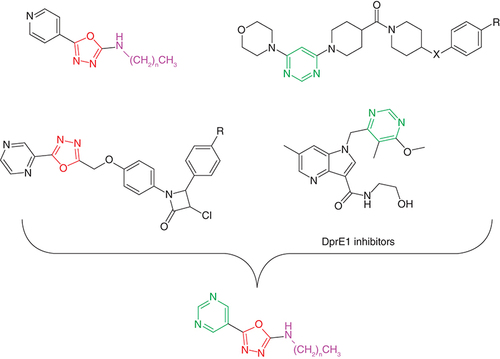
Abstract
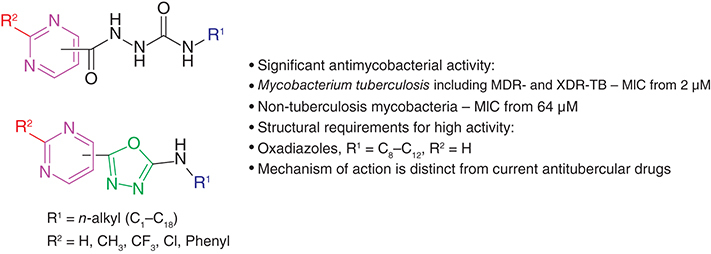
Background: Molecular hybridization and isostery are proven approaches in medicinal chemistry, and as such we used them to design novel compounds that we investigated as potential antimycobacterials to combat drug-resistant strains. Methods & results: Prepared N-alkyl-2-(pyrimidine-5-carbonyl)hydrazine-1-carboxamides were cyclized to N-alkyl-5-(pyrimidin-5-yl)-1,3,4-oxadiazol-2-amines along with their analogues. A total of 48 compounds were tested against Mycobacterium tuberculosis H37Rv, Mycobacterium avium and Mycobacterium kansasii, with oxadiazoles and C8–C12 alkyls being the most effective from a concentration of 2 μM. Multidrug-resistant strains were inhibited at same concentrations as the susceptible strain. For the most potent N-dodecyl-5-(pyrimidin-5-yl)-1,3,4-oxadiazol-2-amine, the mechanism of action related to cell wall biosynthesis was investigated. Conclusion: Pyrimidine-1,3,4-oxadiazole hybrids are unique antimycobacterial agents inhibiting mainly M. tuberculosis strains without cross-resistance to current drugs and are thus promising drug candidates.
Graphical Abstract
Tuberculosis (TB) – a transmissible disease caused by Mycobacterium tuberculosis complex – is, together with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the leading causes of mortality from a single infectious agent. Their symptoms and incidence also overlap to some extent. Although TB is curable, its management requires a long period and compliance with a treatment regimen by patients and healthcare professionals. It is estimated that approximately one-quarter of the global population is infected with Mycobacterium tuberculosis in latent form i.e., without any signs of active disease. However, it can be activated under a variety of relatively common conditions [Citation1,Citation2].
A crucial complication of TB treatment is development of an acquired resistance, in some cases even during treatment. M. tuberculosis strains resistant to initially very potent anti-TB drugs have been identified. Multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB forms have been defined as follows: MDR-TB is insusceptible to both isoniazid (INH) and rifampicin (RIF). According to the updated definition, XDR-TB also includes, in addition, resistance to any fluoroquinolone (FQ) and at least one group A drug (linezolid or bedaquiline) [Citation3].
Other species of mycobacteria with a significant pathogenic potential for humans are also known and increasingly recognized as causative agents of many infections (pulmonary, skin, joint, pleural, etc.). These nontuberculous mycobacteria (NTM) are ubiquitous pathogens that cause mycobacterioses, particularly in immunocompromised patients [Citation4,Citation5].
Treatment of NTM is complicated, due to their intrinsic and acquired resistance. Treatment is based on concomitant administration of multiple antimycobacterial drugs to which the causative strains are (or should be) susceptible, usually one of the rifamycins and/or macrolides (clarithromycin, azithromycin), ethambutol (EMB), FQ, amikacin (AMK) or streptomycin (STM); rarely INH [Citation4,Citation5]. It is therefore indisputable that the development of new antimycobacterial drugs, especially against resistant strains, is necessary and of a critical importance [Citation6].
Five- and six-membered nitrogen heterocycles are often present in both established (INH, RIF, pyrazinamide, FQ, delamanid, bedaquiline, linezolid, etc.) and new low-molecular-weight antimycobacterial agents. The antitubercular activity of pyrimidine derivatives has recently been reviewed [Citation7]. This heterocycle appears in many naturally occurring compounds and antimycobacterial pyrimidine derivatives have exhibited diverse mechanisms of action (MoA) unrelated to established drugs [Citation7,Citation8]. One such innovative MoA is inhibition of decaprenylphosphoryl-β-D-ribose 2′-epimerase (DprE1), which is involved in the biosynthesis of arabinogalactan, an essential component of the mycobacterial cell wall. This flavoprotein represents a validated target for development of anti-TB drugs [Citation7,Citation9]. From an alternative point of view, pyrimidine may serve as an isoster of both pyridine (present in INH) and pyrazine (e.g., from pyrazinamide). Among others, 2,5-disubstituted 1,3,4-oxadiazoles have been described as efficient antitubercular agents with various MoAs; for example, see [Citation10–14], and also reviewed in depth in [Citation15–17]. Alkylamine groups linked to a heteroaromatic cycle have also been successfully utilized in the development of potential antimycobacterial agents [Citation11,Citation18].
On the basis of the facts presented here, using molecular hybridization approach, the aim of this strategy is to combine two (or more) molecules (or key fragments thereof) into a new, single chemical entity. It has proven to be successful, for example, in drug candidates to treat cancer and parasitic and neurodegenerative diseases [Citation19]. Considering pyrimidine as a pyridine isostere (proven, e.g., for kinase inhibitors [Citation20]), we combined pyrimidine, 1,3,4-oxadiazole and alkylamine scaffolds () into a single entity in order to design novel antimycobacterial agents. To the best of our knowledge, these ‘triple’ hybrid molecules have been published here for the first time, both in terms of synthesis and antimycobacterial activity. Alternatively, these compounds can be considered as isosters of previously reported INH-based 5-(pyridin-4-yl)-1,3,4-oxadiazol-2-amines [Citation11]. In addition, we also investigated their synthetic precursors, positional isomers and substituted derivatives.
The validity of this choice has been previously supported by successful pyrimidine-1,3,4-oxadiazole hybrids designed for different biological activity: anticancer 2-[(5-phenyl-1,3,4-oxadiazol-2-yl)thio]-N-(4-phenylpyrimidin-2-yl) acetamides with thioacetamide linkers [Citation21] or dual antiviral/anticancer furo[2,3-d]pyrimidin-2-one-1,3,4-oxadiazoles with a methylene bridge on the pyrimidine nitrogen [Citation22].
Material & methods
Chemistry
General
All chemicals were purchased from Merck KGaA (Darmstadt, Germany), Acros Organics B.V.B.A. (Geel, Belgium), Avantor (Stříbrná Skalice, Czech Republic), Penta Chemicals Unlimited (Prague, Czech Republic) and Fluorochem (Dublin, Ireland), were used as supplied. 1H and 13C NMR spectra were measured in dimethyl sulfoxide (DMSO)-d6 or acetone-d6 at ambient or higher temperature with a Varian VNMR S500 (500 MHz for 1H and 126 MHz for 13C; Varian, CA, USA) or a JEOL JNM-ECZ 600R (600 MHz for 1H and 151 MHz for 13C; JEOL Ltd., Tokyo, Japan). Chemical shifts (δ) are reported in parts-per-million (ppm) and are referenced internally by the residual signals of solvents (DMSO-d6: 2.50 for 1H, 39.70 for 13C; acetone-d6: 2.09 for 1H, 30.60 and 205.87 for 13C). Infrared (IR) spectra were acquired on a Nicolet 6700 FT-IR spectrometer (Thermo Fisher Scientific, MA, USA) using the attenuated total reflectance (ATR) technique on a Ge crystal (600–4000 cm-1). Elemental analysis was conducted on a Vario MICRO Cube Element Analyzer (Elementar Analysensysteme, Hanau, Germany). Calculated and detected values are given as percentages. Melting points (mp) were recorded using a Büchi B-545 apparatus (BÜCHI Labortechnik AG, Flawil, Switzerland). Retention factors (Rf) of compounds and progress of reactions were analyzed by thin-layer chromatography (TLC); TLC plates were coated with 0.2 mm silica gel Merck 60 F254 (Merck KGaA) and visualized by UV light (254 nm). For column chromatography, the Merck Kieselgel 60 Å (0.040–0.063 mm; Merck KGaA) was used. A mixture of dichloromethane and methanol (93:7, v/v) was used as the mobile phase. Calculated logP values (ClogP), the logarithms of partition coefficients for octan-1-ol/water, and chemical drawings were acquired using ChemDraw Professional 20.0 (PerkinElmer Inc., MA, USA).
NMR and IR spectra of the compounds are presented in supplemental information.
Synthesis
Synthesis of pyrimidine-5-carbohydrazide (2)
2 mmol (276.0 mg) of commercially available methyl pyrimidine-5-carboxylate were placed to a small round-bottomed flask and dissolved in 5 ml of methanol. Then three equivalents (6 mmol, 300.0 mg, 291 μl) of hydrazine monohydrate were added in one portion. The reaction mixture was allowed to react for 3 h at room temperature (the reaction time should not be exceeded). Liquid was evaporated on a rotary vacuum evaporator. Traces of water were separated by azeotropic distillation with anhydrous acetonitrile (2 × 2 ml). The desired compound 2 was obtained as pale yellow oil with quantitative conversion (according to TLC) and was immediately used for the following reaction sequence.
The substituted analogues (2-methyl-, 2-trifluoromethyl-, 2-phenyl-, 2-chloro-pyrimidine-5-carbohydrazides) and isomeric pyridimine-2-carbohydrazide were prepared from commercially available precursors (acids, esters, acyl chlorides) and their preparation was followed analogously.
Synthesis of N-alkyl-2-(pyrimidine-5-carbonyl)hydrazine-1-carboxamides & their analogues (3a–3w)
A total of 2 mmol (276.0 mg) of freshly prepared pyrimidine-5-carbohydrazide 2 was dissolved in 15 ml of anhydrous acetonitrile. Then 1.1 equivalents (2.2 mmol) of an appropriate alkyl isocyanate were added at once. The reaction mixture was heated under reflux for 2 h. The hot reaction mixture was filtered through a glass vacuum suction filter and the filtrate was cooled to -20°C. The formed precipitate was filtered, washed with a small amount of acetonitrile and dried to give pure hydrazinecarboxamides.
Methyl, tridecyl, pentadecyl and heptadecyl isocyanates are not commercially available. They were prepared from the corresponding acyl chlorides (in some cases prepared in house by treatment of carboxylic acid with thionyl chloride and a catalytic amount of N,N-dimethylformamide under heating) and sodium azide by Curtius rearrangement [Citation23].
