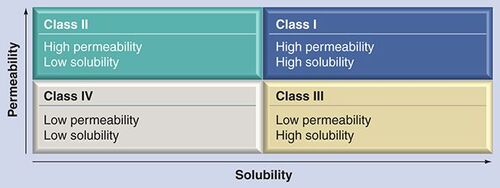
The changing paradigm in formulation development
The acceleration of the drug-discovery process resulting from improved screening approaches and automation is putting increasing demands on the formulation capabilities in drug development. A recent article by Cuatrecasas stressed the importance of developing improved formulations and dosage forms Citation[1]. Typically, preformulation and formulation screening occurs during the preclinical and clinical development phases of pharmaceutical R&D. Preformulation involves an early characterization of the physicochemical properties of an active pharmaceutical ingredient (API). The US FDA has provided guidance on the classification of APIs based on their solubility and permeability . This information is important in the eventual development and characterization of a clinical formulation. Issues addressed in the preformulation stage typically involve solubility, compatability with excipients and short-term stability. Proper attention to preformulation is recognized as an important time and cost saver in the optimization of the development of a successful clinical formulation.
Given the increasing number of chemicals being evaluated in discovery and development, a compelling case can be made for earlier attention to formulation development in the discovery phase . Failure to do this runs the risk of formulation development becoming a significant bottleneck in the drug-development process.
PD: Pharmacodynamic; PK: Pharmacokinetic.
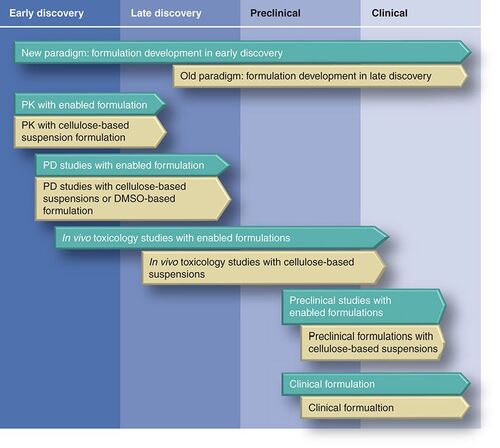
Oral delivery remains the preferred and most frequent route of small-molecule administration. Thus, earlier focus on improving oral bioavailability could have a significant benefit in increasing the likelihood of success of early proof-of-concept studies and in reducing attrition due to poor apparent oral bioavailability. The main reasons frequently expressed for the relatively limited focus on improving formulation earlier in drug discovery appear to be limited compound availability and the requirement for a very rapid turnaround time. We have recently developed and implemented a screening strategy that addresses these concerns and is now being routinely applied to lead identification and optimization with significant success Citation[2].
Much of the current focus in formulation technologies is concerned with approaches to improve the API solubility. This is self evident since it is well established that the solutions will typically have higher oral absorption and that a drug’s dissolution is by and large linearly correlated with the amount absorbed. In addition, approaches for improving permeability remain few, and of comparatively limited success. Thus, formulations and excipients remain the major approach by which formulators attempt to improve the absorption of orally administered compounds. However, optimizing the oral absorption of APIs continues to be a significant challenge.
A considerable number of excipients and technologies currently exist to address solubility and permeability limitations with oral administration of drugs Citation[3–5]. In addition, newer excipients and technologies (e.g., nanoparticles, self-emulsifying drug delivery systems and self microemulsifying drug delivery systems) provide additional approaches for improving formulation success.
However, most of these advances have been focused towards compounds in drug development, and there has been comparatively little focus on the application of these formulation strategies and technologies to the discovery phase of drug development Citation[6–8]. This often results in the use of a common formulation (e.g., cellulose-based) irrespective of whether the formulation is a solution or suspension. Alternatively, if a compound cannot be formulated for oral delivery, other routes are frequently selected to obtain proof-of-principle (e.g., intraperitoneal, subcutaneous or diet) even though these can be far removed from the intended eventual route of administration for the drug. This is not surprising, and is often justifiable, given the increasing lypophilicity that we are seeing for compounds being tested in drug discovery. However, with a little more attention on the evaluation of different excipients earlier in drug discovery, compounds that are often administered as unsatisfactory suspensions, could be administered as solutions, or at least as more homogenous emulsions or suspensions.
