Abstract
Aim: The impact of diabetes mellitus (DM) on survival in patients with colorectal cancer and the impact of colorectal cancer on glycemic control were examined. Materials & methods: Patients with colorectal cancer with and without DM were matched 1:1 (2007–2015). Characteristics were compared between the two groups and survival assessed with the Kaplan–Meier method. Mixed models compared hemoglobin A1c and glucose levels over time. Results: In both groups, glucose values decreased during the year following cancer diagnosis (p < 0.001). 5-year overall survival was 56% (95% CI: 42–68%) for DM patients versus 57% (95% CI: 43–69%) for non-DM patients (p = 0.62). Conclusion: DM did not adversely impact survival of patients with colorectal cancer. Colorectal cancer did not affect glycemic control.
Lay abstract The aim of this study was to evaluate the effect of diabetes mellitus (DM) on survival of patients with colorectal cancer and to determine whether colorectal cancer affected glycemic control. From an institutional cancer registry, 170 patients with colorectal cancer were identified and grouped by the presence (n = 85) or absence of DM (n = 85). The groups were matched by age and year of colorectal cancer diagnosis. DM did not decrease the survival and colorectal cancer did not significantly affect glucose levels of patients with DM.
Colorectal cancer is the second most common cause of cancer death in USA [Citation1]. Furthermore, according to data from the Global Burden of Disease Study, mortality globally from colorectal cancer increased annually from the years 1990 through 2013 [Citation2]. In the USA, however, yearly rates of death from colorectal cancer have declined since the mid-1980s [Citation3]. Risk factors for colorectal cancer include genetic syndromes, inflammatory bowel disease and history of abdominal radiotherapy, dietary factors (red meat, alcohol and high-fat/low-fiber diets) and tobacco use. Other risk factors (obesity and lack of physical activity) have commonality with those for diabetes mellitus (DM) [Citation4]. Inflammation associated with DM may also contribute to development and progression of colorectal cancer [Citation5].
Various studies have reported on the relationship between DM and colorectal cancer. Some studies showed that DM conferred an increased risk of developing colorectal cancer or that DM was associated with higher mortality in patients with colorectal cancer [Citation6–11]. In contrast, other studies have shown that DM does not confer worse survival for patients with colorectal cancer [Citation12,Citation13].
Given the complex interplay between DM and colorectal cancer, and the conflicting reports in the literature, this study aimed to analyze data from our outpatient oncology practice to better understand how colorectal cancer might impact glycemic control and how DM might impact survival from colorectal cancer. In a prior analysis, we [Citation14] did not find a statistically significant difference in overall survival (OS) when patients with colorectal cancer and coexisting DM were compared with patients with colorectal cancer but without DM. In our original analysis, however, data for many variables that may have affected our conclusions were not available. Therefore, for this study, we pulled comprehensive data for DM and colorectal cancer variables to investigate whether DM affected survival of patients with colorectal cancer and to analyze whether colorectal cancer and its treatment affected glycemic control among patients with DM.
Methods
Case selection
Case selection was similar to that described for previous studies [Citation15–18]. Mayo Clinic Institutional Review Board approval was obtained for this retrospective, case–control study. Electronic health records of patients with colorectal cancer newly diagnosed from 1 January 2007 to 31 December 2015, were obtained from the institutional cancer registry. In addition to demographic data, the registry contained the date of cancer diagnosis and the grade/stage of the tumor. This initial data file was linked to electronic records to determine which patients had a diagnosis of DM during the study period (International Classification of Diseases, Ninth Revision, diagnostic code 250.00). We excluded patients who received full or partial cancer treatment at another institution or who had another cancer preceding their colorectal cancer diagnosis. As previously described, patients with colorectal cancer and DM (cases) were matched (1:1 using the Greedy algorithm) [Citation19] to patients with colorectal cancer but no DM (controls). Variables included in the matching algorithm were age, sex and year of colorectal cancer diagnosis.
Glucose and hemoglobin A1c (HbA1c) values were derived from the laboratory information system. The electronic health records were then reviewed for additional detailed information: type of colorectal cancer treatment (surgery, chemotherapy, radiotherapy and targeted therapy) and data related to DM (date of DM diagnosis, type of diabetic therapy and diabetic complications).
