Abstract
This article is part 3 of the review series on ‘Recent progress and new development of post-combustion carbon capture technology using reactive solvents’. This review focuses on alkanolamine absorption during post-combustion CO2 capture from coal-fired flue gas, looking at a range of absorbents, including those that are commonly used, as well as blended and new solvents. The effects on corrosion of blended and new absorbents and process parameters (e.g., amine concentration, CO2 loading, oxygen concentration, SO2 concentration and temperature) are reviewed. Also reviewed is the effect of corrosion on the formation of heat-stable salts in the absorbent stream, as well as the presence of impurities in flue gas. Finally, corrosion mechanisms are discussed and corrosion inhibition approaches are reviewed.
Fossil fuel power plants are a major source of CO2 emissions, which gives rise to an enhanced GHG effect. To control emissions and reduce this effect, a CO2 capture technique may be integrated into the power plant. In the case of low pressure coal-derived flue gas streams, the application of a chemical-absorption process using an aqueous alkanolamine solution has been found to be the most effective of such a CO2 capture approach. However, corrosion is a major drawback, which prevents the amine process from achieving its highest possible efficiency. Corrosion is an electrochemical process involving the transfer of electrons within the construction material, resulting in its deterioration. The electrochemical process is a redox reaction, consisting of two chemical reactions known as oxidation and reduction Citation[1,2]. When a metal is immersed in a given solution, redox occurs at the surface of the metal, causing the metal to corrode. For example, corrosion of zinc in an acid environment proceeds according to the following reactions:
Overall reaction:
Equation 1
Oxidation reaction:
Equation 2
Reduction reaction:
Equation 3
At equilibrium, the electrode potential (E) is referred to as reversible electrode potential (Erev). The corrosion electrode potential (ECORR) is equivalent to Erev and used specifically for corrosion reactions. When a potential is applied to an equilibrium system, it causes the potential to shift and deviate from ECORR to E. The potential deviation is known as polarization (η), which can be either positive or negative. When η is positive, the metal surface is driven toward the anodic side of the reactions and loses its electrons, which is termed as anodic polarization, while cathodic polarization occurs when η is negative.
When a number of half-reactions occur simultaneously on the metal surface, ‘mixed potential theory’ must be utilized to create polarization curves. The theory states that, at equilibrium, the net oxidation rate must be equal to the net reduction rate. That is, the sum of anodic oxidation currents must equal the sum of cathodic reduction currents and the net measurable current is zero.
Equation 4
Electrochemically, corrosion rate measurement is based on the determination of the oxidation current (also called corrosion current, iCORR) at the corrosion potential. The corrosion current is calculated as follows:
Equation 5
Equation 6
Application of mixed potential theory allows the determination of the corrosion rate using a method known as Tafel plot.
Since the corrosion current is related directly to the corrosion rate, Tafel plot and potentiodynamic techniques are performed to experimentally determine iCORR, from which the corrosion rate is calculated.
This paper reviews corrosion studies in a CO2 capture plant. Effects of various factors including type of amine and its concentration, CO2 loading, process parameters (e.g., temperature) and flue gas contaminants (e.g., O2 and SO2) on corrosion are presented. Corrosion prevention strategies using corrosion inhibitors are also reviewed.
Background of amine absorption
Alkanolamines as absorbents for acid-gas removal in gas-treating plants have been used in gas purification processes for more than 60 years. Commercial interest in gas purification has focused primarily on two amines, monoethanolamine (MEA) and diethanolamine (DEA), but can include other alkanolamines such as triethanolamine, diisopropanolamine, methyldiethanolamine (MDEA) and diglycolamine. Triethanolamine was first used in gas-treating plants, but was replaced by MEA, due to the latter’s low reactivity and relatively low stability. MDEA has been proven to be a selective solvent for removal of H2S in the presence of CO2Citation[3]. MDEA is less basic and has greater capacity to react with acid gas due to the fact that it is used in higher concentrations than MEA. MEA is the most widely used solvent for CO2 absorption, due to the fact that it has the highest alkalinity and highest volume of acid- gas removal at the fastest rate. The alkalinity of amines increases in the order of MDEA < DEA < MEA Citation[4]. On the other hand, MEA also has some drawbacks based on its own physical characteristics, such as the high energy consumption required for CO2 regeneration and high vapor pressure compared with other alkanolamines, which leads to considerable vaporization and solvent loss. In addition, amines are degraded upon contact with oxygen, following which the degradation products become corrosive agents and consequently cause equipment corrosion in the absorption plant Citation[5,6]. The corrosion rates of amines increase in the order of MEA > DEA > MDEA Citation[4].
Rooney and DuPart reviewed the role of oxygen in oxidative degradation of amines and corrosivity of these degradation products Citation[7]. To reduce the corrosion rate, the solvent strength is kept at 20–30% amine by weight in water, resulting in relatively large equipment sizes and solvent regeneration costs. Also, there have been attempts to use blended solvents, such as pairing the slower rate of MDEA with the faster rate of DEA Citation[3] or mixing MDEA with piperazine (PZ), a rate-promoting agent Citation[4,8,101]. The process might also be modified to incorporate inhibitors that reduce solvent degradation and equipment corrosion. For instance, Bhat et al. reported the use of inorganic inhibitors in an aqueous solution for oil and gas applications Citation[102]. The corrosion inhibitions combine the effects of anodic inhibitors (ammonium heptamolybdate or sodium orthovanadate), cathodic inhibitors (cerium chloride) and metal complexing ligand (trisodium citrate-2-hydrate). Khusnutdinov et al. disclosed 2-propyl-3-ethyl-8-oxychinoline-ZnCl2 complex as a steel corrosion inhibitor Citation[103]. The complex inhibitor was applied in petroleum production in mineralized media with high O2 content. Abdrakhmanov et al. proposed an organic inhibitor, N-acetyl-2-(2,3-dihydroxycilopentenyl) aniline in mineralized water petroleum solutions including H2S Citation[104].
Post-combustion CO2 capture from coal-fired flue gas
CO2 comprises the largest fraction of GHG emitted into the atmosphere. In order to comply with anticipated future environmental regulations, CO2 in flue gas will need to be captured prior to release into the atmosphere. CO2 capture using chemical or physical absorption is a likely technology for efficiently reducing large volumes of CO2 at emission sources to comply with these anticipated regulations. An effective technique is chemical absorption using aqueous alkanolamine solutions Citation[9]. Amine absorption technology for gas processing has been readily adapted for capturing CO2 from coal-fired power plants and could be for other worldwide large CO2 emission sources, such as steel manufactories, cement production, chemical industries and oil refineries . However, power plants using fossil fuels generate the most CO2 emissions Citation[201].
Natural gas feed composition is typically composed of 75% light hydrocarbons (C1–C5), 13–15% CO2 and no O2Citation[10], while flue gas composition from coal combustion also includes O2, N2 and trace contaminants such as SO2 and NOxCitation[11,12]. Fly ash is also present in flue gas streams and typically consists of inorganic oxides of SiO2, Al2O3, Fe2O3, CaO, MgO, Na2O, K2O and P2O5, which have undesirable effects on the amine solution and must be removed before entering into the treating unit. Oxygen in the flue gas originates from air and excess combustion in the boiler. The presence of O2 introduces oxidative degradation of alkanolamine solvents and causes the formation of heat-stable salts (HSS) Citation[13,14,101].
As amine technology for acid-gas absorption has been adopted for capturing CO2 from flue gas, most of the process configurations from natural gas processing are still used in CO2 capture. However, modification and optimization of the processes are still needed to reduce energy consumption and decrease solvent losses and corrosion problems. Nielsen and Hansen Citation[15] reviewed corrosive agents, including acid gases, NH3, O2, HSS and amine degradation products; they specifically looked at the mechanisms of corrosion of carbon steel in refinery amine systems. These variable parameters in amine absorption must be reviewed and become well understood for CO2 capture from flue gas.
Successful precommercial demonstration of CO2 capture from a coal-fired power plant using aqueous MEA absorption occurred at Boundary Dam in Canada Citation[9]. However, the existing problems, especially corrosion from using coal fuel, may be more complicated than seen in natural gas systems. These SO2, NOx, CO and particulate matter, if passing through the filter into the amine solution, could participate in the amine reactions and subsequently induce corrosion. Thus, a pretreatment of the flue gas feed is required.