The increasing role of the pharmaceutical scientist
In recent years we have seen an increasing role for the pharmaceutical scientist in drug discovery Citation[9,10]. There is both a growing interest, and compelling reason, for applying the expertise of formulation and the pharmaceutical scientist at an earlier stage in the drug-discovery and development process. By enhancing the solubility of a compound, excipients can have a profound effect on bioavailability, pharmacokinetics and pharmacodynamics of the API Citation[2,11,12]. Solution formulations can optimize the properties of an API (e.g., by maximizing the exposure following administration, enhancing the onset of action due to the elimination of the dissolution phase, reducing variability and improving ease of administration). Suboptimal formulation approaches may result in the elimination of potentially promising drug candidates owing to lower exposure and providing inaccurate assessment of potential efficacy and toxicity Citation[7].
Factors impacting oral delivery & absorption
Solubility, increased lipophilicity, permeability, gastrointestinal (GI) metabolism and p-glycoprotein efflux can be limitations in oral delivery. The excipients in discovery formulations should be chosen with consideration of the effect excipients could have on the efficacy and toxicity of the compound. The physicochemical properties of a compound influence the excipients employed. More hydrophilic compounds (i.e., ClogP value < 3.5) can be solubilized in an aqueous solution, with or without co-solvents or surfactants, whereas, for more lipophilic compounds with higher CLogP values, nonaqueous formulations may be required to achieve a solution Citation[13].
The animal species employed and disease model limitations influence the choice of excipients utilized. FDA-approved excipients for human use may not be well tolerated in certain animal species Citation[14]. For example, repeated oral administration of cyclodextrins has been shown to cause GI distress in rodents, leading to loss in body weight Citation[15]. Dextrose-based formulations should be avoided in diabetes disease animal models, and ethanol avoided when investigating behavioral effects of compounds. The animal model and length of a study also affect the choice and concentration of excipients used. For longer term studies, milder formulations are preferred; for example, polyethylene 400 can be employed at 30% w/v concentration orally for a short-term study. However, in long-term repeat-dose studies, polyethylene glycol can lead to significant changes in GI motility, potentially confounding pharmacodynamic and toxicological study outcomes. Thus, the choice of excipients in discovery formulations should be chosen with consideration for their possible impact on the efficacy and toxicity of the compound Citation[16,17]. Some of the more commonly used excipients and techniques employed in the preparation of oral liquid formulations are listed in .
Moving forward
Excipients and techniques commonly employed in the preparation of oral liquid formulations have been previously described Citation[8,18] and include: pH adjustment and in situ salt formations, surfactants, co-solvents, cyclodextrins, lipids and suspending agents. The formulation strategy we have developed employs excipients from all of these classes and a combination of techniques is often utilized, to achieve solubilization. The formulation screen we have developed is both rapid and requires minimal amounts of compound (6 mg) and time (3 h) to evaluate the suitability of the various excipient classes. It is important to stress that the formulation at this stage in no way reflects an optimal and final formulation. However, and most importantly, it does test whether a solution can be achieved and, as we have seen from our studies, significantly improve oral bioavailability and thereby chances of success in lead identification and optimization Citation[2]. It will be evident from , that this earlier focus on improving formulation significantly moves the information and understanding of the compound and its formulation much sooner in the discovery process than is routinely applied. As mentioned, this strategy not only improves the chances of successfully achieving proof-of-concept earlier, but also may potentially shorten the development time of optimal formulation development later in the development cycle.