Yellowish solid. Yield 35%. Mp 199–200°C. 1H NMR (600 MHz, acetone-D6) δ 9.79 (1H, s, Ar-CO-NH-NH), 9.26 (1H, s, Ar-CO-NH-NH), 9.19 (1H, s, H2), 7.32 (2H, s, H4, H6), 6.23 (1H, s, CO-NH), 2.67 (3H, d, J = 4.6 Hz, CH3). 13C NMR (151 MHz, acetone-D6) δ 163.89, 160.60, 156.10, 156.03, 102.12, 25.72. IR (ATR): 632, 718, 763, 903, 929, 1035, 1137, 1171, 1266, 1329, 1412, 1465, 1527, 1561, 1582, 1644, 1666, 1698, 2917, 2934, 3263, 3391 cm-1. Elemental analysis for C7H9N5O2 (195.18) calculated C, 43.08; H, 4.65; N, 35.88, found C, 43.45; H, 5.01; N, 35.51. Rf = 0.1.
White solid. Yield 72%. Mp 168–169°C. 1H NMR (600 MHz, acetone-D6) δ 10.00 (1H, s, Ar-CO-NH-NH), 9.24 (1H, s, Ar-CO-NH-NH), 9.18 (1H, s, H2), 7.53 (2H, s, H4, H6), 6.38 (1H, t, J = 5.9 Hz, CO-NH), 3.15 (2H, qd, J = 7.1, 5.7 Hz, CH2-CH3), 1.03 (3H, t, J = 7.1 Hz, CH2-CH3). 13C NMR (151 MHz, acetone-D6) δ 163.72, 160.56, 158.39, 156.04, 126.68, 34.57, 14.92. IR (ATR): 632, 646, 718, 735, 902, 1038, 1145, 1188, 1231, 1282, 1315, 1343, 1410, 1462, 1512, 1560, 1584, 1631, 1664, 1690, 2981, 3216, 3321 cm-1. Elemental analysis for C8H11N5O2 (209.21) calculated C, 45.93; H, 5.30; N, 33.48, found C, 46.32; H, 5.60; N, 33.40. Rf = 0.1.
Yellowish solid. Yield 73%. Mp 161–162°C. 1H NMR (600 MHz, DMSO-D6) δ 10.37 (1H, s, Ar-CO-NH-NH), 9.31 (1H, s, H2), 9.16 (2H, s, H4, H6), 7.93 (1H, s, Ar-CO-NH-NH), 6.60 (1H, s, CO-NH), 2.95 (2H, dt, J = 7.6, 6.1 Hz, CH2-CH2-CH3), 1.40–1.33 (2H, m, CH2-CH2-CH3), 0.79 (3H, t, J = 7.4 Hz, CH2-CH2-CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.73, 160.85, 158.56, 156.65, 127.04, 41.52, 23.58, 11.77. IR (ATR): 637, 726, 913, 1043, 1144, 1196, 1263, 1346, 1415, 1563, 1584, 1647, 1681, 2877, 2969, 3048, 3224, 3342 cm-1. Elemental analysis for C9H13N5O2 (223.24) calculated C, 48.42; H, 5.87; N, 31.37, found C, 48.58; H, 5.71; N, 31.52. Rf = 0.1.
Yellow solid. Yield 84%. Mp 149–150°C. 1H NMR (600 MHz, DMSO-D6) δ 10.43 (1H, s, Ar-CO-NH-NH), 9.31 (1H, s, H2), 9.16 (2H, s, H4, H6), 7.92 (1H, s, Ar-CO-NH-NH), 6.58 (1H, s, CO-NH), 2.99 (2H, td, J = 7.0, 5.7 Hz, C1H2), 1.34 (2H, tt, J = 7.8, 6.3 Hz, C2H2), 1.28–1.18 (2H, m, C3H2), 0.83 (3H, t, J = 7.4 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.73, 160.85, 158.53, 156.65, 127.04, 39.40, 32.51, 19.96, 14.26. IR (ATR): 720, 760, 903, 934, 1030, 1118, 1191, 1255, 1325, 1412, 1438, 1583, 1659, 1683, 1704, 2872, 2932, 2958, 3044, 3308 cm-1. Elemental analysis for C10H15N5O2 (237.12) calculated C, 50.62; H, 6.37; N, 29.52, found C, 50.99; H, 6.52; N, 29.89. Rf = 0.15.
Yellowish solid. Yield 71%. Mp 160–161°C. 1H NMR (600 MHz, DMSO-D6) δ 10.43 (1H, d, J = 1.8 Hz, Ar-CO-NH-NH), 9.31 (1H, s, H2), 9.16 (2H, s, H4, H6), 7.92 (2H, d, J = 1.8 Hz, Ar-CO-NH-NH), 6.58 (2H, t, J = 6.2 Hz, CO-NH), 2.98 (2H, q, J = 6.9 Hz, C1H2), 1.36 (2H, p, J = 7.2 Hz, C2H2), 1.29–1.14 (4H, m, C3H2, C4H2), 0.82 (3H, t, J = 7.2 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.73, 160.85, 158.52, 156.65, 127.04, 39.65, 30.05, 29.04, 22.42, 14.48. IR (ATR): 629, 642, 716, 755, 907, 1039, 1137, 1195, 1263, 1323, 1412, 1488, 1561, 1588, 1656, 2871, 2932, 3280 cm-1. Elemental analysis for C11H17N5O2 (251.29) calculated C, 52.58; H, 6.82; N, 27.87, found C, 52.79; H, 6.93; N, 28.01. Rf = 0.15.
Yellowish solid. Yield 88%. Mp 139–140°C. 1H NMR (600 MHz, DMSO-D6) δ 10.43 (1H, s, Ar-CO-NH-NH), 9.30 (1H, s, H2), 9.15 (2H, s, H4, H6), 7.92 (1H, s, Ar-CO-NH-NH), 6.59 (1H, s, CO-NH), 2.97 (2H, q, J = 6.7 Hz, C1H2), 1.35 (2H, p, J = 7.1 Hz, C2H2), 1.27–1.16 (6H, m, C3H2, C4H2, C5H2), 0.86–0.78 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.74, 160.85, 158.53, 156.64, 127.04, 39.70, 31.58, 30.34, 26.49, 22.62, 14.44. IR (ATR): 636, 719, 916, 1039, 1142, 1193, 1225, 1268, 1329, 1417, 1445, 1514, 1583, 1650, 2858, 2930, 2955, 3029, 3268 cm-1. Elemental analysis for C12H19N5O2 (265.32) calculated C, 54.32; H, 7.22; N, 26.40, found C, 54.52; H, 7.39; N, 26.55. Rf = 0.15.
Yellowish solid. Yield 92%. Mp 147–148°C. 1H NMR (600 MHz, DMSO-D6) δ 10.43 (1H, s, Ar-CO-NH-NH), 9.30 (1H, s, H2), 9.15 (2H, s, H4, H6), 7.92 (1H, s, Ar-CO-NH-NH), 6.58 (1H, s, CO-NH), 2.97 (2H, q, J = 6.6 Hz, C1H2), 1.35 (2H, p, J = 7.1 Hz, C2H2), 1.27–1.15 (8H, m, C3H2, C4H2, C5H2, C6H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.73, 160.85, 158.54, 156.64, 127.04, 39.76, 31.81, 30.38, 29.01, 26.79, 22.58, 14.47. IR (ATR): 640, 716, 905, 1038, 1136, 1194, 1262, 1323, 1412, 1469, 1489, 1558, 1585, 1650, 2852, 2924, 2953, 3281 cm-1. Elemental analysis for C13H21N5O2 (279.34) calculated C, 55.90; H, 7.58; N, 25.07, found C, 56.19; H, 7.82; N, 24.84. Rf = 0.2.
Beige solid. Yield 89%. Mp 156–158°C. 1H NMR (600 MHz, DMSO-D6) δ 10.43 (1H, d, J = 1.9 Hz, Ar-CO-NH-NH), 9.30 (1H, s, H2), 9.15 (2H, s, H4, H6), 7.92 (1H, d, J = 2.0 Hz, Ar-CO-NH-NH), 6.58 (1H, t, J = 6.2 Hz, CO-NH), 2.97 (2H, q, J = 6.7 Hz, C1H2), 1.34 (2H, p, J = 6.9 Hz, C2H2), 1.27–1.15 (10H, m, C3H2, C4H2, C5H2, C6H2, C7H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.73, 160.85, 158.53, 156.64, 127.04, 39.77, 31.78, 30.37, 29.31, 29.24, 26.84, 22.62, 14.47. IR (ATR): 628, 649, 716, 907, 1038, 1136, 1195, 1266, 1324, 1413, 1469, 1489, 1560, 1590, 1654, 1681, 2852, 2919, 3276 cm-1. Elemental analysis for C14H23N5O2 (293.37) calculated C, 57.32; H, 7.90; N, 23.87, found C, 57.46; H, 7.92; N, 23.99. Rf = 0.2.
Yellowish solid. Yield 56%. Mp 164–165°C. 1H NMR (600 MHz, DMSO-D6) δ 10.42 (1H, d, J = 1.7 Hz, Ar-CO-NH-NH), 9.31 (1H, s, H2), 9.15 (2H, s, H4, H6), 7.92 (1H, d, J = 1.7 Hz, Ar-CO-NH-NH), 6.58 (1H, s, CO-NH), 2.97 (2H, q, J = 6.7 Hz, C1H2), 1.34 (2H, p, J = 7.1 Hz, C2H2), 1.22–1.19 (12H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.73, 160.85, 158.53, 156.64, 127.04, 39.77, 31.83, 30.38, 29.54, 29.36, 29.20, 26.83, 22.62, 14.48. IR (ATR): 634, 648, 718, 902, 1038, 1138, 1194, 1264, 1291, 1415, 1470, 1584, 1644, 2851, 2921, 3304 cm-1. Elemental analysis for C15H25N5O2 (307.40) calculated C, 58.61; H, 8.20; N, 22.78, found C, 58.91; H, 8.53; N, 22.71. Rf = 0.2.
Yellowish solid. Yield 90%. Mp 155–156°C. 1H NMR (600 MHz, DMSO-D6) δ 10.43 (1H, d, J = 1.7 Hz, Ar-CO-NH-NH), 9.30 (1H, s, H2), 9.15 (2H, s, H4, H6), 7.92 (1H, d, J = 1.7 Hz, Ar-CO-NH-NH), 6.58 (1H, s, CO-NH), 2.97 (2H, q, J = 6.6 Hz, C1H2), 1.34 (2H, p, J = 7.0 Hz, C2H2), 1.23–1.17 (14H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.73, 160.85, 158.53, 156.64, 127.04, 39.77, 31.83, 30.38, 29.59, 29.51, 29.36, 29.25, 26.83, 22.63, 14.48. IR (ATR): 630, 644, 718, 901, 1039, 1106, 1145, 1195, 1227, 1261, 1281, 1309, 1332, 1378, 1413, 1646, 1546, 1585, 1628, 2850, 2921, 2952, 3041, 3310 cm-1. Elemental analysis for C16H27N5O2 (321.43) calculated C, 59.79; H, 8.47; N, 21.79, found C, 59.96; H, 8.59; N, 21.70. Rf = 0.2.
