Abstract
Prostate cancer (PCa) has variable biological potential with multiple treatment options. A more personalized approach, therefore, is needed to better define men at higher risk of developing PCa, discriminate indolent from aggressive disease and improve risk stratification after treatment by predicting the likelihood of progression. This may improve clinical decision-making regarding management, improve selection for active surveillance protocols and minimize morbidity from treatment. Discovery of new biomarkers associated with prostate carcinogenesis present an opportunity to provide patients with novel genetic signatures to better understand their risk of developing PCa and help forecast their clinical course. In this review, we examine the current literature evaluating biomarkers in PCa. We also address current limitations and present several ideas for future studies.
Lay abstract: Several biological markers as well as collections of genetic mutations have been identified by researchers that are associated with the development and aggressiveness of prostate cancer. They can also predict death from prostate cancer in combination with other known risk factors. Some are commercially available for use in current clinical practice. These personalized genetic profiles can help select patients that are most likely to benefit from definitive treatment with either surgery or radiation therapy. Future studies, however, need to focus on biological manipulation of known high-risk genes to reduce the incidence of this disease for future generations.
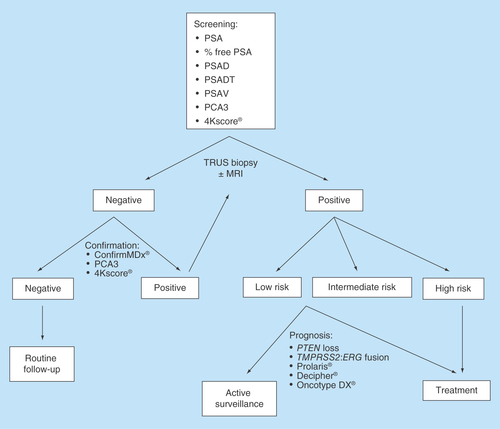
Figure 1. Proposed algorithm for prostate cancer management.
MRI: Magnetic resonance imaging; PCA3: Prostate cancer antigen 3; PSA: Prostate specific antigen; PSAD: PSA density; PSADT: PSA doubling time; PSAV: PSA velocity; TRUS: Transrectal ultrasound.
Background
Prostate cancer (PCa) remains the most common urologic malignancy and the second most common cause of cancer death for men in developed countries [Citation1]. Since the advent of early detection efforts such as PSA screening and increased public awareness, PCa is frequently diagnosed in its initial stages. Reports over the past decade also indicate that a significant percentage of men with newly diagnosed PCa may be managed by active surveillance (AS) [Citation2]. PCa screening, however, is still controversial since early detection can lead to the diagnosis and overtreatment of clinically insignificant disease with long-term effects on patient quality of life [Citation3,Citation4]. Additionally, no reliable screening tool exists that can consistently differentiate indolent versus potentially life-threatening disease [Citation5].
A personalized, patient-centered approach to both the diagnosis and treatment of PCa is necessary due to the variability in disease behavior as well as the multitude of treatment options. Additionally, an individualized approach may improve prognostic criteria and minimize the risk of unnecessary treatment and any associated impairment in quality of life.
Improvements in technology aimed at genetic analysis have led to the discovery of an abundance of new biomarkers that may be utilized in the prediction of PCa incidence, outcomes and response to therapy [Citation6]. The characterization of genetic mutations in tumor tissue through advanced technologies, such as microarray analyses and next-generation sequencing, can subsequently create personalized road maps to guide clinical decision-making due to a better understanding of a patient's risk of progression [Citation7].
We have summarized below the utilization of PCa biomarkers in current clinical practice, their limitations and possible future considerations for their use. We discuss the potential application of biomarkers as a screening or diagnostic tool in men at high risk of developing PCa and as a prognostic tool to discriminate indolent versus aggressive disease, aiming to improve treatment strategies in PCa patients at either a localized or advanced stage. Metabolomic PCa biomarkers, which have recently come to the forefront as a noninvasive investigational tool for PCa detection using in vivo magnetic resonance spectroscopy (MRS), are not discussed in the following review as they have yet to develop mainstream utilization in current clinical practice [Citation8,Citation9]. There is a growing body of evidence, however, that supports the inclusion of MRS in PCa diagnosis and treatment due to their improved sensitivity and specificity in predicting disease prognosis and for patient stratification through the metabolic fingerprint of different biofluids [Citation10–14].
Genetic risk factors in PCa
Family history of PCa in first-generation male relatives is a well-recognized risk factor for developing the disease with twin studies suggesting that greater than 40% of PCa risk is inheritable [Citation15]. Through the Human Genome Project, analysis of constitutional DNA has shown over 60 PCa susceptibility loci explaining approximately a third of the familial risk have been identified, and multiple single nucleotide polymorphisms (SNPs) have been correlated with risk of developing disease [Citation16,Citation17]. Individuals carrying five of these risk alleles were almost tenfold more likely to develop PCa than men carrying no risk alleles (odds ratio [OR]: 9.46), but inclusion of this genotype did not add significantly in the prediction of PCa when added to established risk factors such as age and family history [Citation18].
Rarer variants may be associated with higher relative PCa risk [Citation19]. Coding variants in the homeobox B13 (HOXB13) were found in <0.1% of controls, but 1.4% of patients with a strong family history of early-onset PCa [Citation20]. Although PCa risk stratification including genetic variants are currently limited since they do not add appreciably to the utility of more established clinical risk factors, inclusion of higher-risk variants may improve the performance of these models.
Germline BRCA mutations, which have dramatic genetic implications in breast and ovarian cancer, have also been associated with PCa. BRCA1 and BRCA2 play central roles in DNA repair by homologous recombination, which is the mechanism that cells use to repair double-stranded breaks induced, for example, by platinum-based chemotherapeutic agents or ionizing radiation (XRT). Previous studies have demonstrated that BRCA1 and BRCA2 mutations are predictive factors for response to platinum-based chemotherapy in breast and ovarian cancer [Citation21,Citation22]. BRCA2 mutations have subsequently been shown to confer a higher risk of PCa in men ≤65 years of age (OR: 8.6) [Citation23], and they were an independent prognostic factor for disease-specific survival (DSS) in all stages of PCa including localized disease [Citation24]. Castro et al. evaluated tumor features and outcomes of 1302 patients with local or locally advanced PCa (including 67 BRCA mutation carriers) and found that at 3, 5 and 10 years after treatment (radical prostatectomy [RP] or XRT), noncarriers were more likely to be free from metastasis than carriers (97 vs 90%, 94 vs 72% and 84 vs 50%, respectively, [p < 0.001]). Noncarriers also had better 3-, 5- and 10-year cancer-specific survival (CSS) rates than carriers (99 vs 96%, 97 vs 76% and 85 vs 61%, respectively, [p < 0.001]) [Citation25].
Genetic characterization may play an important clinical role in the future. HOXB13, for example, is associated with low-risk PCa, while BRCA2 is associated with intraductal carcinoma, an early-onset aggressive PCa with a poor prognosis [Citation26]. These susceptibility genes are also racially and ethnicity dependent and act in an additive fashion. Genotyping all men at susceptibility loci, however, could also lead to the overdiagnosis and overtreatment of potentially indolent PCa, subjecting more patients to the morbidity associated with treatment. It is our hope that screening and preventative therapy could be tailored according to risk stratification algorithms incorporating genetic mapping along with family history [Citation27].
Biomarkers in PCa diagnosis
Prostate-specific antigen & its derivatives
The most common current screening test for PCa is a measurement of the serum concentration of prostate-specific antigen (PSA), which currently cannot reliably predict adverse pathological features [Citation28]. Additionally, there is no single cutoff value for PSA that can accurately distinguish patients with PCa from those without [Citation29]. Age-specific upper reference limits for serum total PSA have been created and adjusted according to race (i.e., Caucasian, African–American, etc.) [Citation30,Citation31], and percent-free PSA (free-to-total PSA ratio) may also improve the performance of PSA-based testing [Citation32]. SNPs at six loci have additionally been identified that indicate a significant association between PSA levels and patients without PCa, suggesting that PSA thresholds for biopsy could be personalized based on the patient's genotype at these loci [Citation33]. A brief description of the various PSA-based tests frequently utilized in clinical practice is listed in , and a summary of commercially available biomarkers commonly used in clinical practice for PCa detection is listed in .
PSA kinetics (velocity & doubling time)
There has been considerable uncertainty about PSA kinetics for decisions about prostate biopsy for diagnosis. Recent studies, including analyses of cohorts from all the major randomized trials of localized PCa, have failed to find any evidence that PSA velocity (PSAV) and application of PSA cut points are of benefit in screening. Additionally, PSA dynamics are related to cancer growth rates only partially due to both malignant and nonmalignant processes contributing to PSA fluctuations. Nevertheless, PSA dynamics have been claimed to aid in the long-term prediction of PCa detection [Citation38], and guidelines for PCa diagnosis include a recommendation that men with a PSAV greater than 0.35 ng/ml per year should consider biopsy even if their total PSA is low [Citation39]. Others, however, have refuted the concept that the rate of change of PSA is of any value [Citation40]. A brief definition of PSA kinetics is provided in .
Ulmert et al. tested whether PSAV improved the accuracy of a model using PSA level to predict long-term risk of PCa diagnosis in 4907 screened men (of which 443 [9%] were diagnosed with PCa) [Citation41]. The authors found that PSAV correlated strongly with PSA level (r = 0.93) and that adding PSAV did not significantly increase the accuracy of PSA to predict PCa development. These results were also unchanged, if the analysis was restricted to patient with advanced cancer at diagnosis.
In a systemic review of PSA kinetics in the diagnosis of PCa, PSAV added little predictive value to PSA alone (area under curve [AUC] = 0.83 vs 0.81) [Citation42]. Several other groups have reported that PSAV and PSA doubling time (PSADT) failed to improve the specificity of PSA for biopsy [Citation43,Citation44]. PSAV was not shown to aid in PCa detection in men with prior negative biopsies [Citation45]. Data from the REDUCE trial also clearly indicate that changes in PSAV are unable to predict the risk of a repeat positive biopsy after initially negative findings [Citation46]. In a study from the PCPT, PSAV added very little to the AUC of standard predictors alone (0.709 vs 0.702) with no improvement in the detection of high-grade cancer (0.792 reduced to 0.791) [Citation47]. Using PSAV did not improve the sensitivity of PSA and would lead to many additional biopsies per year without a corresponding increase in the number of high-grade cancers detected.