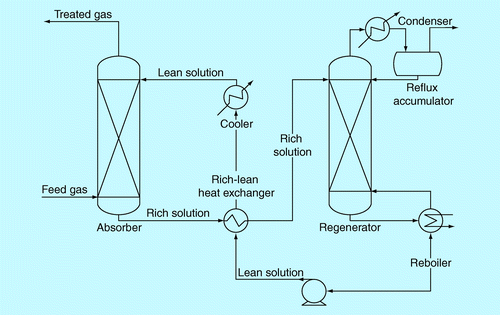
A generalized process diagram of CO2 absorption using aqueous alkanolamine (MEA) solutions is illustrated in . Flue gas from coal firing is first filtered to remove particulate and scrubbed to remove SOx and NOx. The flue gas is fed into the bottom of the absorber column, flowing upward against the aqueous amine solution, where it is introduced to the top of absorber column. CO2 in the flue gas stream rapidly reacts with MEA and remains in the solution. This stream is then called a rich-amine solution. The free CO2 flue gas leaves the solution at the top section of the absorber. The temperature of the rich amine solution, while leaving the absorber, is approximately 60°C. The rich-amine solution exits at the bottom section of the absorber, is heated to a higher temperature in the rich-lean heat exchanger, and then returned to the top section of the stripper. In the stripper, the rich-amine solution is heated by the hot vapor steam from the reboiler at the bottom of the column resulting in gas mixtures of CO2, water and amine. These gas mixtures exit at the top section of the stripper, flow to the condenser and reflux accumulator to recover, and return both water and amine back to the stripper. The aqueous amine solution containing a low concentration of CO2 (called lean-amine solution) at the stripper bottom, now at a solution temperature of approximately 120°C, is pumped and cooled down through the rich-lean heat exchanger before being reintroduced to the absorber to complete the process cycle.
It is suggested that the stripper be operated at temperatures below 120°C to avoid corrosion Citation[16,17]. Types of corrosion, which can be both uniform and localized, include pitting, galvanic, erosion, stress cracking and inter-granular corrosion Citation[4]. Kittel et al. presents the major results of corrosion testing from two pilot plants (coal-fired power station in Esbjerg [Denmark] and natural gas burner at the International Test Centre for CO2 Capture in Regina [Canada], under MEA operation Citation[18]. In addition, the highest corrosion rates were always found in the hottest parts of the unit, including the inlet and outlet of the stripper. Gao et al. also reported that the most serious corrosion occurs at the rich-liquid side outlet of the heat exchanger Citation[19].
Corrosion
Corrosion in CO2 absorption processes is one of the most severe operational problems in gas purification plants Citation[6]. Carbon steel is usually used as plant construction material for absorption and stripping units to reduce investment cost. However, carbon steel is more vulnerable to corrosion than stainless steel. Nevertheless, it is possible that corrosion in amine plants might not be inevitable and could be minimized or controlled. Before any corrosion prevention or protection is performed, it is important to understand the system being operated and the effects of impurities and process parameters on corrosion.
▪ Types of amines
Different amines for CO2 capture yield different degrees of system corrosion. The order of absorption efficiency of amines is MEA > DEA > MDEA, but the degree of corrosiveness is also MEA > DEA > MDEA. Corrosiveness of amines were tested under conditions in ranges of 1–5 kmol/m3 amine concentration, 30–80°C, 0–0.4 CO2 loading, and 0–10% feed gas O2 concentration. For primary amines, the sterically hindered amine of 2-amino-2-methyl-1-propanol (AMP) has slightly less CO2 solubility than MEA, but the corrosion rate of AMP is much higher than that of MEA under the same conditions Citation[20]. AMP’s greater corrosiveness is explained in terms of the preferable formation of HCO3- rather than carbamate, while MEA and DEA favor more stable carbamate formation in the solution. HCO3- is known to be one of corrosive agents responsible for corrosion in an amine system.
For blended amines, including MDEA–DEA, MEA–MDEA, and MEA–AMP, the corrosion rates of mixed amine systems at equal amine mixing ratio and CO2 saturation appear to be a combination of the rates seen in single amine systems. The corrosion behavior of the mixed amine systems for MEA–MDEA and DEA–MDEA also exhibit cathodic and anodic current density values in between those produced in the individual single amines constituting the mixed systems. For MEA–AMP, the corrosion behavior shows only cathodic current. It has been explained that this was probably caused by differences in the amounts of CO2 absorbed under the condition of CO2 saturation Citation[20]. MDEA in a blended system of MEA–MDEA–H2O–CO2 and, in the presence of O2, is preferentially thermally degraded at high temperature and drastically reduces loss of MEA due to degradation. It also decreases the amount of non-environmentally benign degradation products. This allows MEA to be more available for CO2 absorption Citation[21]. Eustaquio-Rincón et al. conducted corrosion experiments using conditions of MDEA, DEA and their various ratio mixtures in a concentration range of 15–60% mass fraction, with and without acid gases, different amounts of H2S and CO2 at a pressure range of 276–5861 kPa and 120°C Citation[22]. The corrosion rate of carbon steel reported after averaged test time of 350 h was determined by the weight loss method in aqueous solutions of individual and blended MDEA and DEA. For a given blended mass ratio, the corrosion rate decreases as the total amine concentration increases in relation to increased amounts of MDEA. The overall corrosion rate was less than 1 mils per year unit (mpy). In the presence of CO2 loading less than 0.35 mol/mol amine and a mass ratio of MDEA to DEA of 3.5/1, the effect of CO2 on corrosion was negligible.
New types of amines have been introduced for CO2 capture in the absorption process, including hetercyclic PZ and its derivatives Citation[23–26]; analog structures of PZ Citation[27]; amino alcohols such as 4-diethylamino-2-butanol Citation[28,29], 2-(isopropylamino)ethanol, 2-(isobutylamino)ethanol, 1-methyl-2-piperidineethanol, and 2-(isopropyl)diethanolamine Citation[30,31]; and sterically hindered amines including AMP, with the intention of increasing absorption and cyclic capacities, which results in a lower circulation rate. This creates energy savings in the CO2 regeneration step as compared with conventional amines Citation[32].
The structure of PZ is a heterocyclic six-member ring containing two secondary amines in the structure. PZ has been found to have similar volatility to MEA but has almost double the CO2 absorption rate and capacity, and is more resistant to oxidative and thermal degradation Citation[25]. A CO2 capture pilot plant using concentrated PZ has also been constructed Citation[33]. The kinetic reaction of PZ and its derivatives with CO2 identified using the stopped-flow technique are in the order of PZ > 2-methyl-PZ > 1-ethyl-PZ > N-(2-hydroxyethyl)-PZ > 1-methyl-PZ Citation[24]. The degradation rates of 1-methyl-PZ and 2-methyl-PZ are faster than PZ under the same conditions at 150°C Citation[27]. For amino alcohols, modification of different chemical structures, including changing an alkyl group relative to the position of an amino group in an alcohol structure, results in a high absorption rate and low heat of reaction when compared with AMP and MDEA Citation[30]. When hydroxyl functions are placed relative to the amino position, some amino alcohols, such as 4-propylamino-2-butanol, show the highest CO2 absorption, and 4-(ethyl-methyl-amino)-2-butanol shows the highest cyclic capacities among the amino alcohols studied in comparison to MEA Citation[28]. These new solvents have a very high potential for application in CO2 absorption processes. However, the physical characteristics, oxidative and thermal degradation, as well as corrosion behavior of these new solvents, must first be studied extensively. Thus, it will take some years for these solvents to be commercialized. Blending the new solvents with existing commercially available amines to improve their CO2 absorption efficiency and reduce operating costs have been reported. However, studies of corrosion on this set of solvents have not been reported.
Several attempts have been made to formulate blended amines in order to overcome the limitations of single amine absorption Citation[19]. The objectives are to increase CO2 capture efficiency, increase CO2 solubility in amine solution Citation[34] and decrease energy requirement in the stripping process Citation[35,36]. MEA–AMP can absorb CO2 with a similar capacity to MEA–MDEA, but at a much higher rate and with higher mass-transfer coefficients Citation[37]. Blended K2CO3-hindered cyclic amine Citation[25], a K2CO3-promoting amine Citation[38], have been claimed to have very low heat absorption, low volatility, and high resistance to thermal and reactive degradation. PZ has been found to rapidly react with CO2 and forms carbamates, which could be used as an effective promoter in existing amine absorption. Extensive study of PZ blends includes MEA–PZ Citation[39,40], MDEA–PZ Citation[41,42], and K2CO3–PZ Citation[23,43]. Blended PZ with MEA for CO2 showed increased absorption 1.5–2.5 times greater than that of MEA alone. The degree of absorption capacity increases in order of MEA–PZ > AMP–PZ or MDEA–PZ and MEA alone Citation[34]. However, PZ not only increases the CO2 absorption capacity, but also increases the corrosion rate. The corrosion rate of carbon steel in the blended MEA–PZ solutions is more corrosive than MEA at the same total concentration and under the same operating conditions. The corrosion rate in the blended MEA–PZ system is more pronounced with increasing total amine concentration, and it also rises with an increase in the ratio of PZ to MEA. Corrosion rates of MEA–PZ system determined under conditions of 5–8.7 kmol/m3 total amine concentration with various mixing ratio, 0.2–0.63 CO2 loading, 0–10.13 kPa feed gas O2 partial pressure, and 80°C (test time was not given) was reported to increase in the presence of O2, CO2 loading and high temperature Citation[39]. also summarizes various corrosion studies conducted using different amines and test conditions.