Deep yellow solid. Yield 52%. Mp 157–158°C. 1H NMR (600 MHz, DMSO-D6) δ 10.42 (1H, d, J = 1.8 Hz, Ar-CO-NH-NH), 9.30 (1H, s, H2), 9.15 (2H, s, H4, H6), 7.92 (1H, d, J = 1.7 Hz, Ar-CO-NH-NH), 6.58 (1H, t, J = 5.9 Hz, CO-NH), 2.97 (2H, q, J = 6.6 Hz, C1H2), 1.34 (2H, p, J = 7.1 Hz, C2H2), 1.23–1.14 (16H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.73, 160.85, 158.53, 156.64, 127.04, 39.78, 31.83, 30.38, 29.59, 29.57, 29.55, 29.36, 29.25, 26.83, 22.63, 14.48. IR (ATR): 654, 719, 914, 1037, 1140, 1193, 1261, 1325, 1416, 1443, 1471, 1510, 1583, 1647, 2849, 2916, 3027, 3265 cm-1. Elemental analysis for C17H29N5O2 (335.45) calculated C, 60.87; H, 8.71; N, 20.88, found C, 60.70; H, 8.59; N, 21.00. Rf = 0.2.
Yellowish solid. Yield 91%. Mp 156–157°C. 1H NMR (500 MHz, DMSO-D6) δ 10.36 (1H, s, Ar-CO-NH-NH), 9.32 (1H, s, H2), 9.18 (2H, s, H4, H6), 7.84 (1H, s, Ar-CO-NH-NH), 6.46 (1H, t, J = 5.9 Hz, CO-NH), 3.03 (2H, q, J = 6.6 Hz, C1H2), 1.40 (2H, p, J = 6.9 Hz, C2H2), 1.30–1.19 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.86 (3H, t, J = 6.8 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 163.61, 160.69, 158.37, 156.49, 127.10, 39.79, 31.71, 30.27, 29.47, 29.46, 29.45, 29.42, 29.24, 29.10, 26.77, 22.47, 14.29. IR (ATR): 634, 719, 748, 1037, 1133, 1192, 1220, 1290, 1327, 1420, 1465, 1512, 1561, 1584, 1641, 1661, 2849, 2920, 3119, 3280 cm-1. Elemental analysis for C18H31N5O2 (349.48) calculated C, 61.86; H, 8.94; N, 20.04, found C, 61.99; H, 9.10; N, 20.00. Rf = 0.2.
Yellowish solid. Yield 92%. Mp 153–155°C. 1H NMR (600 MHz, DMSO-D6) δ 10.42 (1H, s, Ar-CO-NH-NH), 9.30 (1H, s, H2), 9.15 (2H, s, H4, H6), 7.92 (1H, s, Ar-CO-NH-NH), 6.57 (1H, s, CO-NH), 2.97 (2H, q, J = 6.4 Hz, C1H2), 1.34 (2H, p, J = 7.1 Hz, C2H2), 1.23–1.14 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.81 (3H, t, J = 7.0 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.71, 160.84, 158.52, 156.64, 127.05, 39.78, 31.82, 30.38, 29.59, 29.58, 29.57, 29.56, 29.55, 29.36, 29.24, 26.83, 22.62, 14.48. IR (ATR): 636, 719, 916, 1039, 1072, 1139, 1193, 1261, 1280, 1325, 1417, 1444, 1472, 1511, 1583, 1645, 2850, 2932, 3028, 3261 cm-1. Elemental analysis for C19H33N5O2 (363.51) calculated C, 62.78; H, 9.15; N, 19.27, found C, 62.99; H, 9.35; N, 19.00. Rf = 0.2.
Yellowish solid. Yield 96%. Mp 146–147°C. 1H NMR (600 MHz, DMSO-D6, 65°C) δ 10.30 (1H, s, Ar-CO-NH-NH), 9.28 (1H, s, H2), 9.15 (2H, s, H4, H6), 7.78 (1H, s, Ar-CO-NH-NH), 6.40 (1H, t, J = 6.0 Hz, CO-NH), 3.00 (2H, q, J = 6.6 Hz, C1H2), 1.38 (2H, p, J = 7.1 Hz, C2H2), 1.23–1.16 (22H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2, C13H2), 0.83 (3H, t, J = 7.0 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.68, 160.74, 158.43, 156.53, 127.20, 39.88, 31.76, 30.33, 29.52, 29.51, 29.50, 29.49, 29.48, 29.47, 29.30, 29.13, 26.83, 22.52, 14.31. IR (ATR): 621, 719, 914, 1039, 1140, 1193, 1260, 1328, 1417, 1469, 1509, 1584, 1645, 2849, 2919, 3040, 3270 cm-1. Elemental analysis for C20H35N5O2 (377.53) calculated C, 63.63; H, 9.34; N, 18.55, found C, 63.60; H, 9.50; N, 18.77. Rf = 0.2.
Yellowish solid. Yield 59%. Mp 145–147°C. 1H NMR (600 MHz, DMSO-D6, 65°C) δ 10.42 (1H, d, J = 1.9 Hz, Ar-CO-NH-NH), 9.30 (1H, s, H2), 9.15 (2H, s, H4, H6), 7.92 (1H, d, J = 1.9 Hz, Ar-CO-NH-NH), 6.57 (1H, s, CO-NH), 2.97 (2H, q, J = 6.6 Hz, C1H2), 1.34 (2H, p, J = 7.0 Hz, C2H2), 1.19 (24H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2, C13H2, C14H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.72, 160.84, 158.53, 156.64, 127.04, 39.78, 31.83, 30.38, 29.59, 29.58, 29.57, 29.56, 29.55, 29.41, 29.39, 29.37, 29.24, 26.84, 22.62, 14.47. IR (ATR): 626, 718, 914, 1038, 1140, 1193, 1269, 1325, 1417, 1443, 1471, 1511, 1561, 1583, 1648, 2849, 2914, 3265 cm-1. Elemental analysis for C21H37N5O2 (391.56) calculated C, 64.42; H, 9.52; N, 17.89, found C, 64.29; H, 9.31; N, 17.93. Rf = 0.2.
Yellowish solid. Yield 98%. Mp 138–139°C. 1H NMR (600 MHz, DMSO-D6, 65°C) δ 10.30 (1H, s, Ar-CO-NH-NH), 9.28 (1H, s, H2), 9.15 (2H, s, H4, H6), 7.78 (1H, s, Ar-CO-NH-NH), 6.39 (1H, s, CO-NH), 3.00 (2H, q, J = 6.6 Hz, C1H2), 1.38 (2H, p, J = 6.9 Hz, C2H2), 1.27–1.14 (26H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2, C13H2, C14H2, C15H2), 0.83 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.67, 160.74, 158.42, 156.53, 127.20, 39.88, 31.76, 30.33, 29.52, 29.51, 29.50, 29.49, 29.48, 29.47, 29.46, 29.45, 29.30, 29.13, 26.83, 22.52, 14.31. IR (ATR): 625, 635, 719, 916, 1038, 1140, 1193, 1226, 1264, 1329, 1417, 1469, 1511, 1583, 1644, 2849, 2918, 2956, 3270 cm-1. Elemental analysis for C22H39N5O2 (405.59) calculated C, 65.15; H, 9.69; N, 17.27, found C, 65.39; H, 9.80; N, 17.28. Rf = 0.2.
Yellowish solid. Yield 41%. Mp 136–138°C. 1H NMR (600 MHz, DMSO-D6, 65°C) δ 10.28 (1H, s, Ar-CO-NH-NH), 9.28 (1H, s, H2), 9.14 (2H, s, H4, H6), 7.76 (1H, s, Ar-CO-NH-NH), 6.36 (1H, s, CO-NH), 3.01 (2H, q, J = 6.5 Hz, C1H2), 1.38 (2H, p, J = 7.0 Hz, C2H2), 1.25–1.14 (28H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2, C13H2, C14H2, C15H2, C16H2), 0.83 (3H, t, J = 7.0 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.67, 160.73, 158.41, 156.52, 127.23, 39.90, 31.75, 30.32, 29.48, 29.44, 29.43, 29.42, 29.51, 29.40, 29.39, 29.38, 29.37, 29.30, 29.12, 26.83, 22.50, 14.29. IR (ATR): 635, 719, 916, 1038, 1140, 1194, 1261, 1326, 1417, 1470, 1584, 1644, 2850, 2919, 3270 cm-1. Elemental analysis for C23H41N5O2 (419.61) calculated C, 65.84; H, 9.85; N, 16.69, found C, 65.99; H, 9.92; N, 16.70. Rf = 0.25.
Yellow solid. Yield 99 %. Mp 141–142°C. 1H NMR (600 MHz, DMSO-D6, 65°C) δ 10.30 (1H, s, Ar-CO-NH-NH), 9.28 (1H, s, H2), 9.15 (2H, s, H4, H6), 7.78 (1H, s, Ar-CO-NH-NH), 6.39 (1H, t, J = 6.1 Hz, CO-NH), 3.00 (2H, q, J = 6.7 Hz, C1H2), 1.38 (2H, p, J = 7.1 Hz, C2H2), 1.33–1.20 (30H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2, C13H2, C14H2, C15H2, C16H2, C17H2), 0.83 (3H, t, J = 6.8 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 163.68, 160.74, 158.42, 156.53, 127.20, 39.88, 31.76, 30.33, 30.31, 30.30, 30.29, 30.28, 30.27, 30.26, 30.24, 29.52, 29.49, 29.45, 29.30, 29.12, 26.84, 22.52, 14.31. IR (ATR): 618, 635, 719, 915, 1038, 1140, 1193, 1258, 1327, 1417, 1469, 1511, 1584, 1644, 2849, 2860, 2919, 2930, 3270 cm-1. Elemental analysis for C24H43N5O2 (433.64) calculated C, 66.48; H, 10.00; N, 16.15, found C, 66.71; H, 10.31; N, 16.00. Rf = 0.3.
White solid. Yield 77%. Mp 143°C (decomp.). 1H NMR (600 MHz, DMSO-D6) δ 10.34 (1H, s, Ar-CO-NH-NH), 9.04 (2H, s, H4, H6), 7.87 (1H, s, Ar-CO-NH-NH), 6.55 (1H, t, J = 6.1 Hz, CO-NH), 2.97 (2H, q, J = 6.6 Hz, C1H2), 2.65 (3H, s, Ar-CH3), 1.33 (2H, p, J = 7.1 Hz, C2H2), 1.25–1.12 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.81 (3H, t, J = 7.0 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 170.32, 163.89, 158.58, 156.75, 124.08, 39.78, 31.83, 30.38, 29.60, 29.55, 29.36, 29.34, 29.25, 26.84, 26.27, 22.63, 14.47. IR (ATR): 635, 742, 824, 882, 956, 1016, 1081, 1199, 1251, 1271, 1334, 1389, 1446, 1545, 1587, 1627, 1654, 2849, 2922, 3216, 3280 cm-1. Elemental analysis for C19H33N5O2 (363.51) calculated C, 62.78; H, 9.15; N, 19.27, found C, 62.99; H, 9.00; N, 19.11. Rf = 0.3.