Conversely, there are several studies that advocate the use of PSAV and PSADT in addition to total PSA for the detection of PCa. In a retrospective cohort of 219,388 men with at least three PSA measurements, Wallner et al. reported that the annual percent change in PSA accurately predicted the presence of PCa (AUC = 0.963) and the presence of aggressive disease (AUC = 0.955) with more accuracy than a single measurement of PSA alone (AUC = 0.727) [Citation34]. Orsted et al. also showed that when long-term PSAV was added to models already including the baseline PSA value, the age-adjusted hazard ratio (HR) for PCa detection and PCa-related death increased from 2.7 to 5.3 and from 2.3 to 3.4, respectively [Citation48]. The authors, therefore, concluded that long-term PSAV in addition to baseline PSA values improved the classification of PCa risk and mortality.
Due to the conflicting evidence with regards to PSAV and PSADT as a screening tool for PCa diagnosis, several expert consensus recommendations have been developed [Citation49]. These include: first, high PSAV is not an indication for biopsy; second, men with a low PSA but a high PSAV should have another PSA drawn at a shorter time interval; third, men with an indication for biopsy should be biopsied regardless of PSAV; fourth, changes in PSA after negative biopsy findings do not indicate the need for repeat biopsy, and lastly, PSA monitoring over time can aid in clinical decision-making about biopsy but judgment should ultimately be informed by the clinical context.
PCA3
PCA3 is a noncoding RNA with expression confined to the prostate, which is highly overexpressed in 95% of PCa cases compared with benign prostatic tissue [Citation50]. Some men, however, may have a very high PCA3, but no evidence of malignancy [Citation51]. It is commercially available as a diagnostic test that quantitatively detects PCA3 RNA expression in the urine and prostatic fluid after prostatic massage with a score higher than 35 in the urine correlating with an average sensitivity and specificity of 66 and 76%, respectively, for the diagnosis of PCa (compared with a sensitivity of 65% and specificity of 47% for PSA alone) [Citation35]. Elevated PCA3 can also increase the probability of a positive repeat prostate biopsy in men with one or two prior negative biopsies, and it is the US FDA approved for this indication [Citation52]. As such, it may play a complimentary role to PSA alone in PCa screening.
Prostate health index
The Beckman Coulter Prostate Health Index (PHI) combines total, free and [-2]proPSA into a single score to improve PCa detection [Citation53]. As a component of free PSA, [-2]proPSA is more specific for PCa than total or free PSA alone [Citation36,Citation54]. Catalona et al. evaluated the PHI in a prospective, multi-institutional trial of 892 men with a PSA between 2 and 10 ng/ml and a negative digital rectal examination (DRE) [Citation55]. The predictive ability of PHI to diagnose PCa (AUC = 0.703) exceeded that of serum PSA (AUC = 0.525), free PSA (AUC = 0.615), p2PSA (the primary form in PCa tissue) (AUC = 0.557) or percent-free PSA (AUC = 0.648). PHI also performed better than percent-free PSA in its ability to discriminate PCa with a Gleason score (GS) ≥4 + 3 = 7 versus lower grade PCa or benign histology (AUC = 0.724 vs 0.670).
Loeb et al. also externally validated the PHI in a multicenter prospective trial of 658 men aged ≥50 years with a PSA of 4–10 ng/ml and normal DRE who underwent prostate biopsy [Citation56]. Based on Epstein criteria, the PHI was able to detect clinically significant disease (AUC = 0.698) with more accuracy than percent-free PSA (AUC = 0.654), [-2]proPSA (AUC = 0.550) or PSA (AUC = 0.549) alone, suggesting its use to reduce prostate biopsies and the overdiagnosis of indolent disease.
Kallikrein panel
Another promising serum-based biomarker is the kallikrein panel (4k-panel) (OPKO Health Inc., FL, USA) that consists of total PSA, free PSA, intact PSA and human kallikrein-related peptidase 2 (KLK2) [Citation57]. This prostate ‘4Kscore®’ was shown by Vickers et al. to improve the predictive accuracy of PCa detection over PSA alone (AUC: 0.711 vs 0.585) with a reduction in the biopsy rate by 362 for every 1000 men with elevated PSA [Citation58]. Similar results have been seen in externally validated populations in France and The Netherlands with an improvement in the detection of high-grade cancers (AUC increased from 0.77 to 0.87 and 0.76 to 0.87, respectively) [Citation59,Citation60]. When comparing the 4k-panel to the PHI, both similarly improved discrimination when predicting both PCa and high-grade PCa, reducing the number of unnecessary biopsies compared with PSA alone [Citation61]. The 4k-panel has also been shown to enhance the prediction of lethal PCa associated with metastasis compared with PSA alone, providing an additional use as a screening tool for men with elevated PSA [Citation62]. In patients with elevated PSA (>3 ng/ml), low percent-free PSA or suspicious DRE, the 4k-panel of kallikrein markers improved discrimination over age and PSA for high-grade cancer (GS ≥7; AUC = 0.77 vs 0.720; p = 0.002), reducing the number of biopsies by 236 per 1000 to detect 195 of 208 high-grade cancers [Citation63].
TMPRSS2: ERG fusion & β-microseminoprotein
Tomlins et al. recognized one of the most frequent gene-specific alterations in PCa represented by a fusion between theTMPRSS2 gene and the v-ets avian erythroblastosis virus ERG gene [Citation64]. Detection of the TMPRSS2: ERG fusion in the urine after prostatic massage has been reported to yield more than 90% specificity and 94% positive predictive value (PPV) for PCa detection [Citation65]. The sensitivity of this assay, however, is low since 40–50% of prostate tumors carry this fusion, but it can be improved in combination with PCA3. Urinary detection of PCA3 and TMPRSS2: ERG with serum PSA levels has also been reported to improve PCa screening performance compared with PSA alone [Citation66]. Other biomarkers in an active area of research include urinary concentrations of β-microseminoprotein, whose levels are decreased in PCa compared with normal prostate tissue [Citation67]. Decreased urinary levels of β-microseminoprotein have been shown to improve PCa diagnosis over urinary PSA but not serum PSA, so further testing is required to demonstrate any potential utility of this test.
DNA methylation markers
Hypermethylation of CpG islands in the promoter regions of cancer-associated genes is linked to PCa [Citation68]. Three of these genes include GSTP1 [Citation68], which is involved in DNA detoxification; APC, which is involved in cell apoptosis, migration and adhesions [Citation69]; and RSSF1, which is involved in cell cycle regulation [Citation70]. A recent meta-analysis concluded that GSTP1 methylation occurs in up to 90% of PCa cases (on both tissue biopsy and RP specimens), while it is only seen in 5% of noncancerous controls [Citation37]. GSTP1 is also the most widely reported hypermethylated gene in PCa [Citation71]. Since DNA hypermethylation of key genes occurs early during oncogenesis, it is ideally suited for PCa detection [Citation72]. Due to the concept of epigenetic field effect in prostate carcinogenesis [Citation73], investigators have evaluated whether biopsy samples taken with negative pathology findings may produce a positive molecular result since genetic alterations occur in histopathologically nonmalignant tissue that is contiguous with cancerous tissue [Citation74].
In the MATLOC study, Stewart et al. determined the degree of methylation of GSTP1, APC and RASSF1 to detect PCa in initial histopathologically negative biopsy samples from men who were subsequently rebiopsied [Citation75]. This epigenetic assay resulted in a negative predictive value (NPV) of 90% (compared with 70% for histopathology alone), and it was an independent predictor of PCa detection up to 30 months before repeat biopsy. In the follow-up DOCUMENT study, this epigenetic test was externally validated in 350 subjects with an initial negative prostate biopsy across five urological centers in the USA [Citation76]. It resulted in a NPV of 88% and again was an independent predictor of PCa detection on multivariate analysis after correcting for age, PSA, DRE, histopathological characteristics on first biopsy and race. Negative findings of this assay, therefore, could be used to decrease concern about unsampled cancer and avoid unnecessary repeat prostate biopsies. The impact of this epigenetic test (commercially available as ConfirmMDx® [MDxHealth, CA, USA]) on rebiopsy rates was recently surveyed at five centers, and among 138 patients with a negative assay, only six patients (4%) underwent repeat biopsy [Citation77].
Biomarkers in PCa prognosis
PSA kinetics & PSA density
The role of PSA kinetics (PSAV and PSADT) as a tool for PCa prognosis has been extensively studied and is still highly ambiguous as a predictive factor in disease aggressiveness [Citation78]. Khatami et al. and Soloway et al. both reported that preoperative PSADT was a statistically significant predictor of disease progression and PSA relapse after RP [Citation79,Citation80]. Ross et al., however, reported a 35% rate of disease progression on repeat biopsy in an AS program at median follow-up of 2.9 years, and neither PSAV nor PSADT was a significant predictor of progression on univariate analysis [Citation28]. Whitson et al. similarly revealed that PSADT was not significantly associated with risk of biopsy disease progression (grade or volume increase) [Citation81]. Iremashvili et al. reported that PSAV significantly predicted tumor progression in certain subgroups, including men undergoing their fourth or later surveillance biopsy, but in the overall population, there was no significant increase in the predictive accuracy compared with PSA alone [Citation82]. Additionally, PSADT was not associated with biopsy progression in any group in this study. Conversely, San Francisco et al. found that PSAV along with PSADT and family history was highly predictive of disease progression (≥3 positive cores, GS ≥7 or >50% core volume) [Citation83]. Finally, Patel et al. examined PSAV risk count (number of times PSAV exceeded 0.4 ng/ml per year), which outperformed standard PSAV as a predictor of disease progression on AS [Citation84]. The 5-year probability of biopsy reclassification (GS >6, ≥3 positive cores, >50% core volume) was 9.7, 18.7 and 39.5%, respectively, with risk counts of 0, 1 and 2 (p < 0.01), respectively, and men with a risk count higher than 1 had a four-times greater risk of reclassification on multivariate analysis. For men with a risk count of 0–1, the NPV for reclassification the following year was 91%, providing a potential means to reduce invasive biopsies. Use of PSA kinetics as a stand-alone test to determine PCa progression, such as in AS programs, yields highly mixed results, but it could be used to trigger further diagnostic intervention such as MRI or saturation biopsy.
PSA density (PSAD), on the other hand, may predict GS upgrading in AS protocols for low-risk disease [Citation85]. Barayan et al. and Dall'Era et al. both reported that a PSAD >0.15 ng/ml per cubic centimeter at diagnosis was an important predictor for disease progression and increasing GS on repeat biopsy [Citation86,Citation87]. Venkitaraman et al. also showed that PSAD was a predictor of histologic disease progression (primary GS ≥4, >50% positive cores or GS increase from ≤6 to ≥7) on univariate analysis, but it did not reach statistical significance on multivariate analysis (p = 0.069) [Citation88]. A limitation of PSAD, however, is inaccuracy of assessing prostate volume on transrectal ultrasound [Citation89], and loss of significance in the face of new PSA-based tests such as proPSA and PSAV risk count [Citation90]. Tosoian et al. found percent [-2]proPSA and PHI provided the greatest predictive accuracy (over PSAD) for reclassification to high-grade cancer in patients on AS [Citation91], and Hirama et al. showed baseline [-2]proPSA and PHI (but not PSAD) predicted pathological reclassification at 1 year in patients with low-risk disease [Citation92].