▪ Heat stable salts
The main problem of solvent loss is due to oxidative and thermal degradation experienced in the absorption process. Amine solvents can be degraded. Degradation products such as carboxylic acids can react with amine to form alkanolamine salts or HSS, which cannot be removed under solvent regeneration conditions and remain in the amine absorption solution throughout the plant. Various kinds of HSS and their negative effects are often reported Citation[21,44–47]. Tanthapanichakoon et al. investigated the effects of HSS (oxalate, formate, malonate, glycolate, succinate and acetate) of various concentration on corrosion of carbon steel and stainless steel using 5 kmol/m3 MEA, 0.20 mol/mol CO2 and 80°C (test time was not provided) Citation[48]. Degrees of corrosiveness varied depending on type and concentration of salt. For carbon steel under the same conditions when different HSS were added, oxalate was the most corrosive (corrosion rate increase 54–64% as compared with the solution without added HSS), followed by malonate (14.7–17.2%), formate (12.2%) and the others (<4%). For stainless steel, the most corrosive oxalate did not reduce the corrosion resistance of stainless steel or have any impact on the corrosion behavior. The corrosion rate of carbon steel in MEA–PZ blends at 80°C, with CO2 loading and in the presence of 1% wt of HSS (acetate, formate, oxalate and thiosulfate), is faster and shows greater degrees of deterioration Citation[39]. Anions of HSS were found to be an oxidizing agent in the corrosion process, which altered the corrosion mechanisms on both the anodic and cathodic sides. The order of corrosiveness was formate, followed by acetate, oxalate and thiosulfate; however, in the presence of O2, acetate was more corrosive than formate.
▪ Process parameters
Most corrosion behavior in all amine systems is sensitive to the variations in type of amines, amine concentration, solution temperature, CO2 loading, and amounts of oxygen, HSS and impurities. The effects of the process parameters are typically studied in any new equipment or system process or in testing any new solvent.
Effect of O2
Coal-fired power plant flue gas is composed of CO2, N2, O2, SO2 and NO2. Considerable efforts have focused on understanding O2-induced degradation of MEA. Dissolved oxygen in liquid solvent experiences high temperature at various locations in the capture plant, including the heat exchanger, stripper and reboiler/reclaimer, and this leads to a higher corrosion rate, especially in these areas Citation[47]. Bello and Idem proposed pathways for the formation of O2 degradation products in MEA solution during CO2 absorption Citation[44]. O2 was not initially present in the feed gas stream, but could be produced as a degradation product. Thus, an oxidative degradation environment could be created without the presence of O2 in the flue gas feed. In the absence of O2, corrosion reactions occur due to iron dissolution (anodic reaction) and reduction of oxidizers (H2O and HCO3- in cathodic reactions), and black slime of FeCO3 was also observed during the experiment Citation[48]. Increasing the O2 partial pressure increased O2 solubility in the solution. In an MEA system with CO2 loading, the corrosion rate increased with increasing O2 concentration under the test conditions of 5–9 kmol/m3 MEA concentration, 0.2–0.55 CO2 loading, 40–80°C, 0–10.13 kPa feed gas O2 partial pressure and 0–2000 rpm solution velocity Citation[49]. Similar effect of O2 concentration was also reported using 1–7 kmol/m3 MEA, 0–100% feed gas O2, 0–204 ppm SO2, 0–0.5 CO2 and 30–80°C Citation[50,51]. Quantitative information (i.e., corrosion rate) usually dependent on operating parameters (e.g., amine type, temperature and feed gas concentrations) was also given for MEA–H2O-CO2-O2-SO2 system to represent the effects of specific variables (i.e., MEA, O2, CO2 and SO2 concentrations and temperature) on corrosion rate of carbon steel Citation[50]. The rate equation is also given as follows:
Equation 7
Chemical analysis of the amine solution by inductively coupled plasma–MS and capillary electrophoresis found an increase of dissolved iron but no change in carbonate/bicarbonate ions in the solution, and analysis of surface of corroding of specimen using a scanning electron microscope–energy dispersive spectrometer showed a relative decrease in Fe and increase in O and C on the specimen surface. The corrosion products proposed were Fe(OH)2, Fe(OH)3 and FeCO3Citation[50,51].
Effect of amine concentration
Most amines show similar behavior with respect to corrosion rate, in that it tends to increase with increasing amine concentration. The corrosion behavior shows a greater impact on current densities on the anodic side. Higher amine concentrations result in larger amounts of absorbed CO2, carbonate/bicarbonate formation and dissolved iron being present in the solution. The increase in carbonate/bicarbonate anion concentration is responsible for the increased corrosiveness of the system Citation[50,51]. It is noted for AMP and amine concentration above 3 kmol/m3, the corrosion rate of AMP gradually deceases as AMP concentration increases. This was explained as being due to the decrease of the hydrolysis of carbamate, resulting in less HCO3- being produced. There is a limitation of amine concentration of 20–30% amine to minimize the corrosion rate, and there have been research attempts to overcome this limitation. For instance, it was reported that there was no MEA degradation when 40% MEA was used with oxidative inhibitors Citation[52].
Effect of CO2
High CO2 absorption capacity of amines is one of the most important parameters in decreasing operating costs of CO2 capture. Increasing the CO2 loading in most systems, however, leads to a greater amount of HCO3- and, consequently, a higher corrosion rate. Higher CO2 loadings result in both higher anodic and cathodic current densities in electrochemical reactions and, thus, higher amounts of HCO3-. The solution pH also becomes more acidic, suggesting increased amounts of H+ or RNH3+ might occur in the solution. Both HCO3- and H+ accelerate the corrosion rate.
Effect of temperature
Temperature is an essential term in the study of kinetic rate of reaction. It shifts many reaction equilibria, including CO2 absorption, carbamate formation, hydrolysis of carbamate, pH of the solution and solubility of chemical species. Raising the solution temperature, therefore, accelerates corrosion reactions Citation[49,50]. A corrosion study conducted at 30–80°C, 0.0–1.0 mol CO2 loading and 1.0–4.0 kmol/m3 AMP concentration showed that for AMP systems operated at a high temperature and lean CO2 loading, the corrosion rate was less than in MEA systems Citation[53]. At elevated temperatures, CO2 solubility of AMP was usually lower than that of MEA, thus a smaller CO2 loading. A decrease in CO2 loading in AMP then produced a smaller concentration of corroding species, particularly bicarbonate (HCO3-), protonated amine (RNH3+), and proton (H+), thus corrosion rates were reduced. On the other hand, an increase of CO2 loading of AMP solution increased the corrosion rate of the carbon-steel material. Based on the polarization curves at different CO2 loadings, the curves exhibited the most change in current density on the cathodic side as compared with a slight change on the anode. This work demonstrated more activity in the cathodic region where reduction normally occurs. This observation led to the conclusion that CO2 loading enhanced the rate of reduction, whereby it provided more oxidizing species (HCO3-, CO32- and H+). The corrosion inhibitors used for CO2 absorption in various amine solutions are summarized in . To our knowledge, no data are available in terms of comparison of temperature effect of different contaminants. Typically, any study is usually conducted to evaluate the effect of parameters of interest in a step-wise manner. It usually compares the effect of temperature on corrosion rates induced by different concentrations of the different species at the same time. An example can be found in a study, which showed the effect of different temperatures on various CO2 loadings Citation[53]. A similar study also applies to effect of O2 on corrosion rates. In the case of SO2, in which very limited studies are available, its effect was only evaluated at 80°C Citation[50]. However, this study proposed a corrosion rate equation, previously given in , which could indicate the effect of temperature on each corrosion species (i.e., MEA, SO2, O2 and CO2).
Effect of SO2
SO2 in flue gas streams arises from coal combustion. Concentration of SO2 is dependent on the quantity of sulfur contained in the original coal and the conditions of the combustion process. High SO2 concentration (>10 ppm) has a marked depolarizing effect on cathodic reactions on polished metal surfaces and also creates low pH in the surface film. Consequently, it generates a low rate of sulfate production and appreciable amounts of tetravalent sulfur (dithionite and S2O4-), which can be reduced to sulfide. Ferrous sulfide is a stable corrosion product.