White solid. Yield 49%. Mp 124–126°C. 1H NMR (500 MHz, DMSO-D6) δ 10.49 (1H, s, Ar-CO-NH-NH), 9.40 (2H s, H4, H6), 7.91 (1H, s, Ar-CO-NH-NH), 6.45 (1H, t, J = 5.8 Hz, CO-NH), 3.05 (2H, q, J = 6.6 Hz, C1H2), 1.42 (2H, p, J = 6.9 Hz, C2H2), 1.31–1.22 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.86 (3H, t, J = 6.8 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 162.38, 158.16, 158.08, δ 156.90 (q, J = 36.7 Hz), 129.17, 119.85 (q, J = 275.5 Hz), 39.79, 31.67, 30.49, 30.23, 29.43, 29.38, 29.20, 29.05, 26.81, 26.75, 22.43, 14.20. IR (ATR): 626, 723, 819, 907, 1038, 1106, 1131, 1150, 1203, 1241, 1268, 1353, 1468, 1576, 1611, 1643, 1680, 2850, 2912, 2955, 3281, 3334 cm-1. Elemental analysis for C19H30F3N5O2 (417.48) calculated C, 54.66; H, 7.24; N, 16.78, found C, 54.26; H, 7.01; N, 16.40. Rf = 0.5.
White solid. Yield 85%. Mp 167–168°C. 1H NMR (600 MHz, DMSO-D6) δ 10.43 (1H, s, Ar-CO-NH-NH), 9.25 (2H, s, H4, H6), 8.46–8.40 (2H, m, H2´, H6´), 7.93 (1H, s, Ar-CO-NH-NH), 7.59–7.49 (3H, m, H3´, H4´, H5´), 2.98 (2H, q, J = 6.6 Hz, C1H2), 1.35 (2H, p, J = 7.0 Hz, C2H2), 1.25–1.12 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.52, 163.74, 158.61, 157.40, 136.87, 132.24, 129.44, 128.78, 124.75, 39.80, 31.83, 30.38, 29.61, 29.58, 29.57, 29.56, 29.36, 29.26, 26.84, 22.63, 14.48. IR (ATR): 688, 744, 898, 1023, 1239, 1385, 1429, 1480, 1539, 1559, 1574, 2848, 2919, 2959, 3170, 3336 cm-1. Elemental analysis for C24H35N5O2 (425.58) calculated C, 67.73; H, 8.29; N, 16.46, found C, 67.95; H, 8.38; N, 16.32. Rf = 0.6.
Yellowish solid. Yield 62%. Mp 134–135°C. 1H NMR (600 MHz, DMSO-D6) δ 9.44 (1H, s, Ar-CO-NH-NH), 8.76 (2H, s, H4, H6), 7.85 (1H, s, Ar-CO-NH-NH), 6.35 (1H, t, J = 6.1 Hz, CO-NH), 2.94 (2H, q, J = 6.6 Hz, C1H2), 1.31 (2H, p, J = 7.1 Hz, C2H2), 1.23–1.14 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.81 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.47, 164.40, 160.02, 158.74, 115.28, 31.82, 30.35, 29.63, 29.61, 29.60, 29.55, 29.37, 29.25, 26.79, 22.62, 14.68, 14.47. IR (ATR): 604, 626, 719, 915, 1038, 1140, 1139, 1270, 1325, 1379, 1418, 1443, 1480, 1512, 1560, 1583, 1649, 2850, 2914, 3245 cm-1. Elemental analysis for C18H30ClN5O2 (383.92) calculated C, 56.31; H, 7.88; N, 18.24, found C, 56.60; H, 8.01; N, 18.00. Rf = 0.25.
White solid. Yield 63%. Mp 188–189°C. 1H NMR (500 MHz, DMSO-D6) δ 10.30 (1H, d, J = 2.1 Hz, Ar-CO-NH-NH), 8.97 (2H, d, J = 4.9 Hz, H4, H6), 7.93 (1H, d, J = 2.0 Hz, Ar-CO-NH-NH), 7.70 (1H, t, J = 4.9 Hz, H5), 6.39 (1H, t, J = 5.7 Hz, CO-NH), 3.00 (2H, q, J = 6.8 Hz, C1H2), 1.38 (2H, p, J = 7.1 Hz, C2H2), 1.29–1.16 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.84 (3H, t, J = 6.8 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 162.59, 158.23, 158.11, 158.02, 123.73, 39.65, 31.76, 30.29, 29.53, 29.52, 29.51, 29.50, 29.30, 29.18, 26.79, 22.56, 14.41. IR (ATR): 721, 919, 1036, 1135, 1201, 1245, 1315, 1411, 1420, 1467, 1561, 1590, 1665, 1715, 2849, 2918, 3188, 3285, 3379 cm-1. Elemental analysis for C18H31N5O2 (425.58) calculated C, 61.86; H, 8.94; N, 20.04, found C, 61.99; H, 9.17; N, 20.20. Rf = 0.25.
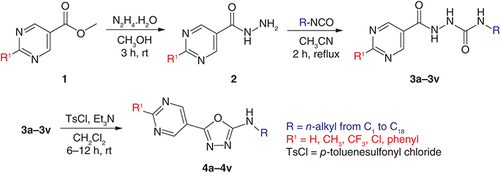
Synthesis of N-alkyl-5-(pyrimidin-5-yl)-1,3,4-oxadiazol-2-amines & their analogues (4a–4w)
1 mmol of appropriate N-alkyl-2-(pyrimidine-5-carbonyl)hydrazine-1-carboxamide 3 was placed to the round bottom flask and suspended in 50 ml of dichloromethane. Then 3 mmol of p-toluenesulfonyl chloride (573 mg) and 5 mmol of triethylamine (505 mg, 696 μl) were added in one portion. The reaction mixture was allowed to react for at least 6 h (up to 12 h) at room temperature (satisfactory conversion was indicated based on TLC). Liquid was evaporated on a rotary vacuum evaporator. The resulting oil was dissolved in ethyl acetate (30 ml) and extracted with a saturated solution of sodium bicarbonate (2 × 30 ml) followed by brine (1 × 30 ml). Ethyl acetate was evaporated on a rotary vacuum evaporator and the crude products 4a–4w were purified by column chromatography.
White solid. Yield 89%. Mp 201–202°C. 1H NMR (600 MHz, DMSO-D6) δ 9.25 (1H, s, H2), 9.13 (2H, s, H4, H6), 7.85 (1H, q, J = 4.8 Hz, NH), 2.85 (3H, d, J = 4.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 165.24, 159.57, 154.05, 153.70, 120.09, 29.60. IR (ATR): 634, 718, 1018, 1071, 1133, 1154, 1190, 1414, 1448, 1543, 1573, 1601, 1633, 3052, 3244 cm-1. Elemental analysis for C7H7N5O (177.17) calculated C, 47.46; H, 3.98; N, 39.53, found C, 47.80; H, 4.25; N, 39.23. Rf = 0.4.
Yellowish solid. Yield 66%. Mp 157–158°C. 1H NMR (600 MHz, DMSO-D6) δ 9.25 (1H, s, H2), 9.12 (2H, s, H4, H6), 7.96 (1H, t, J = 5.6 Hz, NH), 3.26 (2H, qd, J = 7.2, 5.4 Hz, CH2CH3), 1.15 (3H, t, J = 7.1 Hz, CH2CH3). 13C NMR (151 MHz, DMSO-D6) δ 164.55, 159.54, 153.91, 153.69, 120.10, 38.05, 15.02. IR (ATR): 636, 720, 818, 1021, 1041, 1131, 1144, 1190, 1294, 1363, 1415, 1446, 1598, 1619, 2973, 3049, 3234 cm-1. Elemental analysis for C8H9N5O (191.19) calculated C, 50.26; H, 4.74; N, 36.63, found C, 50.60; H, 4.99; N, 36.32. Rf = 0.4.
Yellowish solid. Yield 74%. Mp 165–166°C. 1H NMR (600 MHz, DMSO-D6) δ 9.25 (1H, s, H2), 9.12 (2H, s, H4, H6), 8.00 (1H, t, J = 5.8 Hz, NH), 3.19 (2H, td, J = 7.2, 5.9 Hz, CH2CH2CH3), 1.60–1.51 (2H, m, CH2CH2CH3), 0.88 (3H, t, J = 7.4 Hz, CH2CH2CH3). 13C NMR (151 MHz, DMSO-D6) δ 164.74, 159.53, 153.85, 153.68, 120.12, 44.94, 22.57, 11.73. IR (ATR): 635, 721, 924, 995, 1025, 1043, 1133, 1145, 1189, 1280, 1364, 1385, 1415, 1448, 1573, 1599, 1622, 2986, 3049, 3238 cm-1. Elemental analysis for C9H11N5O (205.22) calculated C, 52.67; H, 5.40; N, 34.13, found C, 52.54; H, 5.22; N, 33.92. Rf = 0.4.
Yellowish solid. Yield 95%. Mp 167–168°C. 1H NMR (600 MHz, DMSO-D6) δ 9.25 (1H, s, H2), 9.12 (2H, s, H4, H6), 7.97 (1H, t, J = 5.6 Hz, NH), 3.22 (2H, td, J = 7.1, 5.7 Hz, C1H2), 1.52 (2H, tt, J = 7.4, 6.5 Hz, C2H2), 1.36–1.27 (2H, m, C3H2), 0.86 (3H, t, J = 7.4 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 164.72, 159.52, 153.84, 153.67, 120.11, 42.84, 31.35, 19.90, 14.13. IR (ATR): 636, 721, 924, 1021, 1048, 1133, 1189, 1277, 1370, 1415, 1448, 1544, 1573, 1621, 2936, 3049, 3236 cm-1. Elemental analysis for C10H13N5O (219.25) calculated C, 54.78; H, 5.98; N, 31.94, found C, 54.92; H, 5.90; N, 32.05. Rf = 0.5.
Yellowish solid. Yield 67%. Mp 164–165°C. 1H NMR (600 MHz, DMSO-D6) δ 9.25 (1H, s, H2), 9.12 (2H, s, H4, H6), 7.98 (1H, t, J = 5.7 Hz, NH), 3.21 (2H, td, J = 7.0, 5.7 Hz, C1H2), 1.58–1.50 (2H, m, C2H2), 1.32–1.23 (4H, m, C3H2, C4H2), 0.84 (3H, t, J = 7.2 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 164.71, 159.53, 153.84, 153.68, 120.11, 43.12, 28.93, 28.90, 22.31, 14.43. IR (ATR): 635, 721, 924, 1023, 1046, 1132, 1189, 1286, 1373, 1415, 1447, 1572, 1597, 1621, 2859, 2933, 2959, 3049, 3240 cm-1. Elemental analysis for C11H15N5O (233.28) calculated C, 56.64; H, 6.48; N, 30.02, found C, 56.85; H, 6.62; N, 30.00. Rf = 0.5.
Yellowish solid. Yield 62%. Mp 157–158°C. 1H NMR (600 MHz, acetone-D6) δ 9.20 (1H, s, H2), 9.12 (2H, s, H4, H6), 7.00 (1H, t, J = 6.1 Hz, NH), 3.42–3.38 (2H, m, C1H2), 1.68 (2H, p, J = 7.3 Hz, C2H2), 1.40 (2H, tt, J = 9.5, 5.7 Hz, C3H2), 1.31 (4H, tq, J = 6.7, 3.1 Hz, C4H2, C5H2), 0.86 (3H, t, J = 7.6 Hz, CH3). 13C NMR (151 MHz, acetone-D6) δ 164.57, 159.12, 153.93, 153.12, 120.19, 43.26, 31.39, 29.26, 26.28, 22.42, 13.45. IR (ATR): 635, 721, 925, 1022, 1056, 1131, 1189, 1374, 1415, 1447, 1480, 1572, 1596, 1620, 2922, 2956, 3050, 3250 cm-1. Elemental analysis for C12H17N5O (247.30) calculated C, 58.28; H, 6.93; N, 28.32, found C, 58.55; H, 7.18; N, 28.54. Rf = 0.5.