Current recommendations suggest making treatment decisions such as AS versus surgery versus XRT or adjuvant therapy, irrespective of PSAV, PSADT or PSAD, using established prediction models based on disease stage, grade and total PSA. PSAV, however, at the time of recurrence should be entered into prognostic models to aid patient counseling since PSA changes after treatment for advanced disease can help indicate therapeutic effectiveness with a rising PSA on treatment indicating likely nonresponse.
PCA3 & TMPRSS2: ERG fusion
The data on PCA3 and PCa prognosis are limited. Tosoian et al. examined urinary PCA3 in men with very low-risk PCa prospectively in an AS cohort and found that PCA3 had poor discrimination (AUC = 0.589) and was not significantly associated with short-term biopsy progression on multivariate analysis after accounting for age and diagnosis date (p = 0.15) [Citation93]. Newer reports, however, have found a significant association between elevated PCA3 and GS ≥7 on subsequent prostate biopsy (p = 0.02) as well as an improved overall detection of PCa (82.1% sensitivity and 79.3% specificity) [Citation94].
Although gene fusions, specifically E26 transformation-specific (ETS) fusions such as the TMPRSS2: ERG translocation, have been associated with the early onset of PCa, its clinical utility as a prognostic tool is still unclear. Nam et al. reported that the expression of the TMPRSS2: ERG gene fusion on polymerase chain reaction (PCR) of prostate specimens was independently predictive of biochemical recurrence (BCR) after RP (HR: 8.6) [Citation95] as well as disease progression in a small, selected cohort of intermediate-risk patients with GS 7 adenocarcinoma [Citation96]. In contrast, Saramaki et al. demonstrated lower BCR risk after RP associated with the TMPRSS2: ERG gene fusion based on fluorescence in situ hybridization (FISH) [Citation97]. Steurer et al. found that the TMPRSS2: ERG gene fusion was associated with low-grade tumors in younger patients but not with overall outcomes [Citation98], and Dal Pra et al. demonstrated no association between the TMPRSS2: ERG gene fusion and BCR in almost 250 men treated with intensity-modulated radiation therapy (IMRT) [Citation99]. Gopalan et al., Hoogland et al. and Minner et al. similarly found no association between the TMPRSS2:ERG gene fusion and BCR or local recurrence after RP [Citation100–102]. A large meta-analysis of 5074 men following RP also found no significant association between the TMPRSS2: ERG gene fusion and BCR or disease progression [Citation103]. Further analysis beyond the overall expression of the gene fusion, however, can provide more significant prognostic value. FitzGerald et al. did not observe an association between the TMPRSS2: ERG gene fusion and overall clinical outcomes in PCa patients, but men with increased copy numbers showed poorer survival [Citation104]. Additionally, Boormans et al. reported that the TMPRSS2: ERG Exon 0 gene fusion was associated with a lower risk of BCR compared with the Exon 1 fusion [Citation105].
Presence of the TMPRSS2: ERG gene fusion may play more of a role in predicting outcomes in AS populations or watchful waiting (WW) cohorts undergoing palliative transurethral resection of the prostate (TURP). Berg et al. evaluated 265 men on AS and reported that expression of TMPRSS2: ERG gene fusion in biopsy specimens was independently associated with progression risk (58.6 vs 21.7%; HR: 2.45) [Citation106]. Lin et al. found that on univariate analysis, urinary PCA3 and TMPRSS2: ERG expression were significantly associated with higher volume disease and higher grade disease, but this was not significant with biopsy reclassification on multivariate analysis [Citation107].
Attard et al. and Hagglof et al. both showed that the TMPRSS2: ERG gene fusion was independently predictive of CSS and overall survival (OS) based on both FISH and immunohistochemistry (IHC) in metastatic patients undergoing palliative TURP [Citation108,Citation109], and this was similarly reproduced by Demichelis et al. and Qi et al. [Citation110,Citation111]. It was thought, therefore, that TMPRSS2: ERG expression could be related to response to androgen deprivation therapy (ADT). Boormans et al., however, found no association between the TMPRSS2: ERG gene fusion with ADT response in PCa patients with lymph node metastases [Citation112]. Similarly, Leinonen et al. found no association between the TMPRSS2: ERG gene fusion and disease progression in ADT-treated patients [Citation113].
Although the TMPRSS2: ERG gene fusion alone has little prognostic impact, its predictive value improves in combination with other investigative markers including other gene-expression panels [Citation114–116]. As result, its clinical utility remains in flux.
Copy number variations
Gains or losses of areas of somatic DNA (i.e., NKX3.1) can have carcinogenic consequences, and PCa overall is characterized by loss of genomic material [Citation117]. Specific gains or deletions as well as the overall burden of copy number variations (CNVs) may have a prognostic role in PCa pathogenesis.
Tsuchiya et al. investigated specific chromosome 8 abnormalities, and loss of 8p22 was associated with an increased risk of BCR and metastatic progression [Citation118]. Loss of 8p22 in prostate biopsy specimens was also associated with PCa radioresistance after image-guided XRT with increased positive biopsies posttreatment [Citation119]. Liu et al. studied the 20 most significant CNVs (15 deletions, five amplifications) in two RP cohorts and found that gain of MYC and deletion of PTEN were significantly associated with PCa-related death (after controlling for pathological stage, GS and initial PSA level) [Citation120]. CNVs of MYC and PTEN were similarly found to be prognostic factors for disease relapse in PCa patients undergoing XRT (HR: 2.58) [Citation121].
Paris et al. found that specific DNA-based biomarkers were associated with advanced disease stage (loss at 8p23.2) and were found to be predictive of postoperative recurrence (gain at 11q13.1) independent of tumor stage and grade [Citation122]. The authors also suggest that a combined set of 39 loci termed the Genomic Evaluators of Metastatic Prostate Cancer (GEMCaP) is associated with PCa recurrence and metastasis. Subsequently, the GEMCaP was demonstrated to offer additional prognostic information above the Kattan nomogram for disease recurrence in high-risk node-negative PCa cases after RP (accuracy = 78% of nomogram + GEMCaP vs 65% of nomogram alone) [Citation123].
As noted from the above studies, CNV analysis may have a prognostic role in PCa patients, but standardization of methods and additional validations studies are required before clinical applications can be planned.
PTEN
One of the most frequently deleted genes in human cancer is a PTEN deletion on chromosome 10, which dephosphorylates lipid-signaling intermediates resulting in deactivation of PI3K signaling, thus controlling cell proliferation and growth [Citation124]. Saal et al. initially correlated PTEN loss with poor outcomes in a variety of malignancies [Citation125]. The prognostic value of PTEN deletion in PCa has subsequently been investigated in several studies. Leinonen et al. demonstrated a higher frequency of PTEN loss in more advanced cases (castrate-resistant PCa [CRPC] compared with localized disease in RP cases) and that PTEN loss was associated with shorter progression-free survival (PFS) in ERG-positive tumors [Citation126]. Yoshimoto et al. reported that homozygous PTEN deletion in prostate specimens was independently associated with BCR risk after RP and was clearly associated with TMPRSS2: ERG gene expression [Citation127]. Furthermore, PTEN loss at the time of RP correlated with clinical parameters of more advanced disease, such as extraprostatic extension and seminal vesicle invasion [Citation128]. Krohn et al. examined over 4700 RP specimens and 57 CRPC cases, and the authors concluded that PTEN loss was independently associated with adverse clinicopathological features and was predictive of BCR and worse PFS after surgery [Citation129]. ERG status, however, did not affect the predictive value of PTEN loss. Reid et al. showed that PTEN loss without TMPRSS2: ERG gene fusion on FISH and IHC was associated with worse CSS in castrate-sensitive cases. This is contrast to prior studies linking PTEN loss and positive TMPRSS2: ERG gene expression with worse outcomes [Citation130]. Finally, McCall et al. compared PTEN status in both castration-sensitive and CRPC, noting that PTEN loss was independently associated with worse CSS but only in castrate-sensitive cases [Citation131]. PTEN-negative tumors, however, have been associated with shorter survival in CRPC in the postchemotherapy setting during treatment with abiraterone [Citation132].
Other markers have been tested in combination with PTEN loss for prognostic information including tumor protein p27 gene loss, HO-1 overexpression and HER2/3 overexpression. Halvorsen et al. reported that combined loss of PTEN and p27 expression defined a group of tumors based on RP specimens associated with increased tumor diameter, seminal vesicle invasion, increased pathological stage and elevated tumor cell proliferation detected by antigen KI-67 [Citation133]. Loss of PTEN and p27 expression was an independent predictor of time to BCR and clinical recurrence. Li et al. assessed the occurrence of both HO-1 expression and PTEN deletion in men with localized and CRPC [Citation134]. HO-1 epithelial expression was statistically different between benign prostate tissue, high-grade PIN, localized PCa and CRPC (p < 0.001). Although neither HO-1 overexpression nor PTEN deletion alone in localized PCa was independently associated with PSA relapse, the combined status of both markers correlated with disease progression (p = 0.01). Inhibition of HO-1 also strongly reduced cell growth and invasion in vitro and inhibited tumor growth and lung metastasis formation in mouse models. Finally, Ahmad et al. showed that patients who developed prostate tumors with low levels of PTEN and high levels of HER2/3 have a poor prognosis [Citation135]. These tumors, however, responded favorably to MAPK enzyme (MEK) inhibition by restoring the PTEN loss.
Loss of the PTEN gene and protein also shows promise as a prognostic biomarker in PCa patients on AS. PTEN status on RP specimens assessed by FISH has shown to be an independent predictor for preoperative PSA and GS with PTEN homozygous deletion associated with worse disease stage [Citation136]. Tumors with PTEN protein loss were also more likely to be upgraded at the time of RP than those without loss, even after adjusting for age, preoperative PSA, clinical stage and race (OR: 3.04; p = 0.035) [Citation137]. PTEN loss in Gleason 6 biopsy specimens identified a unique subset of prostate tumors at increased risk of upgrading at the time of RP. These data provide evidence that the assessment of PTEN status can improve Gleason accuracy and highlight a path toward the clinical use of molecular markers to augment pathologic grading.