Although it is present in small amounts, SO2 is more acidic than CO2; its regenerative removal requires the use of weaker bases than primary amines Citation[54]. The strong basicity of primary amines results in an irreversible reaction with SO2 that produces corrosive and HSS, reducing the CO2 absorption rate and capacity of the absorbent. However, it has been reported that the absorption of CO2 in industrial gas streams that contain a typical concentration of SO2 might lead to a loss of only 2 mol of MEA/(mol of SO2) Citation[55]. Thus, very low concentrations of SO2 (<10 ppm) might be desirable in order to avoid excessive loss of costly solvent Citation[56]. So far, the information on the effects of SO2 and NOx on amine degradation and corrosion is scant. Uyanga and Idem have made a comprehensive study of the effects of SO2 and O2 on the degradation of MEA Citation[57]. The effect of SO2 as well as other process parameters (CO2 loading, O2 concentration and temperature) on degradation of MEA was also reported in Citation[13]. The SO2-O2-N2 gas mixture was in contact with aqueous MEA solutions at elevated temperatures. In the absence of CO2, the rate of MEA degradation increased with raising O2 concentration and further increased when SO2 was added, continuing to climb as the SO2 concentration rose. In the presence of CO2, the effects of O2 and SO2 became negligible. Later, Kladkaew et al. investigated the effect of SO2 (0–204 ppm) on corrosion of carbon steel in a similar system and simulated the conditions of absorption–regeneration sections Citation[50,51]. The corrosion rate increased with increasing SO2 due to the increase of hydronium ion formation from the reactions of SO2 and H2O, as well as SO2, O2 and H2O. However, the corrosion rate was slight as compared with the effects of MEA concentration, CO2 loading and operating temperature.
Gao et al. evaluated the influence of SO2 on the corrosion of carbon steel and stainless steel, and degradation of blended amine solvent in a pilot plant Citation[19]. More serious amine degradation and HSS formation occurred with increasing SO2 concentration. In addition, SO2 has been found to play an important role in formation of corrosion protection films.
Corrosion mechanism
Corrosion in amine-treating units is normally quantified using a corrosion rate measured by two major techniques: weight loss and electrochemical measurements. The weight loss technique uses a metal coupon of known weight immersed into the amine solution at predetermined conditions. The weight after a specific time is determined and used to calculate the corrosion rate during the test period. The electrochemical technique measures current densities of the anode and cathode, dictated by the strengths of oxidation (metal dissolution) and reduction (reduction of corroding species) when corrosion occurs. In any technique, the corrosion rate is normally expressed in mpy. In electrochemical measurement, electrochemical polarization consists of an anodic reaction (oxidation of metal or iron dissolution, ) and cathodic reactions (reduction of oxidizing agents, ). Although redox potentials are important, all of the works reviewed did not include the potentials for the reactions; all of the studies proposed a specific system of various amines and corrosion inhibitors. Objectives set by these works were mostly focused on showing corrosiveness, effects of the studied parameters, and how corrosion occurred in the systems. Redox potentials were not used to explain any reported results, thus they were not available from their studies.
Anodic reaction:
Equation 8
Cathodic reaction:
Equation 9
In an aqueous amine-CO2 system, the reaction of amine (primary and secondary) with CO2 yields reversible formations of carbamate and protonated amine as follows:
Formation of carbamate Citation[58]:
Equation 10
In the aqueous amine solution, water as a bulk phase plays an important role in hydrolysis of carbamate.
Hydrolysis of carbamate Citation[59]:
Equation 11
Hydrolysis of bicarbonate:
Equation 12
Under conditions in ranges of 1–5 kmol/m3 amine concentration, 30–80°C, 0–0.4 CO2 loading, and 0–10% feed gas O2 concentration, 3 kmol/m3 amine and 80°C, the corrosion rates of different amines are in order MEA > AMP > DEA > MDEA and for the blended amines (1:1) are MEA–AMP > MEA–MDEA > DEA–MDEA, which the corrosion rates are in between of the amine precursors. The polarization curves of the amines also show higher anodic and cathodic current densities in the same order. The different corrosion rates of the amines are due to the different CO2 absorption capacity, 0.565, 0.554, 0.442 and 0.243 mol/molCO2, for MEA, AMP, DEA and MDEA, respectively. For low CO2 loading, ex 0.2 mol/mol CO2 loading, 3 kmol/m3 amine and 80°C, the corrosion rates are AMP >> MEA ≥ DEA, corresponding to 30, 20 and 19 mpy, respectively. Higher corrosion of AMP is explained due to the presence of HCO3-, corrosive species. Carbamate compound formation of MEA and DEA is stable, while that of AMP is unstable and thus undergone hydrolysis to generate HCO3-Citation[20].
For MEA–PZ system evaluated at 5–8.7 kmol/m3 total amine concentration with various mixing ratio, 0.2–0.63 CO2 loading, 0–10.13 kPa feed gas O2 partial pressure and 80°C (test time was not given), specifically at 0.2 CO2 loading, 80°C, constant total amine 6.2 kmol/m3 (MEA 5 kmol/m3–PZ 1.2 kmol/m3 vs MEA 6.2 kmol/m3), the mixed system (21.79 mpy) is more corrosive than MEA (19.23 mpy). The corrosiveness of PZ is more pronounced when PZ is increased and the evidence is also seen in the increase of the cathodic current densities. Possibly, there was a change in reduction of oxidizing agents in the presence of PZ; for example, metal complex formation Citation[39].
Corrosiveness of HSS can be viewed by polarization. There are also changes in anodic (βa) and cathodic (βc) Tafel slopes. Linear polarization resistance is decreased as HSS concentration increased. For oxalic acid, both anodic and cathodic current densities in the presence of oxalic acid are greater than that of the same system with no acid, implying the change in iron dissolution and chelation of iron. With increasing oxalic acid concentration, βa is decreased, while βc remains unchanged as compared with no acid system, and linear polarization resistance is also decreased. The polarization parameters are more pronounced as the acid concentration greater than 1000 ppm for oxalic and 10,000 ppm for formic and malonic acids Citation[48].
Reviews of mechanisms are mostly relied on analysis of electrochemical polarization and its data. Postulation is based on electrochemistry and changes in polarization plot (potential related to standard electrode versus logarithm of current density) due to what are added and cause change in the environment. There is no confirmation of what definite chemical species are taking part in corrosion or inhibition. Most recent studies report, 13C-NMR was used to determine AMP–protonated AMP (AMP/AMPH+), AMP carbamate (AMPCOO-) and HCO3-/CO32- in 30 wt% aqueous solution of AMP with different amount of CO2 at 25°C Citation[60]. Yamada et al. also used 13C-NMR to demonstrate that the product ratios of carbamate to bicarbonate of MEA are greater than that of AMP Citation[61].
In the amine–CO2 aqueous system, CO2 is bound in the form of carbamate, bicarbonate and carbonate. Increased CO2 loading accelerates the formation of these chemical species.
Equation 13
An increase in CO2 loading also shows a shift of cathodic current density towards a greater value. This also indicates an increase of bicarbonate concentration in the solution, thus enhancing the corrosion rate Citation[50,62]. Bicarbonate as a primary oxidizer in an aqueous MEA solution was also identified as playing an important role in corrosion in a mechanistic corrosion model Citation[62]. Corrosion occurs due to iron dissolution (anodic reaction) and reductions of oxidizers (H2O and HCO3- in cathodic reactions). Insoluble black particles of FeCO3 were also observed Citation[49].
Oxidized iron can be stabilized in the solution and formed into complexes with HSS anions such as formate and acetate Citation[46]:
Equation 14
Complex formation Citation[63]:
Equation 15
where n = 1 to 6. The complexation with HSS anions will increase dissolution of FeCO3 into the solution with increased HSS anion concentration. As demonstrated by Cummings et al., solubility of FeCO3 was significantly increased in the presence of bicine due to the complex formation of iron-hydroxide-bicine Citation[63]. Diamines as degradation products of primary and secondary amines have no effect on corrosion, but can be strong chelators of iron and promote corrosion by CO2.
Similar to the effect of CO2, an increase in amine concentration intensifies the formation of carbamate, bicarbonate and carbonate. Also, increased amine concentration has a more pronounced effect on the anodic side of reactions, which accelerates the corrosion rate, as seen from the increase of carbonate/bicarbonate in the solution. There is also an increase in carbamate formation, as well as protonated amine and carbonate/bicarbonate Citation[64].