Statistical analysis
The statistical analyses conducted were similar to those used for our previous studies [Citation15–18]. Patient demographic and clinical characteristics were compared between patients with colorectal cancer with and without DM. Continuous variables were compared using paired t-tests; categorical variables were compared using the McNemar test or Bowker test for symmetry. HbA1c levels during the first year after colorectal cancer diagnosis were evaluated with a linear mixed model in DM cases only (HbA1c values were unavailable for most patients without DM). A fixed effect for time (days) and an individual-specific random effect were included. A similar approach was used for modeling glucose values during that year. Fixed effects included days, case or control designation, an interaction term (days × case – control designation), and patient-specific and matched pair-specific random effects. Optimal glycemic control was defined as a mean glucose value < 126 mg/dl.
OS was defined as the time from colorectal cancer diagnosis until death from any cause. For OS, patients were considered censored at the last known follow-up date when death was not documented in the health records. Progression-free survival (PFS) was defined as the time from colorectal cancer diagnosis until disease progression or death from any cause. Patients were considered censored at the last known date they were alive when disease progression or death had not occurred. 5-yearOS and PFS were estimated with the Kaplan–Meier method, and survival curves were compared between groups by using the log-rank test. A Cox proportional hazards regression was used and included matched pairs as the strata variable. Sample size was based on the number of available cases from 2007 to 2015; it provided 80% power to detect a difference in hazard ratio (HR) of 2.2 or greater for OS. The p-values < 0.05 were considered statistically significant. SAS version 9.4 (SAS Institute, Inc, NC, USA) was used for analysis.
Results
Patient characteristics
There were 85 matched pairs (n = 170 patients) analyzed. Mean age of the entire colorectal cancer cohort at diagnosis was 68 years, 32% had stage III disease and most (89%) were white (). Most patients were married (74%), had a history of smoking (current: 8%; former: 49%), were retired (46%), and had a European Cooperative Oncology Group score of 1 (61%). BMI was significantly higher for patients with DM than patients without DM (p = 0.002).
Table 1. Colorectal cancer patient characteristics by diabetes mellitus status.
DM and colorectal cancer treatment characteristics
The median (range) self-reported time since DM diagnosis was 14.5 (3–53) years (). Most patients (64%) were receiving oral medications at the time of their colorectal cancer diagnosis. Only 8% of patients changed their DM therapy within 1 year following cancer diagnosis, with 24% of patients using insulin within 1 year of cancer diagnosis. DM complications were documented in only 18% of patients at the time of cancer diagnosis. Of the patients, 45% had chemotherapy, whereas 23% of patients received radiotherapy. Among patients with DM, seven had their DM therapy changed within 1 year of colorectal cancer diagnosis: two patients to diet management for their DM, three to insulin, and two to oral medication plus insulin. Corticosteroids were taken by 12% of patients without DM and 19% of patients with DM ().
Table 2. Diagnosis and treatment of diabetes mellitus for patients with colorectal cancer.
Colorectal cancer effect on DM and metabolic control
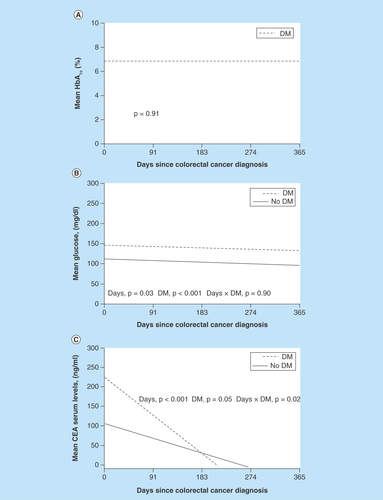
The HbA1c data measured within 1 year after colorectal cancer diagnosis were available for 47 patients with DM. Of these patients, the mean (standard deviation) HbA1c level was 6.8 (1.3) and 45% had at least 1 HbA1c measurement of 7.0% or greater. HbA1c did not change over 1 year (). Mean glucose was significantly different between patients with (144 mg/dl) and without DM (108 mg/dl) (p < 0.001). In a mixed model, both groups had a decline in glucose values during the 1-year period after diagnosis (p = 0.03) ().
Figure 1. Carcinoembryonic antigen during year following cancer diagnosis.