Yellowish solid. Yield 90%. Mp 154–155°C. 1H NMR (600 MHz, acetone-D6) δ 9.20 (1H, s, H2), 9.12 (2H, s, H4, H6), 7.00 (1H, t, J = 6.1 Hz, NH), 3.40 (2H, q, J = 6.7 Hz, C1H2), 1.68 (2H, p, J = 7.2 Hz, C2H2), 1.45–1.36 (2H, m, C3H2), 1.36–1.32 (2H, m, C4H2), 1.32–1.24 (4H, m, C5H2, C6H2), 0.85 (3H, t, J = 6.8 Hz, CH3). 13C NMR (151 MHz, acetone-D6) δ 164.57, 159.12, 153.92, 153.12, 120.19, 43.26, 43.14, 31.70, 29.26, 26.57, 22.42, 13.49. IR (ATR): 634, 721, 924, 1021, 1057, 1131, 1188, 1375, 1415, 1447, 1479, 1572, 1597, 1620, 2855, 2920, 2958, 3049, 3253 cm-1. Elemental analysis for C13H19N5O (261.33) calculated C, 59.75; H, 7.33; N, 26.80, found C, 59.95; H, 7.47; N, 26.82. Rf = 0.5.
Yellowish solid. Yield 55%. Mp 154–155°C. 1H NMR (600 MHz, DMSO-D6) δ 9.25 (1H, s, H2), 9.12 (2H, s, H4, H6), 7.97 (1H, t, J = 5.7 Hz, NH), 3.21 (2H, td, J = 7.0, 5.7 Hz, C1H2), 1.57–1.49 (2H, m, C2H2), 1.33–1.15 (10H, m, C3H2, C4H2, C5H2, C6H2, C7H2), 0.80 (3H, t, J = 7.0 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 164.71, 159.53, 153.83, 153.66, 120.12, 43.15, 40.61, 31.76, 29.25, 29.18, 26.71, 22.61, 14.46. IR (ATR): 634, 721, 925, 1021, 1051, 1084, 1130, 1188, 1415, 1447, 1481, 1573, 1598, 1620, 2852, 2918, 2954, 3254 cm-1. Elemental analysis for C14H21N5O (275.36) calculated C, 61.07; H, 7.69; N, 25.43, found C, 61.29; H, 7.81; N, 25.39. Rf = 0.5.
Yellowish solid. Yield 48%. Mp 155–157°C. 1H NMR (600 MHz, DMSO-D6) δ 9.25 (1H, s, H2), 9.12 (2H, s, H4, H6), 7.97 (1H, t, J = 5.7 Hz, NH), 3.21 (2H, q, J = 6.7 Hz, C1H2), 1.53 (2H, p, J = 7.2 Hz, C2H2), 1.35–1.12 (12H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2), 0.81 (3H, t, J = 7.0 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 164.71, 159.53, 153.83, 153.66, 120.12, 43.15, 31.81, 29.48, 29.25, 29.21, 29.18, 26.70, 22.62, 14.46. IR (ATR): 634, 721, 735, 924, 1021, 1047, 1131, 1188, 1415, 1470, 1480, 1572, 1596, 1620, 2852, 2918, 3254 cm-1. Elemental analysis for C15H23N5O (289.38) calculated C, 62.26; H, 8.01; N, 24.20, found C, 62.51; H, 8.32; N, 24.11. Rf = 0.5.
Yellowish solid. Yield 77%. Mp 150–151°C. 1H NMR (600 MHz, DMSO-D6) δ 9.24 (1H, s, H2), 9.12 (2H, s, H4, H6), 7.81 (1H, t, J = 5.7 Hz, NH), 3.23 (2H, q, J = 6.7 Hz, C1H2), 1.56 (2H, p, J = 7.2 Hz, C2H2), 1.36–1.17 (14H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2), 0.82 (3H, t, J = 7.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 164.85, 159.49, 153.86, 153.64, 120.20, 43.28, 31.76, 29.44, 29.40, 29.30, 29.14, 29.14, 26.68, 22.52, 14.33. IR (ATR): 634, 721, 734, 925, 1022, 1049, 1131, 1189, 1415, 1447, 1481, 1573, 1597, 1622, 2852, 2918, 3253 cm-1. Elemental analysis for C16H25N5O (303.41) calculated C, 63.34; H, 8.31; N, 23.08, found C, 63.58; H, 8.49; N, 23.00. Rf = 0.6.
Yellowish solid. Yield 49%. Mp 148–148°C. 1H NMR (600 MHz, DMSO-D6) δ 9.24 (1H, s, H2), 9.12 (2H, s, H4, H6), 7.81 (1H, t, J = 6.0 Hz, NH), 3.23 (2H, q, J = 6.8 Hz, C1H2), 1.56 (2H, p, J = 7.1 Hz, C2H2), 1.34–1.21 (16H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2), 0.82 (3H, t, J = 6.7 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 164.85, 159.49, 153.85, 153.63, 120.20, 43.28, 31.76, 29.45, 29.43, 29.41, 29.39, 29.29, 29.14, 26.68, 22.53, 14.33. IR (ATR): 634, 721, 924, 1022, 1054, 1130, 1188, 1415, 1446, 1471, 1481, 1572, 1596, 1620, 2851, 2917, 3254 cm-1. Elemental analysis for C17H27N5O (317.44) calculated C, 64.32; H, 8.57; N, 22.06, found C, 64.51; H, 8.62; N, 22.00. Rf = 0.6.
Yellowish solid. Yield 95%. Mp 147–148°C. 1H NMR (500 MHz, DMSO-D6, 50°C) δ 9.27 (1H, d, J = 0.6 Hz, H2), 9.15 (2H, d, J = 0.7 Hz, H4, H6), 7.94 (1H, t, J = 5.9 Hz, NH), 3.26 (2H, q, J = 6.6 Hz, C1H2), 1.59 (2H, p, J = 7.0 Hz, C2H2), 1.37–1.19 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.85 (3H, t, J = 6.8 Hz, CH3). 13C NMR (126 MHz, DMSO-D6, 50°C) δ 164.23, 158.87, 153.21, 153.01, 119.57, 42.63, 31.15, 28.88, 58.86, 28.85, 28.82, 28.80, 28.66, 28.53, 26.06, 21.91, 13.74. IR (ATR): 635, 721, 625, 1021, 1054, 1130, 1188, 1415, 1446, 1471, 1481, 1571, 1597, 1621, 2851, 2917, 3257 cm-1. Elemental analysis for C18H29N5O (331.46) calculated C, 65.23; H, 8.82; N, 21.13, found C, 65.37; H, 8.99; N, 21.01. Rf = 0.6.
Yellowish solid. Yield 56%. Mp 145–146°C. 1H NMR (600 MHz, DMSO-D6, 75°C) δ 9.23 (1H, s, H2), 9.11 (2H, s, H4, H6), 7.83 (1H, t, J = 6.1 Hz, NH), 3.23 (2H, q, J = 6.8 Hz, C1H2), 1.56 (2H, p, J = 7.1 Hz, C2H2), 1.29–1.15 (20H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2), 0.82 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6, 75°C) δ 164.93, 159.46, 153.85, 153.62, 120.24, 43.32, 31.73, 29.46, 29.45, 29.44, 29.43, 29.42, 29.40, 29.31, 29.12, 26.68, 22.48, 14.26. IR (ATR): 634, 721, 925, 1021, 1057, 1130, 1188, 1414, 1446, 1472, 1480, 1571, 1596, 1621, 2851, 2917, 3255 cm-1. Elemental analysis for C19H31N5O (345.49) calculated C, 66.05; H, 9.04; N, 20.27, found C, 66.29; H, 8.90; N, 20.41. Rf = 0.6.
Yellowish solid. Yield 84%. Mp 141–143°C. 1H NMR (600 MHz, DMSO-D6, 75°C) δ 9.23 (1H, s, H2), 9.11 (2H, s, H4, H6), 7.74 (1H, t, J = 6.2 Hz, NH), 3.24 (2H, q, J = 6.6 Hz, C1H2), 1.56 (2H, p, J = 7.4 Hz, C2H2), 1.39–1.14 (22H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2, C13H2), 0.82 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6, 75°C) δ 164.92, 159.46, 153.87, 153.62, 120.24, 43.33, 31.74, 29.47, 29.46, 29.45, 29.44, 29.43, 29.42, 29.40, 29.32, 29.12, 26.67, 22.48, 14.26. IR (ATR): 612, 635, 721, 925, 1021, 1059, 1130, 1188, 1415, 1446, 1470, 1480, 1572, 1597, 1620, 2851, 2918, 3256 cm-1. Elemental analysis for C20H33N5O (359.52) calculated C, 66.82; H, 9.25; N, 19.48, found C, 66.76; H, 9.11; N, 19.40. Rf = 0.6.
Yellowish solid. Yield 96%. Mp 142–144°C. 1H NMR (600 MHz, DMSO-D6, 75°C) δ 9.23 (1H, s, H2), 9.11 (2H, s, H4, H6), 7.74 (1H, t, J = 5.7 Hz, NH), 3.24 (2H, q, J = 6.7 Hz, C1H2), 1.57 (2H, p, J = 7.1 Hz, C2H2), 1.36–1.21 (24H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2, C13H2, C14H2), 0.83 (3H, t, J = 7.0 Hz, CH3). 13C NMR (151 MHz, DMSO-D6, 75°C) δ 164.91, 159.47, 153.88, 153.64, 120.24, 43.33, 31.74, 29.46, 29.44, 29.42, 29.41, 29.40, 29.38, 26.36, 29.34, 29.32, 29.11, 26.67, 22.49, 14.27. IR (ATR): 634, 721, 954, 1022, 1047, 1131, 1188, 1390, 1414, 1446, 1470, 1480, 1572, 1596, 1620, 2851, 2917, 3258 cm-1. Elemental analysis for C21H35N5O (373.55) calculated C, 67.52; H, 9.44; N, 18.75, found C, 67.31; H, 9.29; N, 18.70. Rf = 0.7.
Yellowish solid. Yield 98%. Mp 142–143°C. 1H NMR (600 MHz, DMSO-D6, 75°C) δ 9.24 (1H, s, H2), 9.11 (2H, s, H4, H6), 7.74 (1H, s, NH), 3.24 (2H, q, J = 6.7 Hz, C1H2), 1.57 (2H, p, J = 7.2 Hz, C2H2), 1.36–1.22 (26H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2, C13H2, C14H2, C15H2), 0.83 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6, 75°C) δ 164.90, 159.47, 153.88, 153.65, 120.24, 43.33, 31.74, 29.45, 29.44, 29.43, 29.42, 29.41, 29.40, 29.38, 26.36, 29.34, 29.33, 29.11, 26.67, 22.49, 14.27. IR (ATR): 634, 721, 954, 1021, 1051, 1080, 1131, 1188, 1390, 1415, 1446, 1472, 1571, 1598, 1621, 2851, 2917, 3257 cm-1. Elemental analysis for C22H37N5O (387.57) calculated C, 68.18; H, 9.62; N, 18.07, found C, 68.32; H, 9.55; N, 18.00. Rf = 0.7.