It is known that the corrosion rates of individual amines are in the order of AMP > MEA > DEA > MDEA. For sterically hindered amines, the stability of carbamate is greatly reduced when the number of carbon atoms between the amine and alcohol groups increases from two to three, which increases bicarbonate formation Citation[65,66]. It was also noted that the corrosion rate of AMP is higher than that of MEA under the same operating conditions because of increasing bicarbonate. The existence of chemical species of AMP–CO2 system confirmed by 13C-NMR consist of the amine/protonated amine AMP/AMPH+ and the carbamate (AMPCOO-) Citation[60,61]. The carbamate stability constant (on mol fraction basis) was calculated to be 0.47 at 25°C Citation[60]. Reactions responsible for AMP–CO2 absorption are given as follows Citation[60]:
Equation 16
Equation 17
Equation 18
Equation 19
Increased dissolved O2 in solution means there is an increased presence of oxidizer in the solution Citation[1,67] and an increased tendency for iron to be oxidized.
The oxidation–reduction reactions of iron and O2 in the solution:
Equation 20
According to Fontana, Fe(OH)2 identified as a reaction intermediate is usually oxidized further to form a more stable compound such as Fe(OH)3Citation[1]:
Equation 21
SO2 is a stronger acid gas than CO2, quickly solubilizing in aqueous solution and producing hydrogen ions Citation[68].
Equation 22
Equation 23
Equation 24
HSO3-, SO3-, and SO4- can react with protonated amine and form inorganic HSS, which cannot be regenerated by heat under stripping conditions. Hydrogen ions decrease solution pH and also act as an oxidizer in the solution. However, since the reduction of H+ provided by SO2 hydrolysis shown in can be controlled by the strength of the amine as a base, there will be less possibility for free hydrogen ions to react with iron:
In the solution, SO2 can react directly with water and O2Citation[69].
Equation 25
Temperature also has a profound effect on corrosion rate in the SO2-amine system. In the MEA–O2-CO2-SO2 system, as temperature raised to 30, 50 and 80°C, the polarization curve shifted toward greater values of both anodic and cathodic current densities, which corresponded to corrosion rates of 13 mpy, 41 mpy and 161 mpy, respectively Citation[50]. These corrosion rates were measured under conditions of 1–7 kmol/m3 MEA, 0–100% feed gas O2, 0–204 ppm SO2, and 0–0.5 CO2, and 30–80°C; however, test time was not specified.
Corrosion inhibition
Equipment corrosion and solvent loss are the essential problems in amine absorption and represent the most expensive costs in operating an amine system. The causes of corrosion include HSS from oxidation of amine, products from degradation of amine, and contaminants in flue gas and preparation water. It is possible that corrosion can be controlled and prevented, depending upon its route, using such approaches as filtering contaminants in flue gas before feeding it into the absorber unit, use of high purity water in preparation of amine solution, or separating Citation[45] or neutralizing Citation[70] HSS and degradation products to controlled levels. Since the anodic and cathodic reactions occurring during corrosion are mutually dependent, it is possible to reduce the rates of either reaction to reduce the corrosion Citation[1]. Corrosion inhibitors slow corrosion processes by interfering with either the anodic and cathodic reactions or both, and injection of corrosion inhibitors is inexpensive. Chemical inhibitors, however, could accumulate and build up concentration over a period of time, causing a change in solution physical properties. Such changes cause surface tension to decrease when corrosion inhibitor is added in the MEA solution, leading to a foaming problem in the CO2 absorption process Citation[71]. In addition, the effectiveness of chemical inhibitors might be affected by variations in the process parameters. As well as the inhibitor type and concentration, operating temperature can have an impact on different corrosion inhibitors. A study showed that sodium metavanadate (VND) inhibition performance was not affected significantly with temperature change while the opposite was true for undisclosed inhibitors F, G and H Citation[72]. It is important, therefore, to understand corrosion behavior when inhibitors are used.
Many corrosion inhibitors have been developed, patented and commercialized, and can be categorized into two types. The inorganic category includes salts of arsenic, vanadium, copper, cobalt, molybdenum, antimony and stannous Citation[105]. The organic category includes nitro-substituted aromatic acids and its salts and naphthoquinone Citation[106]. Inorganic inhibitors are more favored in practice than organic compounds because of their superior inhibition performance. However, these inorganic corrosion inhibitors are not environmentally friendly; as such inhibitors contain toxic arsenic, antimony, and vanadium. Vanadium compounds, particularly VND, are the most extensively and successfully used in amine treating plants. However, VND has been known to be toxic, as indicated by a much lower lethal dose to rat (10 mg/kg LD50-oral) when compared with MEA solvent Citation[72]. In addition, it also has a detrimental effect to MEA by boosting up the solvent degradation rate during CO2 absorption operation Citation[73]. The attractive, less toxic and more environmentally friendly inorganic corrosion inhibitor sodium molybdate, as indicated by a higher LD50-oral of 4,000 mg/kg Citation[202] is widely used in cooling water systems Citation[74]. This inhibitor shows high performance in inhibiting both uniform and localized corrosion and pitting of ferrous and nonferrous metal Citation[75].
Corrosion inhibition of VND and 2-aminothiophenol organic inhibitor (a good inhibitor for hot K2CO3 system) at various concentrations were compared using National Association of Corrosion Engineers and American Standard for Testing and Materials complied static weight loss methods in a 5 kmol/m3 AMP saturated with mixture of 52% CO2 and 48% air (equivalent to 10% O2) under boiling conditions Citation[76]. To evaluate performance of the inhibitors (i.e.,% protection), a base run without inhibitor was initially conducted to obtain uninhibited corrosion rate, which was later used to compare with inhibited corrosion rates obtained from runs with inhibitors. For the tested period (not provided in this work), system containing 75 ppm VND yielded only 0.1 mpy corrosion rate equivalent to 99.9% protection, as compared with uninhibited system. On the other hand, 2-aminothiophenol failed the screen test due to nonreproduced corrosion data. The authors suggested that 2-aminothiophenol spiked AMP solution could be degraded under heat, CO2 and O2 during a test, which was observed from successive change of solution color from light yellow to purple.
For CO2 separation using aqueous solutions of MEA, the possibility of using three types of low-toxic organic corrosion inhibitors (amines, carboxylic acid and sulfoxide), instead of inorganic heavy metal inhibitors, was investigated and compared with the commercial inorganic inhibitor VND in carbon steel and MEA solution under CO2 saturation Citation[72]. Inhibitors A–F respectively identified as imidazole, PZ, hexamethyleneimine, cyclohexylamine, 2,4-lutidine (or 2,4-dimethylpyridine) and long-chained aliphatic amine are amines that have nitrogen functional groups with different molecular structures, that is, aromatic and long-chain aliphatic. Based on this study, no inhibitors contain changes in both aromatic and aliphatic structures. Either aromatic or aliphatic compounds were tested for corrosion inhibition performance. The role of compound structures (except electron density) was rarely discussed in this study. The inhibition efficiency, shown by percentage of corrosion protection, depended on the inhibitor concentration and system temperature. VND provided the highest percentage of protection of 97% followed carboxylic acid with 92%, sulfoxide and long-chain aliphatic amine respectively. Mechanisms of inhibitors F-H were proposed as providing enhancement of corrosion resistance by suppressing reduction of HCO3- at the cathodic side and adsorption of the inhibitor at the surface of metal. Increase of temperature from 40°C to 80°C had no significant effect on the inhibition performance of VND, in which percentage of protection still remained as high as 97%. However, temperature had a significant impact on the F–H inhibitors, in which it increased percentage of protection of all inhibitors when temperature rose from 40°C to 80°C. For example, percentage corrosion protection of system containing inhibitor G doubled when the temperature was raised from 40°C to 80°C.
The inhibition performance of less toxic copper carbonate (CuCO3) with 1350 mg/kg LD50Citation[203], as compared with vanadium based inhibitors such as VND having LD50 of only 10 mg/kg, was also investigated Citation[77]. Under the tested conditions of 5 kmol/m3 MEA, 0.20 CO2 loading, various CuCO3 concentration, 0–10% feed gas O2 concentration, 0–2000 rpm solution velocity and 40–80°C, the addition of copper carbonate decreased the corrosion rate of carbon steel but might induce pitting corrosion. The inhibition efficiency was found to be greater at 40°C than at 80°C. This was probably due to the nature of the passive film. The inhibition performance was found negligibly affected by solution velocity range (i.e., 0–2000 rpm) used in this study, as observed from no apparent change in both anodic passive current density and cathodic Tafel slopes. Solution velocity only accelerated cathodic reactions by inducing more mass transfer rate of corroding species available for the metal surface. To our knowledge, there are no reports in the reviewed papers in regards to structure/nature of passive films. In real systems, corrosion is often determined by placing metal coupons in different location within the process to determine their weight loss after specific times or using a corrosometer. Determination of corrosion in pilot plants using these techniques can be found in literature Citation[18].