Peripheral blood & circulating tumor cells
In addition to genetic information, peripheral blood is a potential source for genomic tumor characterization using free circulating nucleic acids, whole blood transcripts or circulating tumor cells (CTCs). Bastian et al. reported an increasing quantity of circulating cell-free DNA independently associated with the risk of BCR after RP [Citation138]. Ross et al. examined a six-gene panel (consisting of ABL2, SEMA4D, ITGAL, C1QA, TIMP1 and CDKN1A) in CRPC patients with significantly improved prognostic value compared with a clinical model alone (AUC = 0.90 vs 0.65; p = 0.0067) [Citation139], and Olmos et al. identified a nine-gene signature analyzed from blood messenger RNA (mRNA) in CRPC patients that was independently associated with worse OS [Citation140]. Specific circulating miRNAs found not only in tumor tissue but also in the plasma of PCa patients, such as miRNA-375 and miRNA-141, have also been shown to be associated with advanced disease [Citation141].
Danila et al. investigated the expression of five genes frequently detected in PCa cells (KLK3, KLK2, HOXB13, GRHL2 and FOXA1) from whole blood transcripts as well as detection of CTCs [Citation142]. Both an unfavorable CTC count (five or more cells) and detection of two or more gene transcripts had similar significant prognostic value for the risk of PCa-related death, and when combined, additional prognostic value was demonstrated. Additionally, KLK3, PCA3 and TMPRSS2:ERG mRNA could be detected in the peripheral blood of CRPC patients but not in healthy controls [Citation143]. Decreased expression levels of these genes, however, were noted after docetaxel treatment, suggesting a potential role for treatment monitoring. Finally, Scher et al. demonstrated that a biomarker panel using CTC count and lactate dehydrogenase (LDH) was a surrogate for survival at the individual patient level in the Phase III trial of abiraterone plus prednisone versus prednisone alone (COU-AA-301) for patients with CRPC [Citation144]. We suspect that peripheral blood markers and CTCs will continue to gain traction for use in future clinical studies as they can be easily collected with minimal patient morbidity.
Genetic panels in PCa prognosis
Multiple genetic markers are often necessary to provide enough prognostic information for clinical decision-making versus any single genetic abnormality alone. Panels evaluating the differential expression of multiple genes are ideally created using knowledge of well-known carcinogenic pathways in PCa [Citation145]. There is a risk of chance associations due to the number of genetic mutations associated with prostate malignancy, but blinded marker analysis and external validation is essential before any clinical application can be considered [Citation146]. Additionally, the biomarkers in the panel have to provide additional independent predictive ability above and beyond standard clinical and pathological characteristics. Recommendations for reporting the clinical utility of biomarkers in oncology are, therefore, essential to follow [Citation147].
Three commercially available RNA-based genetic panels that have been externally validated in the PCa population and are currently utilized in clinical practice are listed in . It is important to note that there is virtually no overlap between these panels even though they include a total of 85 genes. Additionally, there are no comparative or prospective studies evaluating these expression panels head-to-head in the same patient cohort. The commercial assays may also be less helpful because of the difficulties of using them effectively on the variable amount of tumor present at first diagnosis in needle cores.
Lalonde et al. also recently reported on a panel of DNA-based indices in 126 low-to-intermediate-risk PCa patients in order to develop four genomic subtypes for PCa that were prognostic for relapse after both XRT (HR: 4.5; AUC = 0.70) and RP (HR: 4.0; AUC = 0.57) and whose effect was magnified by intratumoral hypoxia (HR: 3.8; AUC = 0.67) [Citation150]. This novel 100-loci DNA signature was subsequently externally validated in two independent cohorts of RP specimens from low-risk to high-risk patients, and it accurately classified treatment outcomes and identified patients who were most likely to fail treatment within 18 months (HR: 2.9; AUC = 0.68; p = 0.004).
Prolaris®
Cuzick et al. identified a 46-gene-expression model (31 cell-cycle progression genes and 15 housekeeping genes) via quantitative PCR on RNA extracted from PCa tumor samples [Citation145]. This cell cycle progression (CCP) score was evaluated retrospectively in a cohort of RP and TURP patients managed conservatively. The primary end point was BCR in the RP group and OS in the TURP group. The CCP score was an independent predictor of BCR for RP patients on univariate (HR: 1.89) and multivariate (HR: 1.77) analysis, and it was also strongly associated with time to death from PCa for TURP patients on univariate (HR: 2.92) and multivariate (HR: 2.57) analysis. CCP was also found to be a stronger prognostic factor than any other measured variable including PSA.
This gene panel (commercially available as the Prolaris test [Myriad Genetics, UT, USA]) was subsequently externally validated using biopsy and TURP specimens in 349 patients managed conservatively with the primary end point being PCa-specific mortality [Citation151]. The CCP score was again independently associated with PCa-related death on univariate (HR: 2.02) and multivariate (HR: 1.65) analysis with GS and PSA providing significant additional contributions.
The Prolaris test has also been externally validated in RP studies. Cooperberg et al. analyzed 413 men who underwent RP and determined whether the CCP score (analyzed on RP tissue) could predict BCR (defined as two PSA levels ≥0.2 ng/ml) [Citation148]. The CCP score was also assessed for independent prognostic utility beyond a stand postoperative risk assessment instrument such as the Cancer of the Prostate Risk Assessment post-Surgical (CAPRA-S) score, which uses pathological data from RP to predict PCa recurrence and mortality [Citation152] and has been externally validated in over 2500 men across multiple institutions (concordance index [c-index] = 0.72 for BCR and 0.85 for PCa-specific mortality) [Citation149]. CCP was independently predictive of BCR after RP on univariate (HR: 2.1) and multivariate (HR: 1.7) analysis after adjusting for the CAPRA-S score. CCP also was able to substratify patients with low clinical risk as defined by CAPRA-S ≤2 (HR: 2.3). Combining the CCP and CAPRA-S scores improved the c-index for both the overall cohort and the low-risk subset, suggesting that the combined score outperformed both individual scores in clinical decision-making (c-index = 0.77 for genetic + clinical model vs 0.73 for clinical model alone).
Bishoff et al. also evaluated the CCP score as a predictor of BCR and metastatic disease based on pre-RP biopsy tissue in 582 men treated with RP for clinical localized PCa [Citation153]. CCP was the strongest predictor of BCR on univariate (HR: 1.6) and multivariate (HR: 1.47) analysis, and it was also the strongest predictor of metastatic disease on univariate (HR: 5.35) and multivariate (HR: 4.19) analysis after adjusting for other clinical variables.
Freedland et al. tested the prognostic utility of the Prolaris test in 141 PCa patients treated with external beam radiation therapy (EBRT) as their primary curative therapy using pretreatment diagnostic prostate biopsy specimens to predict BCR. BCR was defined using the Pheonix definition, and half of the cohort was African–American. The BCR rate was 13% (n = 19), and the CCP score significantly predicted BCR on univariate (p = 0.0017) and multivariate (p = 0.034) analysis accounting for GS, PSA, percent-positive cores and use of ADT. The CCP score also was associated with PCa-specific mortality at 10 years (p = 0.013).
The impact of the CCP score was prospectively analyzed based on physician-completed surveys regarding PCa treatment recommendations in 305 men before and after they received the Prolaris test results [Citation154]. Clinicians also rated the influence of the test on treatment decisions. Surprisingly, the Prolaris test changed physician treatment recommendations in 65% of cases, and in 40% there was a reduction in treatment burden (interventional treatment changed to noninterventional). The long-term impact of these changes on patient outcomes, however, is not known.
Oncotype DX®
Klein et al. identified 17 genes through PCR based on 441 RP specimens from low- to intermediate-risk patients representing multiple biological pathways in PCa out of 732 candidate genes (288 predicted clinical recurrence and tumor multifocality, and 198 were predictive of aggressive disease after adjustment for PSA, GS and clinical stage) [Citation155]. This 17-gene-expression panel was further confirmed in 167 pre-RP biopsy specimens to create a multigene, expression-based signature called the Genomic Prostate Score (GPS). This algorithm was subsequently internally validated in 395 needle biopsies from contemporary patients who were candidates for AS to determine its ability to predict clinical recurrence, PCa-related death and adverse pathological features at the time of RP. In this validation study, the GPS predicted high-grade (Gleason ≥4 + 3; OR: 2.3) and high-stage (pT3 or higher; OR: 1.9) disease on RP specimens after controlling for established clinical factors (age, PSA, clinical stage, biopsy GS), and this was similarly true after inclusion of a clinical risk model (i.e., CAPRA-S; OR: 2.1).
This test (commercially available as the Oncotype DX test [Genomic Health, CA, USA]) was subsequently externally validated by Cullen et al. in 431 prostate biopsies from men with very low-, low- or intermediate-risk PCa based on National Comprehensive Cancer Network (NCCN) guidelines [Citation156]. GPS scores were correlated with BCR, adverse pathology (primary Gleason patter 4 or any pattern 5, pT3 disease) and metastatic recurrence. GPS scores did not vary across race with similar distributions of results, and it was an independent predictor of BCR (HR: 2.7), time to metastases (HR: 3.8) and adverse pathology (OR: 3.3) after adjusting for the NCCN risk group. The Oncotype DX test performed on a patient's original prostate needle biopsy, therefore, may predict the aggressiveness of PCa and help men make decisions regarding immediate treatment versus AS.
Decipher®
Erho et al. presented a case-control study that analyzed a 22-gene-expression signature in men with early clinical metastasis after rising PSA to predict survival after RP [Citation157]. The 22-gene panel (based on the primary tumor in the RP specimen) was the only significant prognostic factor for early metastasis and PCa-specific death on multivariable analysis, and it had a good correlation with DSS on internal validation (AUC = 0.75). Patients with higher scores experienced earlier death from PCa and reduced OS, suggesting its use as an identifier of aggressive prostatic malignancy.
This 22-gene panel (commercially available as the Decipher test [GenomeDX Biosciences, BC, USA]) was externally validated in multiple studies. Cooperberg et al. compared the CAPRA-S score and the Decipher genomic classifier (GC) as a predictor of PCa-specific mortality in 185 men at high risk of recurrence (PSA >20, GS ≥8, stage pT3b) after RP of whom 25 experienced PCa-associated death [Citation158]. The c-indices for CAPRA-S and Decipher GC were 0.75 and 0.78, respectively, and Decipher GC reclassified many men stratified to high-risk based on the CAPRA-S score ≥6. Both high CAPRA-S and Decipher GC scores were independently predictive of PCa-specific mortality (HR: 2.36 and 11.26, respectively) with a cumulative incidence of PCa-related death of 45% at 10 years. This suggests that integration of genomic and clinical classifiers may enable better identification of post-RP patients at high-risk for death who should be considered for more aggressive secondary therapies and clinical trials.