Yellowish solid. Yield 50%. Mp 143–144°C. 1H NMR (600 MHz, DMSO-D6, 75°C) δ 9.23 (1H, s, H2), 9.11 (2H, s, H4, H6), 7.71 (1H, s, NH), 3.24 (2H, q, J = 6.7 Hz, C1H2), 1.57 (2H, p, J = 7.1 Hz, C2H2), 1.36–1.15 (28H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2, C13H2, C14H2, C15H2, C16H2), 0.83 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6, 75°C) δ 164.91, 159.47, 153.87, 153.65, 120.24, 43.33, 31.74, 29.45, 29.44, 29.43, 29.42, 29.41, 29.40, 29.39, 29.38, 26.36, 29.34, 29.33, 29.11, 26.67, 22.49, 14.27. IR (ATR): 634, 721, 925, 1021, 1051, 1131, 1181, 1390, 1415, 1446, 1473, 1482, 1571, 1596, 1621, 2851, 2917, 3260 cm-1. Elemental analysis for C23H39N5O (401.60) calculated C, 68.79; H, 9.79; N, 17.44, found C, 68.84; H, 9.50; N, 17.69. Rf = 0.7.
Yellowish solid. Yield 93%. Mp 141–142°C. 1H NMR (600 MHz, DMSO-D6, 75°C) δ 9.23 (1H, s, H2), 9.11 (2H, s, H4, H6), 7.71 (1H, s, NH), 3.24 (2H, q, J = 6.7 Hz, C1H2), 1.56 (2H, p, J = 7.2 Hz, C2H2), 1.36–1.22 (30H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2, C12H2, C13H2, C14H2, C15H2, C15H2, C16H2, C17H2), 0.83 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6, 75°C) δ 164.90, 159.49, 153.87, 153.66, 120.24, 43.33, 31.74, 29.45, 29.44, 29.43, 29.42, 29.41, 29.40, 29.39, 29.38, 29.37, 26.36, 29.34, 29.33, 29.11, 26.67, 22.49, 14.27. IR (ATR): 633, 721, 925, 1020, 1052, 1085, 1130, 1188, 1390, 1414, 1446, 1472, 1481, 1571, 1596, 1621, 2851, 2916, 3258 cm-1. Elemental analysis for C24H41N5O (415.63) calculated C, 69.36; H, 9.94; N, 16.85, found C, 69.49; H, 10.11; N, 16.70. Rf = 0.7.
White solid. Yield 75%. Mp 145–146°C. 1H NMR (600 MHz, DMSO-D6, 75°C) δ 9.03 (2H, s, H4, H6), 7.70 (1H, t, J = 5.3 Hz, NH), 3.26 (2H, q, J = 6.7 Hz, C1H2), 2.70 (3H, s, Ar-CH3), 1.60 (2H, p, J = 7.1 Hz, C2H2), 1.39–1.27 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.86 (3H, t, J = 6.8 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 169.02, 164.72, 154.19, 153.77, 117.32, 43.32, 31.74, 29.47, 29.44, 29.43, 29.41, 29.31, 29.11, 26.67, 26.12, 22.48, 14.26. IR (ATR): 673, 718, 734, 748, 1020, 1058, 1093, 1268, 1394, 1449, 1471, 1482, 1571, 1600, 1619, 2851, 2917, 2954, 3016, 3267 cm-1. Elemental analysis for C19H31N5O (345.49) calculated C, 66.05; H, 9.04; N, 20.27, found C, 66.21; H, 8.92; N, 20.20. Rf = 0.6.
Colourless liquid. Yield 32%. 1H NMR (500 MHz, DMSO-D6, 75°C) δ 9.36 (2H, s, H4, H6), 7.97 (1H, t, J = 5.6 Hz, NH), 3.29 (2H, q, J = 6.6 Hz, C1H2), 1.60 (2H, p, J = 7.0 Hz, C2H2), 1.38–1.19 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.86 (3H, t, J = 6.8 Hz, CH3). 13C NMR (126 MHz, DMSO-D6) δ 164.49, 154.81 (q, J = 36.5 Hz), 154.18, 152.55, 121.43, 119.32 (q, J = 275.3 Hz), 42.65, 31.08, 29.90, 28.82, 28.79, 28.75, 28.61, 28.46, 26.21, 25.99, 21.83, 13.60. IR (ATR): 719, 726, 819, 905, 1038, 1106, 1131, 1150, 1202, 1212, 1269, 1354, 1472, 1533, 1587, 1645, 1682, 2850, 2919, 2955, 3284 cm-1. Elemental analysis for C19H28F3N5O (399.46) calculated C, 57.13; H, 7.07; N, 17.53, found C, 57.29; H, 7.00; N, 17.66. Rf = 0.6.
White solid. Yield 97%. Mp 124–126°C. 1H NMR (600 MHz, DMSO-D6, 80°C) δ 9.23 (2H, s, H4, H6), 8.47–8.43 (3H, m, H3´, H4´, H5´), 7.75 (1H, s, NH), 7.59–7.53 (2H, m, H2´, H6´), 3.29 (2H, q, J = 6.6 Hz, C1H2), 1.62 (2H, p, J = 6.9 Hz, C2H2), 1.47–1.20 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.86 (3H, t, J = 7.0 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 164.83, 164.52, 154.27, 137.09, 131.92, 129.32, 128.61, 125.85, 117.96, 43.38, 31.74, 29.48, 29.47, 29.46, 29.45, 29.41, 29.34, 29.12, 26.68, 22.48, 14.25. IR (ATR): 692, 744, 931, 1026, 1063, 1157, 1249, 1270, 1392, 1434, 1471, 1478, 1602, 1614, 2850, 2916, 2953, 3017, 3273 cm-1. Elemental analysis for C24H33N5O (407.56) calculated C, 70.73; H, 8.16; N, 17.18, found C, 70.90; H, 8.32; N, 17.31. Rf = 0.9.
White solid. Yield 12%. Mp 110–111°C. 1H NMR (600 MHz, DMSO-D6, 80°C) δ 9.11 (2H, s, H4, H6), 7.65 (1H, t, J = 5.9 Hz, NH), 3.30 (2H, q, J = 6.2 Hz, C1H2), 1.61 (2H, p, J = 7.0 Hz, C2H2), 1.34–1.20 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.86 (3H, t, J = 6.9 Hz, CH3). 13C NMR (151 MHz, DMSO-D6) δ 166.14, 164.85, 159.02, 150.64, 130.28, 43.37, 31.70, 29.46, 29.44, 29.43, 29.41, 29.29, 29.11, 26.67, 26.12, 22.48, 14.26. IR (ATR): 650, 718, 776, 906, 1020, 1041, 1085, 1172, 1223, 1260, 1285, 1297, 1312, 1438, 1472, 1485, 1525, 1557, 1610, 1635, 1722, 2850, 2915, 3100, 3273 cm-1. Elemental analysis for C18H28ClN5O (365.91) calculated C, 59.09; H, 7.71; N, 19.14, found C, 58.87; H, 7.94; N, 19.00. Rf = 0.8.
White solid. Yield 60%. Mp 175–176°C. 1H NMR (500 MHz, DMSO-D6) δ 8.93 (2H, d, J = 4.8 Hz, H4, H6), 8.06 (1H, t, J = 5.8 Hz, NH), 7.58 (1H, t, J = 4.9 Hz, H5), 3.25 (2H, q, J = 6.2 Hz, C1H2), 1.56 (2H, p, J = 7.1 Hz, C2H2), 1.34–1.19 (18H, m, C3H2, C4H2, C5H2, C6H2, C7H2, C8H2, C9H2, C10H2, C11H2), 0.83 (3H, t, J = 6.8 Hz, CH3). IR (ATR): 636, 740, 808, 1027, 1050, 1131, 1376, 1421, 1467, 1555, 1566, 1624, 2849, 2918, 3280 cm-1. Elemental analysis for C18H29N5O (331.46) calculated C, 65.23; H, 8.82; N, 21.13, found C, 65.50; H, 9.00; N, 21.29. Rf = 0.6.
The synthesis and characterization of N-dodecyl-2-(pyrimidine-4-carbonyl)hydrazine-1-carboxamide 3x and N-dodecyl-5-(pyrimidin-4-yl)-1,3,4-oxadiazol-2-amine 4x were published previously by our group [Citation24].
Biological activity
Antimycobacterial activity
Antimycobacterial activity of the pyrimidine derivatives 3 and 4 was evaluated as described previously [Citation25,Citation26]. The micromethod for the determination of the minimum inhibitory concentration (MIC) was performed using the Sula's liquid semisynthetic medium (SEVAC, Prague, Czech Republic) in P-plates. The stock bacterial suspensions had a concentration of 1 mg of bacterial mass per 1 ml. Mycobacterial strains involved were drug-sensitive M. tuberculosis strain 331/88 (H37Rv; dilution of the stock suspension was 10-3) and two nontuberculous mycobacterial species: Mycobacterium avium 330/88 (resistant to INH, RIF, rifabutin, ofloxacin [OFX] and EMB; dilution 10-5) and a clinical isolate of Mycobacterium kansasii (6509/96; strain dilution 10-4). The compounds studied were added to the medium as solutions in DMSO; the final volume contained 1.0% DMSO (v/v) and did not affect mycobacterial growth. Twofold serial dilution from 1000 to 1 μM was used. The MIC was determined after incubation at 37°C for 14 and 21 days, and additionally for 7 days for M. kansasii. The MIC [μM] is the lowest concentration at which mycobacterial growth is completely inhibited. First-choice drugs INH and EMB were used to compare MIC values [Citation26].
The most effective derivatives from both series (3l and 4l) were evaluated against seven drug-resistant TB strains (dilution of these strains was 10-3) with different resistance patterns. All strains were resistant to INH, RIF, rifabutin and STM, and in some cases, additional resistance to other drugs was present: strain 7357/1998 was additionally resistant to EMB and OFX; strain 234/2005 to EMB; strain 8666/2010 resistant to EMB, OFX and clofazimine (CFZ); strain Praha 1 showed additional resistance to EMB and CFZ; strain Praha 4 showed resistance to EMB, OFX and CFZ (all are MDR-TB strains); and Praha 131 was resistant to INH, rifamycins, STM, EMB, OFX, gentamicin (GEN) and AMK (i.e., XDR-TB strain). For these resistant strains, this involved a twofold serial dilution from 32 to 0.03 μM.
Investigation of mechanism of action
The mechanism of action of 4l was analyzed using metabolic labeling of M. tuberculosis H37Rv with 14C acetate. M. tuberculosis H37Rv was cultured in 7H9 broth supplemented with 0.05% Tween 80 and 10% albumin-dextrose-catalase at 37°C. At optical density (OD)600 ∼0.2, the tested compound 4l dissolved in DMSO was added in two concentrations corresponding to its 10× and 100× MIC values for M. tuberculosis H37Rv (i.e., 20 and 200 μM). A control incubation without 4l was also performed. The final concentration of DMSO was 2% (v/v). To each culture, 14C acetate (ARC; specific activity 106 mCi/mmol) at a final concentration of 0.5 μCi/ml was added at the same time as inhibitors. Cells were harvested after a further 24 h of incubation at 37°C.