Jovancicevic et al. studied inhibition of N-based surfactant inhibitor of imidazolines and their amide/amine counterparts containing different hydrocarbon chain lengths Citation[78]. The corrosion tests were all conducted in CO2-saturated brine done at 1 atm, 150°C and 6000 rpm rotating speed of electrode using inhibitor concentration in a range of 3–50 ppm. The corrosion rate was measured at steady state after 3–4-h test periods. In this study, contribution of inhibitor structures helps understand inhibitor molecular arrangements (e.g., spherical/nonspherical micelles) which strongly affect their adsorption strength at the metal surface for corrosion protection. These inhibitors function by forming an ordered structure known as micelle (i.e., an aggregate composing of hydrophilic head regions in contact with surrounding solvent while hydrophobic tails pointing into micelle center) to adsorb as monolayer or bilayer (i.e., molecules are arranged into a two-layered sheet with all of their hydrophobic tails pointing toward the center of the sheet and the hydrophilic heads being exposed to surroundings, such as metal surface and solvent) on hydrophilic surfaces of metal surface to give corrosion protection. Bilayer film specifically formed by surfactant inhibitors analyzed in this work could be considered a type of added passive film. Passive film is generally known as a 2–3 nm thick film composed mainly of iron oxides, Fe(OH)2, Fe3O4 and Fe2O3, which adhere to the metal surface. The film functions as a barrier inhibiting oxidation reaction on the anodic side, thus minimizing corrosion of metals. Some inhibitors, such as CuCO3Citation[77] and VND Citation[72] have been found to promote formation of a passive film on the anodic side. The bilayer film also passivates metal by protecting it from being corroded. However, bilayer film is formed and arranged on the metal surface in a special manner. The molecules of the surfactant inhibitor assemble themselves to a two-layered sheet, where all of the hydrophobic tails point toward the center of the sheet and the hydrophilic heads adhere to the metal surface. The adsorption of surfactant inhibitor can protect the metal surface from contact with oxidizing species in the systems (e.g., H+ and HCO3+ in MEA–CO2–H2O system), thus corrosion is minimized.
Contributions of the constituent parts of the hydrophobic hydrocarbon chain lengths and types of hydrophilic head groups (i.e., imidazoline, amide and amine) on the inhibitor structure were discussed. For imidazoline inhibitors, hydrocarbon chain length was found to affect the minimum effective concentration (MEC), a 2 mpy corrosion rate. A decrease in hydrocarbon chain lengths from C20 to C10 or to C8, for the most part, linearly increased the MEC. Corrosion inhibition was unnoticeable when the chain length reached C8. Therefore, higher chain length imidazolines used smaller concentrations than their lower-chained counterparts to achieve maximum corrosion protection or a corrosion free environment (2 mpy), thus making them more effective inhibitors. The study suggested that longer chain length provided a better metal surface adsorption, due to higher cohesive energy and stability of the thicker bilayer as opposed to the small spherical micelle of the shorter chained C8 inhibitors. Inhibitors with amine and amide head groups were found to work more effectively than imidazoline inhibitors of a comparable hydrocarbon chain length. It was reasoned that amine/amide inhibitors were able to form nonspherical micelles at, or nearer to, the metal surface than their imidazoline counterpart, resulting in the formation of more stable admicelle bilayers, thus creating better corrosion protection. Ramachandran and Jovancicevic used molecular modeling to determine the binding of imidazoline and amide on the iron oxide surface Citation[79]. The conceptual model was based on adsorption and bilayer film formation on a metal surface.
Conclusion
Most corrosion studies in amine system have been carried out to evaluate effect of amine type and concentration, temperature, CO2 loading and O2 concentration in the feed gas. More process parameters used in CO2 absorption using aqueous amine should also be determined for their effects on the corrosion, especially effect of other impurities in gas stream such as NOx and inorganic fly ash, which can lead to corrosion. Major degradation products other than HSS such as NH3 and amides are also important species, which should be included in corrosion studies.
Most corrosion failures in amine service are localized corrosion. Thus, a focus should be given to localized corrosion, since it occurs in specific areas and is often undetected until an unexpected or premature failure occurs. Different forms of localized corrosion, namely pitting, erosion, galvanic, stress corrosion and inter-granular should be identified, as well as proper prevention techniques (e.g., use of effective inhibitors and control of system’s potential to passive regions).
More corrosion inhibitors must be screened, and their performances and roles in preventing corrosion in amine system should be evaluated. Evaluation of blends of two or more potential chemicals should be done to evaluate possible synergistic effect to enhance corrosion inhibition. One should also keep in mind that developed inhibitors should not trigger additional problems in the amine plants, such as solvent degradation and foaming. In addition, long-term exposure test using those inhibitors should be carried out to demonstrate their stability and extended inhibiting power.
Advanced analytical tools have been introduced to assist the electrochemical method to determine corrosive species in amine–CO2 systems. These tools, including micro-Raman, should be applied to investigate corrosion species and to study corrosion inhibition of different types of corrosion inhibitors at various process parameters. The challenge is to find a way to use currently available instruments to perform in situ analysis of corrosion species in the vicinity of the electrodes in an amine–CO2 aqueous solution.
Future perspective
As demand for energy increases, there is a growing reliance on coal combustion for power generation. This is leading to ever-increasing CO2 emissions from large, stationary sources. Post-combustion CO2 capture using alkanolamine absorption is considered by many to be one of the major technologies for reducing these emissions. MEA will likely continue to be the most effective solvent in use over the next 5–10 years; during this time, new candidate solvents with their characteristic properties for CO2 absorption have to be developed and commercialized. In this regard, there will be more blending of other solvents with MEA (formulation) to overcome corrosion and other limitations compared with the use of MEA alone. This will complicate the corrosion behavior in amine systems, as every process parameter can also have an influence on corrosion and on the behavior of the various components of the blended amine solution. The effects of impurities such as SO2 and NOx in flue gas on corrosion need also to be included in future research, as there are few studies on SO2 and none on NOx. As seen in this review, a few advanced analytical instruments have been introduced for use in the study of corrosion. There will be more uses developed for state-of-the-art, advanced analytical instruments in order to explicitly uncover the electrochemistry of corrosion in order to identify the chemical species generated and their paths to the corrosion. Eventually methods will emerge that will demonstrate good methods of corrosion prevention and corrosion that can be inhibited in a cost-effective manner.
Table 1. Worldwide CO2 emissions from large sources as reported by the IPCC.
Table 2. Typical concentrations of coal-fired power plant flue gases after SO2 scrubbing.
Table 3. Summary of various corrosion studies conducted with different amines and test conditions.
Table 4. Various corrosion inhibitor studies of different amine systems.
Electrochemical process involving the transfer of electrons in oxidation–reduction reaction within the construction material, resulting in its deterioration.
Chemical additives added to a process to decrease corrosion rate of construction materials, such as metal or alloy.
Technique used to determine corrosion rate and behavior of a system of interest, based on plot of its potential and current densities, generated on anode and cathode during the test.
▪ Alkanolamine absorption has played a major role in acid-gas removal, both past and present. | |||||||||||||||||||||||
▪ There are many activities using amine absorption in capturing CO2 from power plant coal-fired flue gas. | |||||||||||||||||||||||
▪ Corrosion is one of the drawbacks of capturing CO2 using amine absorption:
| |||||||||||||||||||||||
▪ Generation of corrosion mechanisms is based on information from advanced analysis, using 13C-NMR, inductively coupled plasma–MS, scanning electron microscope and energy-dispersive spectrometers, assisting the electrochemical methods to measure chemical species in identification of corrosion products. | |||||||||||||||||||||||
▪ Corrosion in alkanolamine systems cannot be avoided: mixed anodic and cathodic inhibitors are recommended to change oxidative–reductive reaction behavior of potential corrosive species. | |||||||||||||||||||||||
Financial & competing interests disclosure
The authors would like to acknowledge the research support over recent years to the International Test Centre by the followings organizations: Natural Sciences and Engineering Research Council of Canada, Canada Foundation for Innovation, Saskatchewan Ministry of Energy & Resources, Western Economic Diversification, EnCana Energy Inc., EON Energy, RWE Corp, Saudi Aramco, Doosan Heavy Industries, HTC Purenergy Inc. Saskatchewan Power Corporation, StatOil Hydro (Norway), SaskFerco Inc., Sulzer Chemtech (Switzerland), Fluor Corporation (USA), the Canada Centre for Mineral and Energy Technology, Alberta Energy Research Institute and the Research Institute of Innovative Technology for the Earth. In addition, the authors would also like to acknowledge the recent research supports from Provincial Government of Hunan, Federal Government of China, as well as Hunan University to the Joint International Center for CO2 Capture and Storage. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Fontana MG. Corrosion Engineering (3rd Edition). McGraw-Hill Book Co., NY, USA (1986).
- Kelly RG, Scully JR, Shoesmith DW, Buchheit RG. Electrochemical Techniques in Corrosion Science and Engineering. Marcel Dekker Inc., NY, USA (2003).