Karnes et al. also evaluated 219 men at high risk of recurrence (PSA >20, GS ≥8, stage pT3b) with the Decipher test based on the genomic information from the primary tumor in the RP specimen [Citation159]. The Decipher GC AUC was 0.79 for predicting 5-year metastasis after RP, which exceeded that of clinical models. The Decipher GC was also the predominant predictor of metastasis 5 years after RP on multivariate analysis with a cumulative incidence of 2.4, 6.0 and 22.5% in patients with low (60%), intermediate (21%) and high (19%) scores, respectively (p < 0.001).
In another study, 85 clinically high-risk RP patients who developed BCR after surgery were analyzed using the Decipher GC as a predictor of metastatic disease progression [Citation160]. The AUC of Decipher GC for predicting metastasis after BCR was 0.82 compared with 0.64 for GS, 0.69 for PSADT and 0.52 for time to BCR. It was the only variable associated with metastatic progression on multivariate analysis (p = 0.003), and it performed superiorly to models solely based on clinicopathological features (i.e., CAPRA-S).
Finally, studies have also evaluated the utility of Decipher GC to predict which men would benefit from adjuvant XRT therapy as well as to forecast outcomes after postoperative XRT following RP. Den et al. calculated Decipher GC scores from the primary tumor specimens of 188 patients with pT3 or margin-positive PCa after RP who received post-RP XRT [Citation161]. The primary end point was clinical metastasis, and the prognostic accuracy of Decipher GC was compared with other routine clinical and pathological features. Decipher GC and pre-RP PSA were both independent predictors of metastasis (p < 0.01) with a cumulative incidence at 5 years of 0, 9 and 29% for low, average and high GC scores. For patients with low GC scores, there was no difference in the cumulative incidence of metastasis comparing patients who received adjuvant vs salvage XRT (p = 0.79), but for patients with high GC scores, the cumulative incidence of metastasis at 5 years was significantly lower in patients who received adjuvant vs salvage XRT (6 vs 23%; p < 0.01). These findings suggest that patients with high Decipher GC scores may benefit from adjuvant XRT, while patients with low Decipher GC scores are best treated with salvage XRT.
This same group also reported on the ability of the Decipher GC score to predict BCR and distant metastasis in men receiving XRT after RP [Citation162]. The authors evaluated 139 PCa patients with pT3 or positive margins after RP who received postoperative XRT (both adjuvant and salvage) and found that the addition of Decipher GC improved the AUC of a clinical model (i.e., Stephenson model) from 0.70 to 0.78 and from 0.70 to 0.80 as a predictor of BCR and distant metastasis, respectively. On multivariate analysis, high Decipher GC scores were independent predictors of BCR (HR: 8.1) and distant metastasis (HR: 14.3) with a cumulative incidence of 21, 48 and 81% for BCR and 0, 12 and 17% for distant metastasis for low, intermediate and high GC scores, respectively. The Decipher GC, therefore, may be predictive of oncological outcomes after post-RP XRT and improve decision-making in high-risk cases.
Biomarkers in PCa response to treatment
Genetic markers that can predict response or resistance to treatment could be valuable in the selection of a therapy with the greatest likelihood of efficacy in an individual patient. They may also categorize patients into clinical trials based on the presumed likelihood of response to the tested agents.
It is well known that ADT is first-line therapy for metastatic PCa. Inherited genetic factors, however, may account for variability in response to ADT. Ross et al. identified three polymorphisms (CYP19A1, HSD3B1 and HSD17B4) independently associated with longer time to progression (TTP) during ADT on multivariate analysis [Citation163]. Patients carrying more than one of these polymorphisms demonstrated a better response to ADT than patients carrying none (p < 0.0001). The SLCO1B3 polymorphism, on the other hand, was found to be associated with a shorter time to androgen independence in PCa patients due to its enhanced effect on testosterone import [Citation164]. Yang et al. also identified three SNPs in SLCO2B1 associated with shorter TTP on ADT. Individuals carrying the SLCO1B3 and SLCO2B1 genotype allowed for more efficient import of androgen and enhanced cell growth associated with a median 2-year worse TTP on ADT [Citation165].
Although ADT remains first-line treatment for metastatic PCa, predicting the response of CRPC to various novel agents may have added value. Theoretically, tumors with AR amplification or overexpression would be expected to respond well to abiraterone, which further decreases circulating androgens. Conversely, tumors with AR mutations leading to promiscuity to nonandrogen substrates or loss of AR expression would be expected to respond well to enzalutamide, which prevents AR nuclear import. CRPC mediated through active splice variants lacking the ligand-binding domain at the carboxy-terminal of the AR protein might require novel agents targeting the amino-terminal of the protein [Citation166]. Multiple markers have also been implicated in taxane resistance in CRPC, including elevated class III β-tubulin expression [Citation167,Citation168]. These markers, however, have yet to be prospectively validated.
Peripheral blood genetic information may be useful to predict therapeutic response in CRPC. Pretherapy CTC counts have been demonstrated to predict response to therapy, and a decrease in the number of CTCs after therapy had a greater predictive value than the classic 50% PSA decrease [Citation169]. This was observed after treatment with both docetaxel and abiraterone [Citation170,Citation171]. Antonarakis et al. also recently reported that a splice variant of the androgen receptor (AR-V7) could be detected in CTCs from CRPC patients [Citation172]. AR-V7-positive patients had lower PSA response rates to abiraterone and enzalutamide than AR-V7-negative patients (0 vs 68% [p = 0.004] and 0 vs 53% [p = 0.004], respectively). They also had a shorter PSA PFS (median = 1.3 months vs not reached [p < 0.001] and 1.4 vs 6.0 months [p < 0.001], respectively), shorter clinical or radiographic PFS (median = 2.3 months vs not reached [p < 0.001] and 2.1 vs 6.1 months [p < 0.001], respectively) and shorter OS (median = 10.6 months vs not reached [p = 0.006] and 5.5 months vs not reached [p = 0.004], respectively). One limitation of this study, however, is the fact that AR-V7-positive patients had a greater overall disease burden than AR-V7-negative patients, so it is unclear whether AR-V7 represents a predictive factor of response to abiraterone and enzalutamide in CRPC or simply a marker of more advanced disease.
Conclusion
Newer molecular biomarkers allow the clinician to better evaluate patients for the detection of clinically significant prostate cancer preventing over diagnosis and overtreatment. They are becoming a powerful tool for risk-stratification and for directing treatment.
Future perspective
A possible future algorithm in the diagnosis and prognosis of PCa is demonstrated in . Challenges still remain, however, in the development of genetic markers in the PCa population. PCa is a heterogeneous disease both within a single tumor locus (intrafocal heterogeneity) and between different tumor deposits (interfocal heterogeneity) [Citation173]. Ongoing abnormal mutational processes give rise to extensive branching evolution and cancer clone mixing, which can be demonstrated by the coexistence of multiple cancer lineages harboring distinct ERG fusions within a single PCa nodule [Citation174]. There is a field effect of molecular changes as benign areas of the same prostate can show cancer-related genetic changes [Citation175]. Discordance may also exist between metastatic versus primary lesions, so tissue sampling is of the utmost importance. The morbidity and costs associated with biopsy of metastatic or primary lesions, however, may dissuade patients from participation in clinical trials. CTCs in the peripheral blood may offer an alternative option for disease monitoring since genetic and molecular changes in prostate CTCs have shown to predict response to therapy in metastatic lesions.
Second, although combinations of multiple markers (gene-expression profiles) have been tested as predictors of disease prognosis and clinical response in PCa with three commercially approved assays available, there seems to be no commonality in the prognostic genes identified, raising questions as to the randomness of these identified biomarkers and whether they just represent downstream pathways in carcinogenesis rather than inciting genetic mutations. These genetic panels also have to be validated in future prospective studies to truly gain credibility, and testing them head-to-head with known clinicopathological risk factors will ultimately determine their added prognostic value. Inflammatory biomarker panels may provide an alternative option in the near future as emerging data suggest that an improved understanding of the tumor–host microenvironment within the prostate will lead to new immunobiomarkers that may be very informative.
Finally, although large-scale efforts have been placed into identifying genetic markers and molecular indicators in the diagnosis and prognosis of PCa, investigation into PCa prevention is still lacking. Despite knowledge of inherited alleles that contribute to PCa development, genetic manipulation of these susceptibility loci has still not been possible to reduce the incidence of disease and the burden of treatment in a high-risk population. Additionally, whether genetic or biochemical engineering can be used to change the natural history and the overall aggressiveness of PCa is still not known and may be the subject of future investigation.
Table 1. Description of prostate-specific antigen-based markers utilized in clinical practice for prostate cancer diagnosis.
Table 2. Commercially available biomarkers utilized in clinical practice for prostate cancer diagnosis.
Table 3. Commercially available RNA-based gene panels utilized in clinical practice for prostate cancer prognosis.
Advances in the genotyping and understanding of biochemical pathways involved in prostate cancer (PCa) development and carcinogenesis have led to the possibility of personalized genetic profiling of primary and metastatic tumor cells.
These biomolecular signatures may become readily available for routine clinical decision-making in PCa patients ranging from the need for repeat biopsy, initial treatment selection, decisions about adjuvant therapy or selection of treatments for advanced disease.
Large clinical trials involving multi-institutional collaborations will be necessary to prospectively validate the utility of these genetic biomarkers in the diagnosis and treatment of PCa.
Future research should also focus on biogenetic engineering in PCa prevention as well as targeted agents to minimize disease aggressiveness in order to reduce the incidence and burden of this malignancy.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Siegel R , MaJ, ZouZ, JemalA. Cancer statistics, 2014. CA Cancer J. Clin.64(1), 9–29 (2014).
- Klotz L , VespriniD, SethukavalanPet al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J. Clin. Oncol.33(3), 272–277 (2015).
- Hugosson J , CarlssonS. Overdetection in screening for prostate cancer. Curr. Opin. Urol.24(3), 256–263 (2014).
- Sanda MG , DunnRL, MichalskiJet al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N. Engl. J. Med.358(12), 1250–1261 (2008).
- Klotz L . Prostate cancer overdiagnosis and overtreatment. Curr. Opin. Endocrinol. Diabetes Obes.20(3), 204–209 (2013).
- Barbieri CE , BangmaCH, BjartellAet al. The mutational landscape of prostate cancer. Eur. Urol.64(4), 567–576 (2013).
- Jeronimo C , BastianPJ, BjartellAet al. Epigenetics in prostate cancer: biologic and clinical relevance. Eur. Urol.60(4), 753–766 (2011).
- Giskeodegard GF , BertilssonH, SelnaesKMet al. Spermine and citrate as metabolic biomarkers for assessing prostate cancer aggressiveness. PLoS ONE8(4), e62375 (2013).
- Zhang VY , WestphalenA, Delos SantosLet al. The role of metabolic imaging in radiation therapy of prostate cancer. NMR Biomed.27(1), 100–111 (2014).