Lipids were extracted with 1.5 ml of chloroform/methanol mixture (2:1, v/v) for 2 h at 65°C. Then, 150 μl of water was added to each sample, the samples were mixed and centrifuged at room temperature, 1000×g. The organic phases were transferred to 2-ml tubes, dried under N2 and subjected to biphasic Folch washing [Citation27]. Isolated lipids were dissolved in 30 μl of chloroform/methanol mixture (2:1, v/v) and 5 μl were spotted onto TLC silica gel plates F254 (Merck KGaA). Lipids were separated in a chloroform/methanol/water mixture (20:4:0.5, v/v/v) and visualized using an Amersham™ Typhoon™ Biomolecular Imager (Cytiva; Marlborough, MA, USA).
Analysis of susceptibility of M. tuberculosis H37Ra strains overproducing DprE1/DprE2 was carried out. These proteins were overproduced in M. tuberculosis H37Ra using pVV2-dprE2 and pVV2-dprE1/dprE2 constructs [Citation28]. The production of recombinant proteins was confirmed by western blot using antiHis antibodies. Susceptibility of M. tuberculosis H37Ra overproducing strains along with a control strain carrying empty vector pVV2 to the selected derivative 4l was evaluated by determining MIC values using the drop dilution method. The cultures were grown in 7H9 broth supplemented with 10% albumin–dextrose–catalase and 0.05% Tween 80, and were adjusted to an OD600 of ∼0.5. A total of 4 μl of 1×, 10× and 100× dilutions from each culture were dropped on 7H11 broth supplemented with 10% oleic acid–albumin–dextrose–catalase and various concentrations of the compounds 4l dissolved in DMSO (2% final concentration, v/v). Plates were incubated for 28 days at 37°C. Benzothiazinone BTZ-043 was involved as a positive control.
Results & discussion
Chemistry
The target oxadiazoles were prepared by a multistep procedure (). First, commercially available methyl pyrimidine-5-carboxylate 1 was subjected to hydrazinolysis (suprastoichiometric amount of hydrazine hydrate) to quantitively afford pyrimidine-5-carbohydrazide (2). A total of 2 mmol of freshly prepared compound 2 was mixed with a mild excess (2.2 mmol) of appropriate alkyl isocyanate in anhydrous acetonitrile and then refluxed for 2 h. After cooling, the precipitate was filtered off, washed with acetonitrile and dried to give N-alkyl-2-(pyrimidine-5-carbonyl)hydrazine-1-carboxamides (3; yields in a wide range of 35–99%). Methyl derivative 3a was obtained in the lowest yield. In general, more hydrophilic derivatives with shorter alkyls were obtained in lower yields (3a–3e; 35–84%). Interestingly, among longer substituents (from C9 onwards), the yields were lower for alkyls with an odd number of carbons compared to those with an even carbon number (41–92% vs 90–99%, respectively).
Commercially unavailable isocyanates were prepared in house from sodium azide and acetyl, tetradecyl, hexadecyl and octadecyl chlorides by Curtius rearrangement. These chlorides were purchased or prepared from carboxylic acids using thionyl chloride and N,N-dimethylformamide as a catalyst.
For cyclization of hydrazides 3 to oxadiazoles 4, 1 mmol of compound 3 was suspended in dichloromethane and treated with p-toluenesulfonyl chloride (three equivalents) and triethylamine (five equivalents) at room temperature. The reaction mixture was evaporated, and the residue was dissolved in ethyl acetate and extracted with aqueous sodium bicarbonate followed by brine. After evaporation of the solvent, crude N-alkyl-5-(pyrimidin-5-yl)-1,3,4-oxadiazol-2-amines (4) were purified by column chromatography. The yields ranged from 48 to 98% depending on the substitution pattern. The lower yields were associated inconsistently with C2, C5, C6, C8, C9, C11, C13 and C17 (49–67%).
Initially, only pyrimidine-5-yl derivatives with C1–C18n-alkyls (3a–3r, 4a–4r) were synthesized. Based on the results of biological evaluation, we decided to prepare additional analogues: 2-substituted pyrimidine derivatives covering lipophilic substituents with various electronic and steric parameters (methyl, trifluoromethyl, phenyl, chlorine; 3s–3v and 4s–4v; ) as well as positional isomers: pyrimidine-2-yl (3w, 4w) and pyrimidine-4-yl (3x, 4x; we have previously reported their synthesis [Citation24]). For these derivatives, the starting materials were different. Carboxylic acids were converted to their methyl esters by heating in thionyl chloride and subsequent treatment with methanol in the presence of triethylamine. Commercially available chlorides were also handled in this way. The resulting esters were treated as described earlier (hydrazinolysis following a reaction with isocyanates and cyclization). The yields of these substituted derivatives and positional isomers were generally lower than those of the parent compounds 3l and 4l (62–85% for hydrazinecarboxamides 3 and 12–97% for oxadiazoles 4), particularly for halogenated molecules t and v.
The prepared compounds were characterized and their purity was checked.
Antimycobacterial activity
Initially, we evaluated both acyclic 3 and cyclic pyrimidine derivatives 4in vitro for their activity against drug-susceptible M. tuberculosis (331/88, i.e., H37Rv), drug-resistant M. avium 330/88 and M. kansasii 6509/96 (clinical isolate). INH and EMB were used as reference compounds for comparison of MIC values ().
Table 1. Structure and antimycobacterial activity of pyrimidine derivatives 3 and 4.
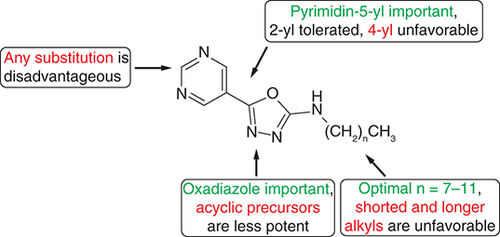
Importantly, a number of compounds 3 and 4 showed antimycobacterial properties, although the evaluation of derivatives with long alkyls (from tridecyl; 3m, 4m, 3n, 4n, 3o, 3p, 4p, 3q, 4q, 3r, 4r) and those with pyrimidine substituted with methyl (3s, 4s) and phenyl (3u, 4u) was complicated by their low solubility in testing media. MIC values started from 2 μM (4l).
Generally, M. tuberculosis was the most sensitive strain followed by M. kansasii. Key structure–activity relationships are summarized in . The short alkyls (C1-C5) provided only moderate activity against all three strains (MIC ≥250 μM). Thereafter, the activity increased and from C13 onwards, the in vitro efficacy dropped again (≥250 μM). Optimal activity was associated with nonyl (4i) and dodecyl (3l, 4l), for hydrazinecarboxamides, and additionally for octyl, decyl and undecyl (3h, 3j, 3k). The oxadiazoles 4 are predominantly more active against M. tuberculosis than their precursors 3 (up to 16-fold for the pair 3l and 4l), but not uniformly for M. kansasii. For this pathogen, oxadiazoles are only more potent if they are N-substituted with a short alkyl to six carbons, then compounds 3 exhibited lower MIC values with the superiority of 3h–3l (64–125 μM). Structure–activity relationships were analogous for polyresistant M. avium: this strain was inhibited by an identical MIC value to M. kansasii (64 μM; however, many compounds were inactive). The most active derivatives were 3i–3k and 4l (64–125 μM). Thus, optimal activity is associated with intermediate-length alkyls, which confer an optimal range of lipophilicity. Lower hydrophilic as well as longer, highly lipophilic compounds are less efficacious.
Regarding the high anti-TB activity of 4l, we prepared and evaluated its 2-substituted derivatives (4s–4v) and positional isomers (4w, 4x). Substitution of pyrimidine at position 2 was generally disadvantageous (methyl 4s, phenyl 4u, chlorine 4v), only CF3 derivatives were slightly more effective (64–125 μM for M. tuberculosis for both 3t and 4t) but still less active than unsubstituted analogues. The activity against NTM was completely abolished (>250 μM). Hence, in this series hydrogen on C2 is important for activity and its substitution is detrimental, possibly due to steric hindrance with target sites or a change in the reactivity of partially positively charged C2. The isomers provided more interesting results. Pyrimidin-2-yl isomers 3w and 4w were equally active, even several MIC values for NTM were better than for their pattern structures l. Pyrimidine-4-yl compounds 3x and 4x did not yield homogeneous results. Oxadiazole 4x was virtually inactive (>250 μM), while acyclic derivative 3x produced comparable activity, even improved for M. avium.
Making a comparison to INH, this highly efficacious drug gave lower MIC values for M. tuberculosis and M. kansasii, but our compounds were generally better in vitro for INH-resistant M. avium. The most effective oxadiazole 4l showed MIC values for M. tuberculosis analogous to another first-line oral drug, EMB.
Based on results on M. tuberculosis H37Rv, we evaluated the most potent derivatives of hydrazinecarboxamides and oxadiazoles, i.e., 3l and 4l, respectively, against drug-resistant TB. We used six strains of MDR-TB and one strain of XDR-TB () with different resistance profiles. All these strains were resistant to INH, rifamycins (i.e., MDR-TB) and STM. Some strains showed additional resistance (EMB, FQ, CFZ, GEN, AMK). Clinical isolate Praha 131 was resistant to INH, rifamycins, STM, EMB, FQ (ofloxacin) and aminoglycosides (GEN, AMK); i.e., an extensively drug-resistant strain according to the ‘traditional’ definition. For comparison, MIC values of clinically used antitubercular drugs for fully susceptible strain M. tuberculosis H37Rv and MDR-TB strains used are presented in Supplementary Table 1.
Table 2. Activity of 3l and 4l against drug-resistant tuberculous strains.
Importantly, both compounds also inhibit drug-resistant (MDR, XDR) strains at identical concentrations to fully susceptible M. tuberculosis H37Rv; i.e., ≥32 μM for 3l and 2–4 μM for 4l. Fortunately, these values do not indicate cross-resistance to first- and second-line antitubercular drugs (INH, rifamycins, various aminoglycosides, EMB, FQ and CFZ) and different mechanism of action.
Investigation of mechanism of action
The mechanism of action of the most active antimycobacterial agent 4l was experimentally investigated. Keeping in mind the design of the target compounds and structural similarity with known pyrimidine inhibitors of mycobacterial cell wall biosynthesis [Citation7], we considered biosynthesis of the mycobacterial cell wall as a potential target, with a special focus on DprE1, which has been also reported as a target structure for pyrimidines [Citation7].
To study the inhibition of cell wall components by 4l, M. tuberculosis H37Rv cells were treated with different concentrations of this compound (10× and 100× MIC) and metabolically labeled with 14C acetate. We took advantage of the fact that the inhibition of some essential components of mycobacterial cell wall leads to specific changes in the lipid profile. For example, blocking the synthesis of arabinogalactan by DprE1 inhibitors results in the accumulation of trehalose monomycolates (TMM) and trehalose dimycolates (TDM) [Citation29]. This is due to lack of arabinan chains in the cell wall core, which serve as attachment sites for mycolic acids. TLC analysis of 14C-labelled lipids of M. tuberculosis H37Rv cells treated with 10× and 100× MIC of 4l showed that there were no changes in comparison to untreated controls in the production of TMM, TDM, cardiolipin and phosphatidylethanolamine. In addition, MIC values of 4l for M. tuberculosis H37Ra strains (without inserted additional genes; carrying an empty vector pVV2; overproducing DprE2 or DprE1/DprE2 complex) were unchanged.
