Abstract
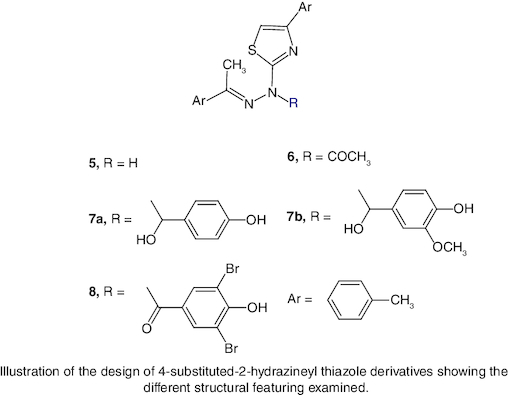
Background: 4-Methylacetophenone is used in the preparation of starting materials, 4-methylphenacyle bromide (2) and 4-methylacetophenone thiosemicarbazole (3). Results: Several novel 2,4-disubstituted-1,3-thiazole analogues were obtained via the treatment of starting materials with 4-methylphenacyl bromide, acetyl chloride, aromatic aldehydes and bromination providing thiazole derivatives 5–8 respectively. Conclusion: Compounds 5–8 were investigated for their cytotoxic activity on MCF-7 and normal breast cells. Active compounds were found and in contrast to staurosporine, compound 8 displayed the most potent cytotoxic action that showed a strong inhibitory effect (aromatase) and (protein tyrosine kinase) enzymes, proving that the novel thiazole derivatives promoted the effective anticancer drug candidates.
Graphical abstract
In this study, a docking simulation investigation between compound 8 and the binding pocket of the tyrosine kinase and aromatase protein receptors showed the hydrophilic and hydrophobic interactions of compound 8.
Thiazole derivatives were investigated for their cytotoxic activity on MCF-7 in vitro and normal breast cells.
Our synthetic route led to the formation of 4-methylphenacyl bromide (2) and 4-methylacetophenone thiosemicarbazone (3).
The majority of the compounds examined demonstrated strong anti-breast cancer effects and IC50 values between 3.36 and 6.09 μg/ml showing higher activity than the reference drug staurosporine (5.25 μg/ml).
The target compound 8 induced apoptosis in the pre-G1 phase and halted the cell cycle in the G1 and S phases.
As the compounds retained their activity against breast cancer cell lines with IC50, this could be a starting point for further development toward new anticancers.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Financial disclosure
The work was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project no. PNURSP2024R155, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, and Deanship of Scientific Research, King Khalid University, Saudi Arabia (research group project no. RGP. 2/12/44). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project (no. PNURSP2024R155), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the large group research project under grant no. RGP2/12/44.