- Polasek J, Bullin JA. Selecting amines for sweetening units. Presented at: GPA Regional Meeting. Tulsa, OK, USA, September 1994.
- DuPart MS, Bacon TR, Edwards DJ. Part 1: understanding corrosion in alkanolamine gas treating plants: proper mechanism diagnosis optimizes amine operations. Hydrocarb. Process. Int. Ed.72,75–80 (1993).
- DuPart MS, Bacon TR, Edwards DJ. Part 2: understanding corrosion in alkanolamine gas treating plants: proper mechanism diagnosis, optimizes amine operations. Hydrocarb. Process. Int. Ed.72,89–94 (1993).
- Kohl AL, Nielsen RB. Gas Purification (Fifth Edition). Gulf Publishing Co., Houston, TX, USA (1997).
- Rooney PC, Dupart MS. Corrosion in alkanolamine plants: causes and minimization. Presented at: Corrosion 2000: NACE International Annual Conference and Exposition. Orlando, FL, USA, 26–31 March 2000.
- Versteeg GF, Vanswaaij WP. On the kinetics between CO2 and alkanolamines both in aqueous and non-aqueous solutions-II. Tertiary amines. Chem. Eng. Sci.43(3),587–591 (1988).
- Wilson M, Tontiwachwuthikul P, Chakma A et al. Test results from a CO2 extraction pilot plant at boundary dam coal-fired power station. Energy29(9–10),1259–1267 (2004).
- Astarita G, Savage D, Bisio A. Gas Treating With Chemical Solvents. J Wiley & Sons, NY, USA (1983).
- Chakma A, Mehrotra AK, Nielsen B. Comparison of chemical solvents for mitigating CO2 emissions from coal-fired power plants. Heat Recov. Syst. CHP15(2),231–240 (1995).
- Xu X, Song C, Wincek R, Andresen JM, Miller BG, Scaroni AW. Separation of CO2 from power plant flue gas using a novel CO2 ‘molecular basket’ adsorbent. Fuel Chem. Div. Preprints48(1),162–163 (2003).
- Supap T, Idem R, Tontiwachwuthikul P, Saiwan C. Kinetics of sulfur dioxide and oxygen induced degradation of aqueous monoethanolamine solution during CO2 absorption from power plant flue gas streams. Int. J. Greenhouse Gas Control3(2),133–142 (2009).
- Closmann F, Rochelle GT. Degradation of aqueous methyldiethanolamine by temperature and oxygen cycling. Energy Procedia4,23–28 (2011).
- Nielsen RB, Hansen DA, Lewis KR. Corrosion in refinery amine systems. Presented at: Corrosion 1995: NACE International Annual Conference and Exposition. Orlando, FL, USA, 26–31 March 1995.
- Chakma A, Meisen A. Corrosivity of diethanolamine solutions and their degradation products. Ind. Eng. Chem. Prod. Res. Dev.25(4),627–630 (1986).
- Davis J, Rochelle GT. Thermal degradation of monoethanolamine at stripper conditions. Energy Procedia1,327–333 (2009).
- Kittel J, Idem RO, Gelowitz D, Tontiwachwuthikul P, Parrain G, Bonneau A. Corrosion in monoethanolamine units for CO2 capture: pilot plant studies. Energy Procedia1,791–797 (2009).
- Gao J, Wang S, Zhou S, Zhao B, Chen C. Corrosion and degradation performance of novel absorbent for CO2 capture in pilot-scale. Energy Procedia4,1534–1541 (2011).
- Veawab A, Tontiwachwuthikul P, Chakma A. Corrosion behavior of carbon steel in the CO2 absorption process using aqueous amine solutions. Ind. Eng. Chem. Res.38(10),3917–3924 (1999).
- Lawal O, Bello A, Idem RO. The role of N-methyldiethanolamine in preventing the oxidative degradation of CO2 loaded and concentrated aqueous monoethanolamine blends during CO2 absorption from flue gases. Ind. Eng. Chem. Res.44(6),1874–1896 (2005).
- Eustaquio-Rinco´n R, Rebolledo-Libreros ME, Trejo A, Molnar R. Corrosion in aqueous solution of two alkanolamines with CO2 and H2S: N-methyldiethanolamine + diethanolamine at 393 K. Ind. Eng. Chem. Res.47(14),4726–4735 (2008).
- Bishnoi S. Rochelle GT. Absorption of carbon dioxide into aqueous piperazine: reaction kinetics, mass transfer and solubility. Chem. Eng. Sci.55(22),5531–5543 (2000).
- Rayer AV, Sumon KZ, Henni A, Tontiwachwuthikul P. Kinetics of the reaction of carbon dioxide (CO2) with cyclic amines using the stopped-flow technique. Energy Procedia4,140–147 (2011).
- Freeman SA, Dugas R, Van Wagener DH, Nguyen T, Rochelle GT. Carbon dioxide capture with concentrated aqueous piperazine. Int. J. Greenhouse Gas Control4(2),119–124 (2010).
- Yoon Y, Nam S, Jung S, Kim Y. K2CO3/hindered cyclic amine blend (SEFY-1) as a solvent for CO2 capture from various industries. Energy Procedia4,267–272 (2011).
- Freeman SA. Rochelle GT. Thermal degradation of piperazine and its structural analogs. Energy Procedia4,43–50 (2011).
- Maneeintr K, Idem RO, Tontiwachwuthikul P, Wee AGH. Synthesis, solubilities, and cyclic capacities of amino alcohols for CO2 capture from flue gas streams. Energy Procedia1,1327–1334 (2009).
- Maneeintr K, Idem RO, Tontiwachwuthikul P, Wee AGH. Comparative mass transfer performance studies of CO2 absorption into aqueous solutions of DEAB and MEA. Ind. Eng. Chem. Res.49(6),2857–2863 (2010).
- Chowdhury FA, Okabe H, Yamada H, Onoda M, Fujioka Y. Synthesis and selection of hindered new amine absorbents for CO2 capture. Energy Procedia4,201–208 (2011).
- Robinson K, McCluskey A, Attalla M. The effect of molecular structural variations has on the CO2 absorption characteristics of heterocyclic amines. Energy Procedia4,224–231 (2011).
- Zhang P, Shi Y, Wei, Zhao W, Qing Y. Regeneration of 2-amino-2-methyl-1-propanol used for carbon dioxide absorption. J. Environ. Sci.20(1),39–44 (2008).
- Plaza JM., Rochelle GT. Modeling pilot plant results for CO2 capture by aqueous piperazine. Energy Procedia4,1593–1600 (2011).
- Tan C, Chen J. Absorption of carbon dioxide with piperazine and its mixtures in a rotating packed bed. Sep. Purif. Technol.49(2),174–180 (2006).
- Zhang J, Agar X, Zhang DW, Geuzebroek F. CO2 absorption in biphasic solvents with enhanced low temperature solvent regeneration. Energy Procedia4,67–74 (2011).
- Hartono A, Hoff KA, Mejdell T, Svendsen HF. Solubility of carbon dioxide in aqueous 2.5 M of diethylenetriamine (DETA) solution. Energy Procedia4,179–186 (2011).
- Bhattacharya S, Aroonwilas A, Veawab A. Kinetics of carbon dioxide absorption into aqueous solutions of monoethanolamine solution of monoethanolamine (MEA)-2-amino-2-methyl-1-propanol (AMP) blends under industrial conditions. Presented at: The 10th International Conference on Greenhouse Gas Technologies. Amsterdam, The Netherlands, 19–23 September 2010.
- Behr P, Maun A, Deutgen K, Tunnat A, Oeljeklaus G, Görner K. Kinetic study on promoted potassium carbonate solutions for CO2 capture from flue gas. Energy Procedia4,85–92 (2011).
- Nainar M, Veawab A. Corrosion in CO2 capture process using blended monoethanolamine and piperazine. Ind. Eng. Chem. Res.48(20),9299–9306 (2009).
- Dang H, Rochelle GT. CO2 Absorption rate and solubility in monoethanolamine/piperazine/water. Sep. Sci. Technol.38(2),337–357 (2003).
- Zhao B, Sun Y, Yuan Y et al. Study on corrosion in CO2 chemical absorption process using amine solution. Energy Procedia4,93–100 (2011)
- Bishnoi S, Rochelle GT. Absorption of carbon dioxide in aqueous PZ/MDEA, AIChE J.48(12),2788–2799 (2002).
- Cullinane JT, Rochelle GT. Carbon dioxide absorption with aqueous K2CO3 promoted by piperazine. Chem. Eng. Sci.59(17),3619–3630, (2004).
- Bello A, Idem RO. Pathways for the formation of products of the oxidative degradation of CO2-loaded concentrated aqueous monoethanolamine solutions during CO2 absorption from flue gases. Ind. Eng. Chem. Res.44(4),945–969 (2005).