Based on these findings, the derivative 4l does not affect the synthesis of investigated lipidic molecules and our hypothesis was not confirmed. It is an analogy of pyridine-1,3,4-oxadiazole hybrids [Citation11].
Conclusion
Despite the introduction of new antituberculosis drugs, there is a need for new antimycobacterial agents due to the development of resistance. Heterocyclic compounds are of great importance in the design of these drugs. The combination of multiple heterocycles in one molecule, both fused and linked directly or by various bridges, is also very common. This approach allows different scaffolds to be combined and thus mutually promote the desired antimicrobial activity and prevent rapid development of resistance. It can also be advantageously used together with isostery. The pyrimidine cycle is present in many naturally occurring essential compounds (nucleobases, etc.) and drugs, whereas 1,3,4-oxadiazole is found in numerous drugs in development.
Based on these premises, the newly designed antimycobacterial molecules combine pyrimidine, 1,3,4-oxadiazole and alkylamine moieties in a single molecular entity that has not been reported previously in terms of both synthesis and antimicrobial activity.
A total of 23 hydrazinecarboxamides were prepared by reacting the corresponding hydrazides with commercially available or in-house prepared isocyanates. They were then cyclized with p-tosyl chloride under basic conditions to give 5-(pyrimidinyl)-1,3,4-oxadiazole-2-amines in sufficient yields. Both series were investigated against three mycobacterial strains. In general, the best activity is associated with an intermediate-length of N-alkyl (C8-C12). Oxadiazoles are more potent than their acyclic precursors against M. tuberculosis. In contrast to pyrimidine ring substitution by various substituents, the pyrimidin-2-yl positional isomer is tolerated. Overall, many of the compounds showed antimycobacterial activity against M. tuberculosis H37Rv, nontuberculous mycobacteria, as well as MDR- and XDR-TB strains without cross-resistance to clinically used anti-TB drugs with MIC values from 2 μM.
The investigation of the mechanism of action did not elucidate any molecular targets involved in the biosynthesis of the lipidic part of the mycobacterial cell wall. Thus, the chosen approaches and molecular design were successful, as we identified several derivatives with activity in the low micromolar range.
Molecular hybridization and isosteric approaches were used to design novel compounds to combat mycobacteria and address drug resistance.
Hybrid compounds combining 1,3,4-oxadiazole, pyrimidine and n-alkylamine scaffolds were prepared and biologically evaluated.
The synthetic methods used provided good-to-excellent yields and they tolerate different substrates.
Both oxadiazoles and their acyclic hydrazinecarboxamide precursors selectively inhibited Mycobacterium tuberculosis and nontuberculous mycobacteria at low micromolar concentrations.
No cross-resistance to currently used drugs was observed, whereas drug-resistant strains were susceptible at the same concentrations as fully susceptible strains.
In general, oxadiazoles are more active than their acyclic precursors, especially when combined with C8–C12 alkylamine groups.
The majority of compounds met criteria for drug-likeness (Lipinski rule of 5).
Investigation of the mechanisms of action showed that their cellular target is not involved in the biosynthesis of the lipid portion of the mycobacterial cell wall.
Author contributions
M Krátký was responsible for conceptualization; V Pflégr, J Stolaříková, J Korduláková and M Krátký were responsible for methodology; V Pflégr, J Stolaříková, A Pál, J Korduláková and M Krátký were responsible for investigation; V Pflégr, J Korduláková and M Krátký were responsible for writing – original draft preparation; J Korduláková and M Krátký were responsible for writing – review and editing; J Korduláková and M Krátký were responsible for supervision. J Korduláková and M Krátký were responsible for funding acquisition.
Supplemental Text 1
Download PDF (12.1 MB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at:www.tandfonline.com/doi/full/10.2217/epi-2016-0184
Financial & competing interests disclosure
This work was supported by the project National Institute of Virology and Bacteriology (Programme EXCELES ID project no. LX22NPO5103) – Funded by the European Union – Next Generation EU); Ministry of Health of the Czech Republic (grant no. NU21-05-00482); Charles University (SVV 260 661); the Slovak Research and Development Agency (grant no. APVV-19-0189); and the OPII, ACCORD, ITMS2014+: 313021X329, co-financed by ERDF. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance has been used in the creation of this manuscript.
References
- WHO . Global Tuberculosis Report 2020. World Health Organization (2020). https://apps.who.int/iris/rest/bitstreams/1312164/retrieve
- WHO . Tuberculosis and COVID-19 (2020). www.who.int/teams/global-tuberculosis-programme/covid-19
- WHO . WHO announces updated definitions of extensively drug-resistant tuberculosis (2021). www.who.int/news/item/27-01-2021-who-announces-updated-definitions-of-extensively-drug-resistant-tuberculosis/
- Rahama O , ThakerH. Atypical mycobacteria: an important differential for the general physician. Clin. Med.13, 504–506 (2013).
- Perez-Miranda J , TraversiL , PolverinoE. Atypical mycobacteria in bronchiectasis. When do we treat it?Arch. Bronconeumol. (Engl. Ed.)55(4), 183–184 (2019).
- Lu HJ , WangHH , ZhaoHY , ZhangDF. Recent advances in oxazolidinones as antituberculosis agents. Future Med. Chem.14, 1149–1165 (2022).
- Finger V , KufaM , SoukupO , CastagnoloD , RohJ , KorabecnyJ. Pyrimidine derivatives with antitubercular activity. Eur. J. Med. Chem.246, 114946 (2023).
- Alexandrova LA , KhandazhinskayaAL , MatyuginaES , MakarovDA , KochetkovSN. Analogues of pyrimidine nucleosides as mycobacteria growth inhibitors. Microorganisms10, 1299 (2022).
- Imran M , KhanSA , AsdaqSMBet al. An insight into the discovery, clinical studies, compositions, and patents of macozinone: a drug targeting the DprE1 enzyme of Mycobacterium tuberculosis. J. Infect. Public Health15, 1097–1107 (2022).
- Navarrete-Vázquez G , Molina-SalinasGM , Duarte-FajardoZVet al. Synthesis and antimycobacterial activity of 4-(5-substituted-1, 3, 4-oxadiazol-2-yl)pyridines. Bioorg. Med. Chem.15, 5502–5508 (2007).
- Vosátka R , KrátkýM , ŠvarcováMet al. New lipophilic isoniazid derivatives and their 1,3,4-oxadiazole analogues: synthesis, antimycobacterial activity and investigation of their mechanism of action. Eur. J. Med. Chem.151, 824–835 (2018).
- Yang R , CaoW , LiuSet al. Evaluation of a novel inhibitor of aspartate semialdehyde dehydrogenase as a potent antitubercular agent against Mycobacterium tuberculosis. J. Antibiot.75, 333–340 (2022).
- Metre TV , JoshiSD , KodasiBet al. L-proline catalyzed ring transformation of 5-substituted tetrazole to 1,3,4-oxadiazoles as anti-tubercular agents. Synth. Commun.52(13–14), 1500–1516 (2022).
- Wang A , XuS , ChaiYet al. Design, synthesis and biological evaluation of nitrofuran-1,3,4-oxadiazole hybrids as new antitubercular agents. Bioorg. Med. Chem.53, 116529 (2022).
- De SS , KhambeteMP , DeganiMS. Oxadiazole scaffolds in anti-tuberculosis drug discovery. Bioorg. Med. Chem. Lett.29(16), 1999–2007 (2019).
- Glomb T , ŚwiątekP. Antimicrobial activity of 1,3,4-oxadiazole derivatives. Int. J. Mol. Sci.22, 6979 (2021).
- Verma SK , VermaR , VermaS , VaishnavY , TiwariSP , RakeshKP. Anti-tuberculosis activity and its structure-activity relationship (SAR) studies of oxadiazole derivatives: a key review. Eur. J. Med. Chem.209, 112886 (2021).
- Servusova-Vanaskova B , JandourekO , PaterovaPet al. Alkylamino derivatives of N-benzylpyrazine-2-carboxamide: synthesis and antimycobacterial evaluation. MedChemComm.6(7), 1311–1317 (2015).
- Ivasiv V , AlbertiniC , GonçalvesAE , RossiM , BolognesiML. Molecular hybridization as a tool for designing multitarget drug candidates for complex diseases. Curr. Top. Med. Chem.19, 1694–1711 (2019).
- Lawhorn BG , PhilpJ , ZhaoYet al. Identification of purines and 7-deazapurines as potent and selective type I inhibitors of troponin I-interacting kinase (TNNI3K). J. Med. Chem.58(18), 7431–7448 (2015).
- Shaikh AS , KiranmaiG , DeviGPet al. Exploration of mercaptoacetamide-linked pyrimidine-1,3,4-oxadiazole derivatives as DNA intercalative topo II inhibitors: cytotoxicity and apoptosis induction. Bioorg. Med. Chem. Lett.65, 128697 (2022).
- El Mansouri A , OubellaA , DânounKet al. Discovery of novel furo[2, 3-d]pyrimidin-2-one-1,3,4-oxadiazole hybrid derivatives as dual antiviral and anticancer agents that induce apoptosis. Arch. Pharm.354, e2100146 (2021).
- Wyeth LLC . Ayral-KaloustianS , MansourTK , TsouHRet al.3-Substituted-1H-pyrrolo[2,3-b]pyridine and 3-substituted-1H-pyrrolo[3, 2-b]pyridine compounds, their use as mTOR kinase and pi3 kinase inhibitors, and their syntheses. WO2010/030727, A1 (2010).
- Pflégr V , Štěpánkovአ, SvrčkováK , ŠvarcováM , VinšováJ , KrátkýM. 5-Aryl-1,3,4-oxadiazol-2-amines decorated with long alkyl and their analogues: synthesis, acetyl- and butyrylcholinesterase inhibition and docking study. Pharmaceuticals15, 400 (2022).
- Pflégr V , HorváthL , StolaříkováJet al. Design and synthesis of 2-(2-isonicotinoylhydrazineylidene)propanamides as InhA inhibitors with high antitubercular activity. Eur. J. Med. Chem.223, 113668 (2021).
- Krátký M , BaranyaiZ , Štěpánkovአ, SvrčkováK , ŠvarcováM , StolaříkováJ , HorváthL , BőszeS , VinšováJ. N-Alkyl-2-[4-(trifluoromethyl)benzoyl]hydrazine-1-carboxamides and their analogues: synthesis and multitarget biological activity. Molecules25, 2268 (2020).
- Folch J , LeesM , SloaneStanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem.226, 497–509 (1957).
- Brecik M , CentarovaI , MukherjeeRet al. DprE1 Is a vulnerable tuberculosis drug target due to its cell wall localization. ACS Chem. Biol.10, 1631–1636 (2015).
- Karabanovich G , DusekJ , SavkovaKet al. Development of 3,5-dinitrophenyl-containing 1,2,4-triazoles and their trifluoromethyl analogues as highly efficient antitubercular agents inhibiting decaprenylphosphoryl-β-D-ribofuranose 2′-oxidase. J. Med. Chem.62, 8115–8139 (2019).