In summary, we believe there is a compelling reason for an increased focus on improving formulations in early discovery, which can have an important impact on drug discovery and development. The field of drug discovery has been increasingly moving to the earlier screening for properties that may delay or impede drug approval and success. For example, it is well documented that issues around absorption, distribution, metabolism and excretion, pharmacokinetics, safety and efficacy are know to be important contributors to late-stage drug failure Citation[18]. Thus, intuitively, it would appear that spending just a little more time on improving the integrity of a formulation early in discovery could significantly improve the chances for success in lead identification and optimization and save considerable time and money. We believe we are starting to see this trend in the pharmaceutical industry, slowly moving towards early preformulation and formulation development.
Table 1. Excipients employed in oral liquid formulations.
Acknowledgements
The authors acknowledge the valuable help of Melinda M Albright in the preparation of this manuscript.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
Bibliography
- Cuatrecasas P . Interview with Pedro Cuatrecasas.Nat. Rev. Drug Discov.8, 446 (2009).
- Gopinathan S , NouraldeenA, WilsonAGE. Development and application of a high throughput formulation screening strategy in drug discovery.Curr. Drug Discovery Tech. (2009) (In Press).
- Sastry SV , NyshadhamJR, FixJA. Recent technological advances in oral drug delivery – a review.Pharmaceut. Sci. Tech. Today3, 138–145 (2000).
- Gupta H , BhandariD, SharmaA. Recent trends in oral drug delivery: a review.Recent Pat. Drug Deliv. Formulation3, 162–173 (2009).
- Singh BN , KimKH. Drug delivery: oral route. In: Encyclopedia of Pharmaceutical Technology (3rd Edition). Informa Healthcare, London, UK 1242–1265 (2006).
- Maas J , KammW, HauckG. An integrated early formulation strategy – from hit evaluation to preclinical candidate profiling.Eur. J. Pharm. Biopharm.66, 1–10 (2007).
- Neervannan N . Preclinical formulations for discovery and toxicology: physicochemical challenges.Expert Opin. Drug Metabol. Toxicol.2, 715–731 (2006).
- Li P , ZhaoL. Developing early formulations: practice and perspective.Int. J. Pharm.341, 1–19 (2007).
- Venkatesh S , LipperRA. Role of the development scientist in compound lead selection and optimization.J. Pharm. Sci.89, 145–154 (2000).
- Lee YC , ZocharskiPD, SamasB. An intravenous formulation decision tree for discovery compound formulation development.Int. J. Pharm.253, 111–119 (2003).
- Gonzalez RCB , HuwylerJ, WalterI, MountfieldR, BittnerB. Improved oral bioavailability of cyclosporin A in male Wistar rats: comparison of a Solutol HS 15 containing self-dispersing formulation and a microsuspension.Int. J. Pharm.245, 143–151 (2002).
- Klein S , WempeMF, ZoellerTet al. Improving glyburide solubility and dissolution by complexation with hydroxybutenyl-β-cyclodextrin. J. Pharm. Pharmacol. 61, 23–30 (2009).
- Lipinski CA , LombardoF, DominyBW, FeeneyPJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings.Adv. Drug Delivery Rev.46, 3–26 (2001).
- Gould S , ScottRC. 2-hydroxypropyl-b-cyclodextrin (HP-b-CD): a toxicology review.Food Chem. Toxicol.43, 1451–1459 (2005).
- Gad SC , CassidyCD, AubertN, SpainhourB, RobbeH. Nonclinical vehicle use in studies by multiple routes in multiple species.Int. J. Toxicol.25, 499–521 (2006).
- Pestel S , Martin H-J, Maier G-M, Guth B. Effect of commonly used vehicles on gastrointestinal, renal, and liver function in rats. J. Pharmacol. Toxicol. Methods54, 200–214 (2006).
- Strickley G . Solubilizing excipients in oral and injectable formulations.Pharm. Res.21, 201–230 (2004).
- Kola I , LandisJ. Can the pharmaceutical industry reduce attrition rates?Nat. Rev. Drug Discov.3(8), 711–715 (2004).