraction from a series of the steam turbines: crossover pipe between intermediate pressure (IP) turbine and low pressure (LP) turbine port and the LP port. | |||||
▪ It is shown that extracting the steam from LP port would be inaccessible. Hence, there are two possible options to extract the steam from the LP turbine: a new design of LP turbine and an LP/LP port by splitting of LP turbine into more than one. | |||||
Acknowledgements
We would like to acknowledge the research supports given to to ITC over the past many years by the followings organizations: Natural Sciences and Engineering Research Council of Canada (NSERC), Canada Foundation for Innovation (CFI), Saskatchewan Ministry of Energy & Resources, Western Economic Diversification, EnCana Energy Inc., E.ON Energy, Stantec, Saudi Aramco, Doosan Heavy Industries, HTC Purenergy Inc. Saskatchewan Power Corporation, StatOil Hydro (Norway), SaskFerco Inc., Sulzer Chemtech (Switzerland), Fluor Corporation (USA), the Canada Centre for Mineral and Energy Technology (CANMET), Alberta Energy Research Institute (AERI) and Research Institute of Innovative and Technology for the Earth (RITE).
We would also like to acknowledge the assistants from Luo X, Svendsen HF (NTNU, Norway), Notz R (University of Stuttgart, Germany), Hoch S, Hasse H (University of Kaiserslautern, Germany), Lemaire E, Alix P (IFP, France), Tobiesen FA, Juliussen O (SINTEF, Norway), Köpcke M (Vattenfall, Sweden), Hopman J (TNO, The Netherlands) and CAPRICE consortium members for the pilot plant data, Hendrick C (BR&E, Texas, USA) for ProMax technical support and Saiwan C (Chulalongkorn University, Thailand) for pre-reviewing this article.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- International Energy Agency (IEA). World Energy Outlook. (2002).
- Gray ML, Soong Y, Champagne KJ et al. Improved immobilized carbon dioxide capture sorbents. Fuel Process. Technol.86(14–15),1449–1455 (2005).
- Yang WC, Ciferno J. Assessment of carbozyme enzyme-based membrane technology for CO2 capture from flue gas. DOE/NETL 401/072606 (2006).
- Anderson JL, Dixon JK, Maginn EJ, Brennecke JF. Measurement of SO2 solubility in ionic liquids. J. Phys. Chem. B.110,15059–15062 (2006).
- Metz B, Davidson O, deConinck H, Loos M, Meyer L. IPCC special report on carbon dioxide capture and storage. Intergovernmental Panel on Climate Change, Cambridge University Press, UK (2005).
- Dooley JJ, Davidson CL, Dahowski RT. An assessment of the commercial availability of carbon dioxide capture and storage technologies as of June 2009. Pacific Northwest National Laboratory, US DOE. (2009).
- Figueroa JD, Fout T, Plasynski S, McIlvried H, Srivastava RD. Advances in CO2 capture technology – the U.S. department of energy’s carbon sequestration program. Int. J. Greenhouse Gas Control.2(1),9–20 (2008).
- Thitakamol B, Veawab A, Aroonwilas A. Environmental impacts of absorption-based CO2 capture unit for post-combustion treatment of flue gas from coal-fired power plant. Int. J. Greenhouse Gas Control.1(3),318–342 (2007).
- Alie CF. CO2 Capture with MEA: integrating the absorption process and steam cycle of an existing coal-fired power plant. MSc thesis. University of Waterloo, Canada (2004).
- Fisher KS, Beitler C, Rueter C, Searcy K, Rochelle G, Jassim M. Integrating MEA regeneration with CO2 compression and peaking to reduce CO2 capture costs. US DOE. (2005).
- Rao AB, Rubin ES. Identifying cost-effective CO2 control levels for amine-based CO2 capture systems. Ind. Eng. Chem. Res.45,2421–2429 (2006).
- Abu-Zahra MRM, Schneiders LHJ, Niederer JPM, Feron PHM, Versteeg GF. CO2 capture from power plants. Part I. A parametric study of the technical performance based on monoethanolamine. Int. J. Greenhouse Gas Control.1,37–46 (2007).
- Romeo LM, Bolea I, Escosa JM. Integration of power plant and amine scrubbing to reduce CO2 capture costs. Appl. Therm. Eng.28(8–9),1039–1046 (2008).
- Adams RG, Alin J, Biede O et al. CAPRICE project – engineering study on the integration of post combustion capture technology into the power plant gas path and heat cycle. Energy Procedia.1(1),3801–3808 (2009).
- Luo X, Knudsen JN, deMontigny D et al. Comparison and validation of simulation codes against sixteen sets of data from four different pilot plants. Energy Procedia.1(1),1249–1256 (2009).
- Sanpasertparnich T, Idem R, Bolea I, deMontigny D, Tontiwachwuthikul T. Integration of post-combustion capture and storage into a pulverized coal-fired power plant. Int. J. Greenhouse Gas Control.4(3),499–510 (2010).
- Pfaff I, Oexmann J, Kather A. Optimised integration of post-combustion CO2 capture process in greenfield power plants. Energy35(10),4030–4041 (2010).
- Tontiwachwuthikul P, Meisen A, Lim CJ. Methodologies for designing packed acid gas absorbers with chemical reaction: a review. Int. Conf. on Adv. Sci. & Technol. Exch. Bangkok, Thailand (1994).
- Tontiwachwuthikul P, Meisen A, Lim CJ. Novel pilot plant technique for sizing gas absorbers with chemical reactions. Can. J. Chem. Eng.67,602–607 (1989).
- Tontiwachwuthikul P. New pilot plant technique for sizing gas absorbers with chemical reactions. PhD. Thesis. University of British Columbia, Canada (1991).