- Huang G , LiuX, JiaoLet al. Metabolomic evaluation of the response to endocrine therapy in patients with prostate cancer. Eur. J. Pharmacol.729, 132–137 (2014).
- Kumar V , DwivediDK, JagannathanNR. High-resolution NMR spectroscopy of human body fluids and tissues in relation to prostate cancer. NMR Biomed.27(1), 80–89 (2014).
- Lucarelli G , RutiglianoM, GalleggianteVet al. Metabolomic profiling for the identification of novel diagnostic markers in prostate cancer. Expert Rev. Mol. Diagn. doi:10.1586/14737159.2015.1069711 (2015) ( Epub ahead of print).
- Shukla-Dave A , HricakH, AkinOet al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int.109(9), 1315–1322 (2012).
- Thapar R , TitusMA. Recent advances in metabolic profiling and imaging of prostate cancer. Curr. Metabolomics2(1), 53–69 (2014).
- Lichtenstein P , HolmNV, VerkasaloPKet al. Environmental and heritable factors in the causation of cancer – analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med.343(2), 78–85 (2000).
- Al Olama AA , DadaevT, HazelettDJet al. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum. Mol. Genet. doi:10.1093/hmg/ddv203 (2015) ( Epub ahead of print).
- Al Olama AA , Kote-JaraiZ, BerndtSIet al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat. Genet.46(10), 1103–1109 (2014).
- Zheng SL , SunJ, WiklundFet al. Cumulative association of five genetic variants with prostate cancer. N. Engl. J. Med.358(9), 910–919 (2008).
- Saunders EJ , DadaevT, LeongamornlertDAet al. Fine-mapping the HOXB region detects common variants tagging a rare coding allele: evidence for synthetic association in prostate cancer. PLoS Genet.10(2), e1004129 (2014).
- Ewing CM , RayAM, LangeEMet al. Germline mutations in HOXB13 and prostate-cancer risk. N. Engl. J. Med.366(2), 141–149 (2012).
- Byrski T , GronwaldJ, HuzarskiTet al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J. Clin. Oncol.28(3), 375–379 (2010).
- Yang D , KhanS, SunYet al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA306(14), 1557–1565 (2011).
- Kote-Jarai Z , LeongamornlertD, SaundersEet al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br. J. Cancer105(8), 1230–1234 (2011).
- Castro E , GohC, OlmosDet al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol.31(14), 1748–1757 (2013).
- Castro E , GohC, LeongamornlertDet al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur. Urol.68(2), 186–193 (2014).
- Risbridger GP , TaylorRA, CloustonDet al. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur. Urol.67(3), 496–503 (2015).
- Macinnis RJ , AntoniouAC, EelesRAet al. A risk prediction algorithm based on family history and common genetic variants: application to prostate cancer with potential clinical impact. Genet. Epidemiol.35(6), 549–556 (2011).
- Ross AE , LoebS, LandisPet al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J. Clin. Oncol.28(17), 2810–2816 (2010).
- Labrie F , DupontA, SuburuRet al. Serum prostate specific antigen as pre-screening test for prostate cancer. J. Urol.147(3 Pt 2), 846–851; discussion 851–842 (1992).
- Morgan TO , JacobsenSJ, MccarthyWF, JacobsonDJ, McleodDG, MoulJW. Age-specific reference ranges for prostate-specific antigen in black men. N. Engl. J. Med.335(5), 304–310 (1996).
- Oesterling JE . Age-specific reference ranges for serum PSA. N. Engl. J. Med.335(5), 345–346 (1996).
- Jansen FH , RoobolM, JensterG, SchroderFH, BangmaCH. Screening for prostate cancer in 2008 II: the importance of molecular subforms of prostate-specific antigen and tissue kallikreins. Eur. Urol.55(3), 563–574 (2009).
- Gudmundsson J , BesenbacherS, SulemPet al. Genetic correction of PSA values using sequence variants associated with PSA levels. Sci. Transl. Med.2(62), 62ra92 (2010).
- Wallner LP , FrencherSK, HsuJWet al. Changes in serum prostate-specific antigen levels and the identification of prostate cancer in a large managed care population. BJU Int.111(8), 1245–1252 (2013).
- Van Gils MP , HesselsD, Van HooijOet al. The time-resolved fluorescence-based PCA3 test on urinary sediments after digital rectal examination; a Dutch multicenter validation of the diagnostic performance. Clin. Cancer Res.13(3), 939–943 (2007).
- Khan MA , PartinAW, RittenhouseHGet al. Evaluation of proprostate specific antigen for early detection of prostate cancer in men with a total prostate specific antigen range of 4.0 to 10.0 ng/ml. J. Urol.170(3), 723–726 (2003).
- Van Neste L , HermanJG, OttoG, BigleyJW, EpsteinJI, Van CriekingeW. The epigenetic promise for prostate cancer diagnosis. Prostate72(11), 1248–1261 (2012).
- Eggener SE , RoehlKA, CatalonaWJ. Predictors of subsequent prostate cancer in men with a prostate specific antigen of 2.6 to 4.0 ng/ml and an initially negative biopsy. J. Urol.174(2), 500–504 (2005).
- Mohler J , BahnsonRR, BostonBet al. NCCN clinical practice guidelines in oncology: prostate cancer. J. Natl Compr. Canc. Netw.8(2), 162–200 (2010).
- Vickers AJ , BrewsterSF. PSA velocity and doubling time in diagnosis and prognosis of prostate cancer. Br. J. Med. Surg. Urol.5(4), 162–168 (2012).
- Ulmert D , SerioAM, O'BrienMFet al. Long-term prediction of prostate cancer: prostate-specific antigen (PSA) velocity is predictive but does not improve the predictive accuracy of a single PSA measurement 15 years or more before cancer diagnosis in a large, representative, unscreened population. J. Clin. Oncol.26(6), 835–841 (2008).
- Vickers AJ , SavageC, O'BrienMF, LiljaH. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J. Clin. Oncol.27(3), 398–403 (2009).
- Vickers AJ , WoltersT, SavageCJet al. Prostate-specific antigen velocity for early detection of prostate cancer: result from a large, representative, population-based cohort. Eur. Urol.56(5), 753–760 (2009).
- Wolters T , RoobolMJ, BangmaCH, SchroderFH. Is prostate-specific antigen velocity selective for clinically significant prostate cancer in screening? European Randomized Study of Screening for Prostate Cancer (Rotterdam). Eur. Urol.55(2), 385–392 (2009).
- Vickers AJ , WoltersT, SavageCJet al. Prostate specific antigen velocity does not aid prostate cancer detection in men with prior negative biopsy. J. Urol.184(3), 907–912 (2010).
- Andriole GL , BostwickD, BrawleyOWet al. The effect of dutasteride on the usefulness of prostate specific antigen for the diagnosis of high grade and clinically relevant prostate cancer in men with a previous negative biopsy: results from the REDUCE study. J. Urol.185(1), 126–131 (2011).
- Thompson IM , Pauler AnkerstD, ChiCet al. Prediction of prostate cancer for patients receiving finasteride: results from the Prostate Cancer Prevention Trial. J. Clin. Oncol.25(21), 3076–3081 (2007).
- Orsted DD , BojesenSE, KamstrupPR, NordestgaardBG. Long-term prostate-specific antigen velocity in improved classification of prostate cancer risk and mortality. Eur. Urol.64(3), 384–393 (2013).
- Vickers AJ , ThompsonIM, KleinE, CarrollPR, ScardinoPT. A commentary on PSA velocity and doubling time for clinical decisions in prostate cancer. Urology83(3), 592–596 (2014).
- Salagierski M , SchalkenJA. Molecular diagnosis of prostate cancer: PCA3 and TMPRSS2: ERG gene fusion. J. Urol.187(3), 795–801 (2012).
- Schroder FH , VenderbosLD, Van Den BerghRCet al. Prostate cancer antigen 3: diagnostic outcomes in men presenting with urinary prostate cancer antigen 3 scores ≥100. Urology83(3), 613–616 (2014).
- Haese A , De La TailleA, Van PoppelHet al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur. Urol.54(5), 1081–1088 (2008).
- Jansen FH , Van SchaikRH, KurstjensJet al. Prostate-specific antigen (PSA) isoform p2PSA in combination with total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur. Urol.57(6), 921–927 (2010).
- Lazzeri M , HaeseA, De La TailleAet al. Serum isoform [-2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2–10 ng/ml: a multicentric European study. Eur. Urol.63(6), 986–994 (2013).
- Catalona WJ , PartinAW, SandaMGet al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J. Urol.185(5), 1650–1655 (2011).
- Loeb S , SandaMG, BroylesDLet al. The prostate health index selectively identifies clinically significant prostate cancer. J. Urol.193(4), 1163–1169 (2015).
- Vickers AJ , CroninAM, AusGet al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Med.6, 19 (2008).
- Vickers AJ , CroninAM, RoobolMJet al. A four-kallikrein panel predicts prostate cancer in men with recent screening: data from the European Randomized Study of Screening for Prostate Cancer, Rotterdam. Clin. Cancer Res.16(12), 3232–3239 (2010).
- Benchikh A , SavageC, CroninAet al. A panel of kallikrein markers can predict outcome of prostate biopsy following clinical work-up: an independent validation study from the European Randomized Study of Prostate Cancer screening, France. BMC Cancer10, 635 (2010).
- Gupta A , RoobolMJ, SavageCJet al. A four-kallikrein panel for the prediction of repeat prostate biopsy: data from the European Randomized Study of Prostate Cancer screening in Rotterdam, Netherlands. Br. J. Cancer103(5), 708–714 (2010).
- Nordstrom T , VickersA, AsselM, LiljaH, GronbergH, EklundM. Comparison between the four-kallikrein panel and prostate health index for predicting prostate cancer. Eur. Urol.68(1), 139–146 (2015).
- Stattin P , VickersAJ, SjobergDDet al. Improving the specificity of screening for lethal prostate cancer using prostate-specific antigen and a panel of kallikrein markers: a nested case-control study. Eur. Urol.68(2), 207–213 (2015).
- Braun K , SjobergDD, VickersAJ, LiljaH, BjartellAS. A four-kallikrein panel predicts high-grade cancer on biopsy: independent validation in a community cohort. Eur. Urol. doi:10.1016/j.eururo.2015.04.028 (2015) ( Epub ahead of print).
- Tomlins SA , RhodesDR, PernerSet al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science310(5748), 644–648 (2005).
- Hessels D , SmitFP, VerhaeghGW, WitjesJA, CornelEB, SchalkenJA. Detection of TMPRSS2–ERG fusion transcripts and prostate cancer antigen 3 in urinary sediments may improve diagnosis of prostate cancer. Clin. Cancer Res.13(17), 5103–5108 (2007).