- Verma N, Verma A. Amine system problems arising from heat stable salts and solutions to improve system performance. Fuel Pro. Technol.90(4),483–489 (2009).
- Rooney PC, DuPart MS, Bacon TR. Effect of heat stable salts on solution corrosivity of MDEA-based alkanolamine plants. Presented at: The 48th Annual Laurance Reid Gas Conditioning Conference. Norman, OK, USA, 2–5 March 1997.
- Bosen SF, Bedell SA. The relevance of bicine in the corrosion of amine gas treating plants. Presented at: Corrosion 2004: NACE International Annual Conference and Exposition. New Orleans, LA, USA, 28 March–1 April 2004.
- Tanthapanichakoon W, Veawab A, McGarvey B. Electrochemical investigation on the effect of heat-stable salts on corrosion in CO2 capture plants using aqueous solution of monoethanolamine. Ind. Eng. Chem. Res.45(8),2586–2593 (2006).
- Soosaiprakasam IR, Veawab A. Corrosion and polarization behavior of carbon steel in MEA-based CO2 capture process. Int. J. Greenhouse Gas Control2(4),553–562 (2008).
- Kladkaew N, Idem RO, Tontiwachwuthikul P, Saiwan C. Corrosion behavior of carbon steel in the monoethanolamine-H2O-CO2-O2-SO2 system: products, reaction pathways, and kinetics. Ind. Eng. Chem. Res.48(23),10169–10179 (2009).
- Kladkaew N, Idem RO, Tontiwachwuthikul P, Saiwan C. Corrosion behavior of carbon steel in the monoethanolamine-H2O-CO2-O2-SO2 system. Ind. Eng. Chem. Res.48(19),8913–8919 (2009).
- Delfort B, Carrette PL, Bonnard L. MEA 40% with improved oxidative stability for CO2 capture in post-combustion. Energy Procedia4,9–14 (2011).
- Veawab A, Tontiwachwuthikul P, Chakma A. Influence of process parameters on corrosion behavior in a sterically hindered amine-CO2 system. Ind. Eng. Chem. Res.38(1),310–315 (1999).
- Bonenfant D, Mimeault M, Hausler R. Estimation of the CO2 absorption capacities in aqueous 2-(2-Aminoethylamino) ethanol and its blends with MDEA and TEA in the presence of SO2. Ind. Eng. Chem. Res.46(26),8968–8971 (2007).
- Rao AB, Rubin ES. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol.36(20),4467–4475 (2002).
- Chávez RH, Guadarrama J de J, Klapp J. CO2 capture for atmosphere pollution reduction. In: Towards a Cleaner Planet: Energy for the Future. Chávez RH, Guadarrama JJ, Klapp J (Eds). Springer, Berlin, Germany (2007).
- Uyanga IJ, Idem RO. Studies of SO2- and O2-induced degradation of aqueous monoethanolamine during CO2 capture from power plant flue gas streams. Ind. Eng. Chem. Res.46(8),2558–2566 (2007).
- Danckwerts PV, McNeil KM. The absorption of carbon dioxide into aqueous amine solutions and the effect of catalysis. Trans. Inst. Chem. Eng.45,T32 (1967).
- Chakraborty AK, Astarita, G, Bischoff KB. CO2 absorption in aqueous solutions of hindered amines. Chem. Eng. Sci.41(4),997–1003 (1986).
- Ciftja AF, Hartono A, Da Silva EF, Svendsen HF. Study on carbamate stability in the AMP/CO2/H2O system from 13C-NMR spectroscopy. Energy Procedia4,614–620 (2011).
- Yamada H. Matsuzaki Y, Okabe H, Shimizu S, Fujioka Y. Quantum chemical analysis of carbon dioxide absorption into aqueous solutions of moderately hindered amines. Energy Procedia4,133–139 (2011).
- Veawab A, Aroonwilas A. Identification of oxidizing agents in aqueous amine–CO2 systems using a mechanistic corrosion model. Corros. Sci.44(5),967–987 (2002).
- Cummings AL, Waite SW, Nelsen DK. Corrosion and corrosion enhancers in amine systems. Presented at: The Brimstone Sulfur Conference. Banff, AB, Canada, April 2005.
- Heuer JK, Stubbins JF. An XPS characterization of FeCO3 films from CO2 corrosion. Corros. Sci.41(7),1231–1243 (1999).
- Singh P, Da Silva EF, Brilman DWF. Determination of structural effects on carbamate stability for various amine based solvents by using ab initio method. Presented at: The 10th International Conference on Greenhouse Gas Technologies. Amsterdam, The Netherlands, 19–23 September 2010.
- Da Silva EF, Svendsen HF. Study of the carbamate stability of amines using Ab Initio methods and free-energy perturbations. Ind. Eng. Chem. Re.45(8),2497–2504 (2006).
- CRC Press. Handbook of Chemistry and Physics (65th Edition). Weast RC (Ed.). CRC Press, FL, USA (1984).
- Beutler D, Renon H. Representation of NH3-H2S-H2O, NH3-CO2- H2O, and NH3-SO2-H2O vapor-liquid equilibria. Ind. Eng. Chem. Process Des. Dev.17(3),220–230 (1978).
- Sastri VS. Corrosion Inhibitors. John Wiley & Sons, Chichester, UK (2001).
- Liu HJ, Dean JW. Neutralization technology to reduce corrosion from heat-stable amine salts. Presented at: Corrosion 1995: NACE International Annual Conference and Exposition. Orlando, FL, USA, 26–31 March 1995.
- Thitakamol B, Veawab A. Foaming behavior in CO2 absorption process using aqueous solutions of single and blended alkanolamines. Ind. Eng. Chem. Res.4(21),216–225 (2008).
- Veawab A, Tontiwachwuthikul P, Chakma A. Investigation of low-toxic organic corrosion inhibitors for CO2 separation process using aqueous monoethanolamine solvent. Ind. Eng. Chem. Res.40(22),4771–4777 (2001).
- Bello A, Idem RO. Comprehensive study of the kinetics of the oxidative degradation of CO2 loaded and concentrated aqueous monoethanolamine (MEA) with and without sodium metavanadate during CO2 absorption from flue gases. Ind. Eng. Chem. Res.45(8),2569–2579 (2006).
- Eldin AMS, Wang LF. Mechanism of corrosion inhibition by sodium molybdate. Desalination107(1),29–43 (1996).
- Haddad F, Benchettara A, Amara SE, Kesri R. Cobalt and molybdate ions effects on the passivation of iron based FeCoC ternary alloys, in non deaerated solution of 10–13 M NaHCO3 + 10–13 M Na2SO4, at 25°C. Portugaliae Electrochimica Acta26,181–198 (2008).
- Veawab A, Tontiwachwuthikul P, Bhole SD. Studies of corrosion and corrosion control in a CO2–2-amino-2-methyl-1-propanol (AMP) environment. Ind. Eng. Chem. Res.36(1),264–269 (1997).
- Soosaiprakasam I, Veawab, A. Inhibition performance of copper carbonate in CO2 absorption process using aqueous MEA. Presented at: Corrosion 2007: NACE International Annual Conference and Exposition. Nashville, TN, USA, 11–15 March 2007.
- Jovancicevic V, Ramachandran S, Prince P, Petrolite B. Inhibition of CO2 corrosion in mild steel by imidazolines and their precursors. Presented at: Corrosion 1998: NACE International Annual Conference and Exposition. San Diego, CA, USA, 22–27 March 1998.
- Ramachandran S, Jovancicevic V, Petrolite B. Molecular modeling of the inhibition of mild steel carbon dioxide corrosion by imidazolines. Presented at: Corrosion 1998: NACE International Annual Conference and Exposition. San Diego, CA, USA, 22–27 March 1998.
▪ Patents
- Shrikar C, Gupta A. US6174506 (2001).
- Oil And Natural Gas Corporation Limited. WO084503 (2008).
- Khusnutdinov RA, Laptev AB, Bugai DE, Shchepetov AE. RU2365584 (2009).
- Abdrakhmanov IB, Mustafin RN, Chernova AG, Chernova VA, Gataullin RR. RU2353708 (2009).
- Mago BF, West CW. US3959170 (1976).
- McCullough JG, Barr KJ. US4502979 (1985).
▪ Websites
- European Commission research and innovation. http://ec.europa.eu/research/energy/nn/nn_rt/nn_rt_co/article_1150_en.htm
- Oxford University. The physical and theoretical chemistry laboratory. http://msds.chem.ox.ac.uk/SO/sodium_molybdate.html
- ScienceLab.com. Chemical and laboratory equipment. www.sciencelab.com/msds.php?msdsId=9923591