- Kohl A, Nielsen R. Gas Purification (5th Edition), Gulf Publishing, Texas, UK (1997).
- Weiland RH. Physical properties of MEA, DEA, MDEA and MDEA-based blends loaded with CO2. GPA Research Report No. 152. (1996).
- Weiland RH, Dingman JC, Cronin DB, Browning GJ. Density and viscosity of some partially carbonated aqueous alkanolamine solutions and their blends. J. Chem. Eng. Data.43(3),378 (1998).
- ProMax 2.0. ProMax® Level I & II. Bryan Research & Engineering, Inc. (2008).
- Aspen Plus. EAP101 Aspen Plus®: Process Modeling. (2009).
- Kister HZ. Distillation Design. McGraw-Hill. NY, USA (1992).
- Perry RH, Green D. Perry’s chemical engineers’ handbook. (6th Edition). Mcgraw-Hill. NY, USA (1984).
- Billet R. Packed Towers in Processing and Environmental Technology. VCH. NY, USA (1995).
- Astarita G. Mass transfer with chemical reaction. Elsevier Publishing. NY, USA (1967).
- Danckwerts PV. Gas Liquid Reactions. McGraw Hill. NY, USA (1970).
- Joshi SV, Astarita G, Savage DW. Prediction of pilot plant performance for a chemical absorption process. Chem. Eng. Symp. Series.77(202),63–77 (1981).
- Treybal RE. Adiabatic gas absorption and stripping in packed towers. Ind. Eng. Chem.61,36–41 (1969).
- Pandya JD. Adiabatic gas absorption and stripping with chemical reaction in packed towers. Chem. Eng. Commun.19,343–361 (1983).
- Kelly RM, Rousseau RW, Ferrell JK. Design of packed, adiabatic absorbers: physical absorption of acid gases in methanol. Ind. Eng. Chem. Process Des. Dev.23,102–109 (1984).
- Raal JD, Khurana MK. Gas absorption with large heat effects in packed columns. Can. J. Chem. Eng.51,162–167 (1973).
- Barth D, Tondre C, Delpuech JJ. Kinetics and mechanisms of the reactions of CO2 with alkanolamines: a discussion concerning the cases of MDEA and DEA. Chem. Eng. Sci.39,1753–1760 (1984).
- Austgen DM, Rochelle GT, Peng X, Chan CC. Model of vapor-liquid equilibria for aqueous acid gas alkanolamine systems using the electrolyte – NRTL equation. Ind. Eng. Chem. Res.28,1060–73 (1989).
- Morsi BI, Charpentier JC. Solubilities and diffusivities of gases in liquids. ASI Series. 72 (1981).
- Rase HF. Chemical Reactor Design for Process Plants: Volume 1 – Principles and Techniques. John Wiley & Sons, NY, USA (1977).
- Astarita G, Savage DW, Bisio A. Gas Treating with Chemical Solvents. John Wiley & Sons. NY, USA (1983).
- DeCoursey WJ, Thring RW. Effects of unequal diffusivities on enhancement factors for reversible and irreversible reaction. Chem. Eng. Sci.44(8),1715–1721 (1989).
- Winkelman JGM, Brodsky SJ, Beenackers AACM. Effects of unequal diffusivities on enhancement factors for reversible reaction: numerical solutions and comparison with DeCoursey’s method. Chem. Eng. Sci.47(2),485–489 (1992).
- DeCoursey WJ. Enhancement factors for gas absorption with reversible reaction. Chem. Eng. Sci.37(10),1483–1489 (1982).
- Danckwerts PV, Alper E. Design of gas absorbers: part III laboratory point model of a packed column absorber. Trans. Instn. Chem. Engrs.53,34–40 (1975).
- Alper E, Danckwerts PV. Laboratory scale-model of a complete column absorber. Chem. Eng. Sci.31(7),599–608 (1976).
- Laurent A, Charpentier JC. The string of spheres column with pools: a new experimental laboratory apparatus to simulate the process of gas–liquid absorption with chemical reaction in an industrial packed column. Proc. Conf. Appl. Chem. Unit Oper. Processes (3rd edition) 419–429 (1977).
- Danckwerts PV, Gillham AJ. Design of gas absorbers: part I methods for predicting rates of absorption. Trans. Instn. Chem. Eng.44,42–54 (1966).
- Sanpasertparnich T, Idem R, Tontiwachwuthikul T. CO2 absorption in an absorber column with a series of intercooler circuits. Proceedings of: The 10th International Conference on Greenhouse Gas Control Technologies, Amsterdam, Netherlands, 19–23 September 2010.
- Veawab A. Corrosion in CO2 capture unit for coal-fired power plant flue gas. Greenhouse Gas Control Technologies: Proceeding of: The 6th International Conference on Greenhouse Gas Control Technologies, 1–4 October 2002.
- Sanpasertparnich T. Monte Carlo simulation of pulverized coal-fired power plants: efficiency improvement and CO2 capture options. MSc thesis. University of Regina, Canada (2007).
- Liang Z. Optimization of post-combustion amine-based carbon dioxide capture process to minimize heat requirements for solvent regeneration. PhD thesis. University of Regina, Canada (2010).
▪ Websites
- IEA Greenhouse Gas R&D Programme. Capturing CO2www.ieaghg.org/docs/general_publications/cocapture.pdf
- NETL. Carbon dioxide capture from existing coal-fired power plants. DOE/NETL-401/110907 www.netl.doe.gov/energy-analyses/pubs/CO2%20Retrofit%20From%20Existing%20Plants%20Revised%20November%202007.pdf
- ITC. International Test Centre for CO2 Capture www.co2-research.ca
- CAPRICE. International Cooperation and Exchange CO2 Capture Using Amine Processes.(2009) www.caprice-project.eu