- Salami SS , SchmidtF, LaxmanBet al. Combining urinary detection of TMPRSS2: ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol. Oncol.31(5), 566–571 (2013).
- Whitaker HC , Kote-JaraiZ, Ross-AdamsHet al. The rs10993994 risk allele for prostate cancer results in clinically relevant changes in microseminoprotein-beta expression in tissue and urine. PLoS ONE5(10), e13363 (2010).
- Harden SV , GuoZ, EpsteinJI, SidranskyD. Quantitative GSTP1 methylation clearly distinguishes benign prostatic tissue and limited prostate adenocarcinoma. J. Urol.169(3), 1138–1142 (2003).
- Jeronimo C , HenriqueR, HoqueMOet al. A quantitative promoter methylation profile of prostate cancer. Clin. Cancer Res.10(24), 8472–8478 (2004).
- Agathanggelou A , CooperWN, LatifF. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res.65(9), 3497–3508 (2005).
- Nakayama M , GonzalgoML, YegnasubramanianS, LinX, De MarzoAM, NelsonWG. GSTP1 CpG island hypermethylation as a molecular biomarker for prostate cancer. J. Cell. Biochem.91(3), 540–552 (2004).
- Mehrotra J , VardeS, WangHet al. Quantitative, spatial resolution of the epigenetic field effect in prostate cancer. Prostate68(2), 152–160 (2008).
- Nonn L , AnanthanarayananV, GannPH. Evidence for field cancerization of the prostate. Prostate69(13), 1470–1479 (2009).
- Chai H , BrownRE. Field effect in cancer-an update. Ann. Clin. Lab. Sci.39(4), 331–337 (2009).
- Stewart GD , Van NesteL, DelvennePet al. Clinical utility of an epigenetic assay to detect occult prostate cancer in histopathologically negative biopsies: results of the MATLOC study. J. Urol.189(3), 1110–1116 (2013).
- Partin AW , Van NesteL, KleinEAet al. Clinical validation of an epigenetic assay to predict negative histopathological results in repeat prostate biopsies. J. Urol.192(4), 1081–1087 (2014).
- Wojno KJ , CostaFJ, CornellRJet al. Reduced rate of repeated prostate biopsies observed in confirmMDx clinical utility field study. Am. Health Drug Benefits7(3), 129–134 (2014).
- Van Den Bergh RC , RoemelingS, RoobolMJ, WoltersT, SchroderFH, BangmaCH. Prostate-specific antigen kinetics in clinical decision-making during active surveillance for early prostate cancer‐‐a review. Eur. Urol.54(3), 505–516 (2008).
- Khatami A , AusG, DamberJE, LiljaH, LoddingP, HugossonJ. PSA doubling time predicts the outcome after active surveillance in screening-detected prostate cancer: results from the European randomized study of screening for prostate cancer, Sweden section. Int. J. Cancer120(1), 170–174 (2007).
- Soloway MS , SolowayCT, WilliamsS, AyyathuraiR, KavaB, ManoharanM. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int.101(2), 165–169 (2008).
- Whitson JM , PortenSP, HiltonJFet al. The relationship between prostate specific antigen change and biopsy progression in patients on active surveillance for prostate cancer. J. Urol.185(5), 1656–1660 (2011).
- Iremashvili V , ManoharanM, LokeshwarSD, RosenbergDL, PanD, SolowayMS. Comprehensive analysis of post-diagnostic prostate-specific antigen kinetics as predictor of a prostate cancer progression in active surveillance patients. BJU Int.111(3), 396–403 (2013).
- San Francisco IF , WernerL, ReganMM, GarnickMB, BubleyG, DewolfWC. Risk stratification and validation of prostate specific antigen density as independent predictor of progression in men with low risk prostate cancer during active surveillance. J. Urol.185(2), 471–476 (2011).
- Patel HD , FengZ, LandisP, TrockBJ, EpsteinJI, CarterHB. Prostate specific antigen velocity risk count predicts biopsy reclassification for men with very low risk prostate cancer. J. Urol.191(3), 629–637 (2014).
- Cary KC , CowanJE, SanfordMet al. Predictors of pathologic progression on biopsy among men on active surveillance for localized prostate cancer: the value of the pattern of surveillance biopsies. Eur. Urol.66(2), 337–342 (2014).
- Barayan GA , BrimoF, BeginLRet al. Factors influencing disease progression of prostate cancer under active surveillance: a McGill University Health Center cohort. BJU Int.114(6b), E99–E104 (2014).
- Dall'Era MA , KonetyBR, CowanJEet al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer112(12), 2664–2670 (2008).
- Venkitaraman R , NormanA, Woode-AmissahRet al. Predictors of histological disease progression in untreated, localized prostate cancer. J. Urol.178(3 Pt 1), 833–837 (2007).
- Loeb S , HanM, RoehlKA, AntenorJA, CatalonaWJ. Accuracy of prostate weight estimation by digital rectal examination versus transrectal ultrasonography. J. Urol.173(1), 63–65 (2005).
- Makarov DV , IsharwalS, SokollLJet al. Pro-prostate-specific antigen measurements in serum and tissue are associated with treatment necessity among men enrolled in expectant management for prostate cancer. Clin. Cancer Res.15(23), 7316–7321 (2009).
- Tosoian JJ , LoebS, FengZet al. Association of [-2]proPSA with biopsy reclassification during active surveillance for prostate cancer. J. Urol.188(4), 1131–1136 (2012).
- Hirama H , SugimotoM, ItoK, ShiraishiT, KakehiY. The impact of baseline [-2]proPSA-related indices on the prediction of pathological reclassification at 1 year during active surveillance for low-risk prostate cancer: the Japanese multicenter study cohort. J. Cancer Res. Clin. Oncol.140(2), 257–263 (2014).
- Tosoian JJ , LoebS, KettermannAet al. Accuracy of PCA3 measurement in predicting short-term biopsy progression in an active surveillance program. J. Urol.183(2), 534–538 (2010).
- Merola R , TomaoL, AntenucciAet al. PCA3 in prostate cancer and tumor aggressiveness detection on 407 high-risk patients: a National Cancer Institute experience. J. Exp. Clin. Cancer Res.34(1), 15 (2015).
- Nam RK , SugarL, YangWet al. Expression of the TMPRSS2: ERG fusion gene predicts cancer recurrence after surgery for localised prostate cancer. Br. J. Cancer97(12), 1690–1695 (2007).
- Nam RK , SugarL, WangZet al. Expression of TMPRSS2: ERG gene fusion in prostate cancer cells is an important prognostic factor for cancer progression. Cancer Biol. Ther.6(1), 40–45 (2007).
- Saramaki OR , HarjulaAE, MartikainenPM, VessellaRL, TammelaTL, VisakorpiT. TMPRSS2: ERG fusion identifies a subgroup of prostate cancers with a favorable prognosis. Clin. Cancer Res.14(11), 3395–3400 (2008).
- Steurer S , MayerPS, AdamMet al. TMPRSS2–ERG fusions are strongly linked to young patient age in low-grade prostate cancer. Eur. Urol.66(6), 978–981 (2014).
- Dal Pra A , LalondeE, SykesJet al. TMPRSS2–ERG status is not prognostic following prostate cancer radiotherapy: implications for fusion status and DSB repair. Clin. Cancer Res.19(18), 5202–5209 (2013).
- Gopalan A , LevershaMA, SatagopanJMet al. TMPRSS2–ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res.69(4), 1400–1406 (2009).
- Hoogland AM , JensterG, Van WeerdenWMet al. ERG immunohistochemistry is not predictive for PSA recurrence, local recurrence or overall survival after radical prostatectomy for prostate cancer. Mod. Pathol.25(3), 471–479 (2012).
- Minner S , EnodienM, SirmaHet al. ERG status is unrelated to PSA recurrence in radically operated prostate cancer in the absence of antihormonal therapy. Clin. Cancer Res.17(18), 5878–5888 (2011).
- Pettersson A , GraffRE, BauerSRet al. The TMPRSS2: ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol. Biomarkers Prev.21(9), 1497–1509 (2012).
- FitzGerald LM , AgalliuI, JohnsonKet al. Association of TMPRSS2–ERG gene fusion with clinical characteristics and outcomes: results from a population-based study of prostate cancer. BMC Cancer8, 230 (2008).
- Boormans JL , PorkkaK, VisakorpiT, TrapmanJ. Confirmation of the association of TMPRSS2(exon 0):ERG expression and a favorable prognosis of primary prostate cancer. Eur. Urol.60(1), 183–184 (2011).
- Berg KD , VainerB, ThomsenFBet al. ERG protein expression in diagnostic specimens is associated with increased risk of progression during active surveillance for prostate cancer. Eur. Urol.66(5), 851–860 (2014).
- Lin DW , NewcombLF, BrownECet al. Urinary TMPRSS2: ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the Canary Prostate Active Surveillance Study. Clin. Cancer Res.19(9), 2442–2450 (2013).
- Attard G , ClarkJ, AmbroisineLet al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene27(3), 253–263 (2008).
- Hagglof C , HammarstenP, StromvallKet al. TMPRSS2–ERG expression predicts prostate cancer survival and associates with stromal biomarkers. PLoS ONE9(2), e86824 (2014).
- Demichelis F , FallK, PernerSet al. TMPRSS2: ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene26(31), 4596–4599 (2007).
- Qi M , YangX, ZhangFet al. ERG rearrangement is associated with prostate cancer-related death in Chinese prostate cancer patients. PLoS ONE9(2), e84959 (2014).
- Boormans JL , HermansKG, MadeACet al. Expression of the androgen-regulated fusion gene TMPRSS2–ERG does not predict response to endocrine treatment in hormone-naive, node-positive prostate cancer. Eur. Urol.57(5), 830–835 (2010).
- Leinonen KA , TolonenTT, BrackenHet al. Association of SPINK1 expression and TMPRSS2: ERG fusion with prognosis in endocrine-treated prostate cancer. Clin. Cancer Res.16(10), 2845–2851 (2010).
- Cheville JC , KarnesRJ, TherneauTMet al. Gene panel model predictive of outcome in men at high-risk of systemic progression and death from prostate cancer after radical retropubic prostatectomy. J. Clin. Oncol.26(24), 3930–3936 (2008).
- Gasi Tandefelt D , BoormansJL, Van Der KorputHA, JensterGW, TrapmanJ. A 36-gene signature predicts clinical progression in a subgroup of ERG-positive prostate cancers. Eur. Urol.64(6), 941–950 (2013).
- Karnes RJ , ChevilleJC, IdaCMet al. The ability of biomarkers to predict systemic progression in men with high-risk prostate cancer treated surgically is dependent on ERG status. Cancer Res.70(22), 8994–9002 (2010).
- Taylor BS , SchultzN, HieronymusHet al. Integrative genomic profiling of human prostate cancer. Cancer Cell18(1), 11–22 (2010).
- Tsuchiya N , SlezakJM, LieberMM, BergstralhEJ, JenkinsRB. Clinical significance of alterations of chromosome 8 detected by fluorescence in situ hybridization analysis in pathologic organ-confined prostate cancer. Genes Chromosomes Cancer34(4), 363–371 (2002).
- Locke JA , ZafaranaG, IshkanianASet al. NKX3.1 haploinsufficiency is prognostic for prostate cancer relapse following surgery or image-guided radiotherapy. Clin. Cancer Res.18(1), 308–316 (2012).
- Liu W , XieCC, ThomasCYet al. Genetic markers associated with early cancer-specific mortality following prostatectomy. Cancer119(13), 2405–2412 (2013).
- Zafarana G , IshkanianAS, MalloffCAet al. Copy number alterations of c-MYC and PTEN are prognostic factors for relapse after prostate cancer radiotherapy. Cancer118(16), 4053–4062 (2012).
- Paris PL , AndayaA, FridlyandJet al. Whole genome scanning identifies genotypes associated with recurrence and metastasis in prostate tumors. Hum. Mol. Genet.13(13), 1303–1313 (2004).
- Paris PL , WeinbergV, AlboGet al. A group of genome-based biomarkers that add to a Kattan nomogram for predicting progression in men with high-risk prostate cancer. Clin. Cancer Res.16(1), 195–202 (2010).
- Cairns P , OkamiK, HalachmiSet al. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res.57(22), 4997–5000 (1997).
- Saal LH , JohanssonP, HolmKet al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc. Natl Acad. Sci. USA104(18), 7564–7569 (2007).
- Leinonen KA , SaramakiOR, FurusatoBet al. Loss of PTEN is associated with aggressive behavior in ERG-positive prostate cancer. Cancer Epidemiol. Biomarkers Prev.22(12), 2333–2344 (2013).
- Yoshimoto M , JoshuaAM, CunhaIWet al. Absence of TMPRSS2: ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod. Pathol.21(12), 1451–1460 (2008).
- Yoshimoto M , CunhaIW, CoudryRAet al. FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br. J. Cancer97(5), 678–685 (2007).
- Krohn A , DiedlerT, BurkhardtLet al. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. Am. J. Pathol.181(2), 401–412 (2012).
- Reid AH , AttardG, AmbroisineLet al. Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. Br. J. Cancer102(4), 678–684 (2010).
- McCall P , WittonCJ, GrimsleyS, NielsenKV, EdwardsJ. Is PTEN loss associated with clinical outcome measures in human prostate cancer?Br. J. Cancer99(8), 1296–1301 (2008).
- Ferraldeschi R , Nava RodriguesD, RiisnaesRet al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur. Urol.67(4), 795–802 (2015).
- Halvorsen OJ , HaukaasSA, AkslenLA. Combined loss of PTEN and p27 expression is associated with tumor cell proliferation by Ki-67 and increased risk of recurrent disease in localized prostate cancer. Clin. Cancer Res.9(4), 1474–1479 (2003).
- Li Y , SuJ, DingzhangXet al. PTEN deletion and heme oxygenase-1 overexpression cooperate in prostate cancer progression and are associated with adverse clinical outcome. J. Pathol.224(1), 90–100 (2011).
- Ahmad I , PatelR, SinghLBet al. HER2 overcomes PTEN (loss)-induced senescence to cause aggressive prostate cancer. Proc. Natl Acad. Sci. USA108(39), 16392–16397 (2011).
- Troyer DA , JamaspishviliT, WeiWet al. A multicenter study shows PTEN deletion is strongly associated with seminal vesicle involvement and extracapsular extension in localized prostate cancer. Prostate75(11), 1206–1215 (2015).
- Lotan TL , CarvalhoFL, PeskoeSBet al. PTEN loss is associated with upgrading of prostate cancer from biopsy to radical prostatectomy. Mod. Pathol.28(1), 128–137 (2015).
- Bastian PJ , PalapattuGS, YegnasubramanianSet al. Prognostic value of preoperative serum cell-free circulating DNA in men with prostate cancer undergoing radical prostatectomy. Clin. Cancer Res.13(18 Pt 1), 5361–5367 (2007).
- Ross RW , GalskyMD, ScherHIet al. A whole-blood RNA transcript-based prognostic model in men with castration-resistant prostate cancer: a prospective study. Lancet Oncol.13(11), 1105–1113 (2012).
- Olmos D , BrewerD, ClarkJet al. Prognostic value of blood mRNA expression signatures in castration-resistant prostate cancer: a prospective, two-stage study. Lancet Oncol.13(11), 1114–1124 (2012).
- Brase JC , JohannesM, SchlommTet al. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer128(3), 608–616 (2011).
- Danila DC , AnandA, SchultzNet al. Analytic and clinical validation of a prostate cancer-enhanced messenger RNA detection assay in whole blood as a prognostic biomarker for survival. Eur. Urol.65(6), 1191–1197 (2014).
- Dijkstra S , LeytenGH, JanninkSAet al. KLK3, PCA3, and TMPRSS2–ERG expression in the peripheral blood mononuclear cell fraction from castration-resistant prostate cancer patients and response to docetaxel treatment. Prostate74(12), 1222–1230 (2014).
- Scher HI , HellerG, MolinaAet al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J. Clin. Oncol.33(12), 1348–1355 (2015).
- Cuzick J , SwansonGP, FisherGet al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol.12(3), 245–255 (2011).
- Pepe MS , FengZ, JanesH, BossuytPM, PotterJD. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J. Natl Cancer Inst.100(20), 1432–1438 (2008).
- Mcshane LM , HayesDF. Publication of tumor marker research results: the necessity for complete and transparent reporting. J. Clin. Oncol.30(34), 4223–4232 (2012).
- Cooperberg MR , SimkoJP, CowanJEet al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J. Clin. Oncol.31(11), 1428–1434 (2013).
- Punnen S , FreedlandSJ, PrestiJCJret al. Multi-institutional validation of the CAPRA-S score to predict disease recurrence and mortality after radical prostatectomy. Eur. Urol.65(6), 1171–1177 (2014).
- Lalonde E , IshkanianAS, SykesJet al. Tumour genomic and microenvironmental heterogeneity for integrated prediction of 5-year biochemical recurrence of prostate cancer: a retrospective cohort study. Lancet Oncol.15(13), 1521–1532 (2014).
- Cuzick J , BerneyDM, FisherGet al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br. J. Cancer106(6), 1095–1099 (2012).
- Cooperberg MR , FreedlandSJ, PastaDJet al. Multi-institutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy. Cancer107(10), 2384–2391 (2006).
- Bishoff JT , FreedlandSJ, GerberLet al. Prognostic utility of the cell cycle progression score generated from biopsy in men treated with prostatectomy. J. Urol.192(2), 409–414 (2014).
- Crawford ED , ScholzMC, KarAJet al. Cell cycle progression score and treatment decisions in prostate cancer: results from an ongoing registry. Curr. Med. Res. Opin.30(6), 1025–1031 (2014).
- Klein EA , CooperbergMR, Magi-GalluzziCet al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur. Urol.66(3), 550–560 (2014).
- Cullen J , RosnerIL, BrandTCet al. A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low- and intermediate-risk prostate cancer. Eur. Urol.68(1), 123–131 (2014).
- Erho N , CrisanA, VergaraIAet al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE8(6), e66855 (2013).
- Cooperberg MR , DavicioniE, CrisanA, JenkinsRB, GhadessiM, KarnesRJ. Combined value of validated clinical and genomic risk stratification tools for predicting prostate cancer mortality in a high-risk prostatectomy cohort. Eur. Urol.67(2), 326–333 (2015).
- Karnes RJ , BergstralhEJ, DavicioniEet al. Validation of a genomic classifier that predicts metastasis following radical prostatectomy in an at risk patient population. J. Urol.190(6), 2047–2053 (2013).
- Ross AE , FengFY, GhadessiMet al. A genomic classifier predicting metastatic disease progression in men with biochemical recurrence after prostatectomy. Prostate Cancer Prostatic Dis.17(1), 64–69 (2014).
- Den RB , YousefiK, TrabulsiEJet al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. J. Clin. Oncol.33(8), 944–951 (2015).
- Den RB , FengFY, ShowalterTNet al. Genomic prostate cancer classifier predicts biochemical failure and metastases in patients after postoperative radiation therapy. Int. J. Radiat. Oncol. Biol. Phys.89(5), 1038–1046 (2014).
- Ross RW , OhWK, XieWet al. Inherited variation in the androgen pathway is associated with the efficacy of androgen-deprivation therapy in men with prostate cancer. J. Clin. Oncol.26(6), 842–847 (2008).
- Sharifi N , HamadaA, SissungTet al. A polymorphism in a transporter of testosterone is a determinant of androgen independence in prostate cancer. BJU Int.102(5), 617–621 (2008).
- Yang M , XieW, MostaghelEet al. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J. Clin. Oncol.29(18), 2565–2573 (2011).
- Andersen RJ , MawjiNR, WangJet al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell17(6), 535–546 (2010).
- Ploussard G , TerryS, MaillePet al. Class III beta-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy. Cancer Res.70(22), 9253–9264 (2010).
- Terry S , PloussardG, AlloryYet al. Increased expression of class III beta-tubulin in castration-resistant human prostate cancer. Br. J. Cancer101(6), 951–956 (2009).
- Danila DC , FleisherM, ScherHI. Circulating tumor cells as biomarkers in prostate cancer. Clin. Cancer Res.17(12), 3903–3912 (2011).
- Danila DC , AnandA, SungCCet al. TMPRSS2–ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate. Eur. Urol.60(5), 897–904 (2011).
- Okegawa T , ItayaN, HaraH, TamboM, NutaharaK. Circulating tumor cells as a biomarker predictive of sensitivity to docetaxel chemotherapy in patients with castration-resistant prostate cancer. Anticancer Res.34(11), 6705–6710 (2014).
- Antonarakis ES , LuC, WangHet al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med.371(11), 1028–1038 (2014).
- Boutros PC , FraserM, HardingNJet al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat. Genet.47(7), 736–745 (2015).
- Cooper CS , EelesR, WedgeDCet al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat. Genet.47(4), 367–372 (2015).
- Vaananen RM , LiljaH, KaukoLet al. Cancer-associated changes in the expression of TMPRSS2–ERG, PCA3, and SPINK1 in histologically benign tissue from cancerous vs noncancerous prostatectomy specimens. Urology83(2), 511.e1–7 (2014).