(A) Mean HbA1c level after colorectal cancer diagnosis over 1 year for patients with DM. No change was shown in mean HbA1c level (p = 0.91, linear mixed model). (B) Mean glucose level after colorectal cancer diagnosis over 1 year for patients with and without DM. Mean glucose values were significantly different for patients with and without DM (p < 0.001), and glucose values decreased significantly over 1 year (p = 0.03, linear mixed model). (C) Mean CEA level during the year following colorectal cancer diagnosis. CEA levels decreased significantly in both groups (p < 0.001, linear mixed model).
CEA: Carcinoembryonic antigen; DM: Diabetes mellitus; HbA1c: Hemoglobin A1c.
DM effect on colorectal cancer survival
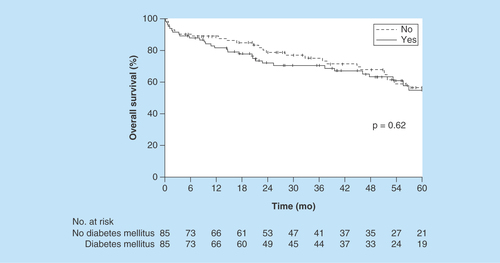
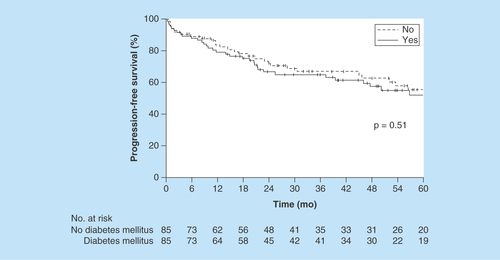
5-year OS (median follow-up, 49.3 months) was estimated at 56% (95% CI: 42–68%) for patients with DM versus 57% (95% CI: 43–69%) for patients without DM (p = 0.62, Kaplan–Meier method) (). The HR for matched pairs was 1.26 (95% CI: 0.69–2.31). 5-year PFS was estimated at 54% (95% CI: 40–65%) for patients with DM versus 57% (95% CI: 43–68%) for patients without DM (p = 0.51, Kaplan–Meier method) (). The HR for matched pairs was 1.24 (95% CI: 0.70–2.20).
Carcinoembryonic antigen
A nonsignificant tendency was shown for carcinoembryonic antigen (CEA) between groups (p = 0.05), but there was a significant time (p < 0.001) and interaction effect (p = 0.02). The patients with DM had higher CEA levels initially; then, the levels declined at a higher rate than those for patients without DM (the slope of the lines differed by group). In both groups, CEA levels declined significantly (p < 0.001) (). However, no correlation was shown between CEA and glucose or HbA1c levels (both p > 0.05).
Discussion
Additional data about patient-centered outcomes are needed for the interaction of DM and cancer. We previously investigated the effects of several different solid tumors (breast, prostate and lung) and DM on patient outcomes and care [Citation15–18]. In all of these matched case–control studies, DM did not impact patients’ short-term survival, and the cancer did not affect glycemic control in patients with DM. In this study, we used this methodology to study colorectal cancer. With a matched case–control analysis, we investigated how DM affected survival of patients with colorectal cancer and how colorectal cancer affected metabolic control in DM. Our prior analysis showed that there was no difference in survival between patients with colorectal cancer and coexisting DM and patients with colorectal cancer but without DM [Citation14]. The results of the current study also showed that DM did not affect survival of patients with colorectal cancer. Colorectal cancer also did not appear to negatively affect glycemic control.
Prior studies have reported that patients with DM and colorectal cancer fare worse than patients without DM. A meta-analysis of 36 cohort studies reported the effect of DM on prognosis for colorectal cancer [Citation9]. This study concluded that DM had a negative effect on survival of patients with colorectal cancer. However, this meta-analysis incorporated studies that used different methodologies (for example, both retrospective and prospective cohort studies). Another meta-analysis also showed that DM was associated with a higher mortality for patients with colorectal cancer [Citation10], but this report included observational studies and some studies that did not adjust for age and cancer stage. According to one report [Citation11], older persons with colorectal cancer and DM also had an increased risk of death, but the mortality was related primarily to cardiovascular disease than to colorectal cancer. In addition, administrative and public health surveillance data were used in this study, and other key variables such as those included in this study were not incorporated. Other conflicting reports also exist in the literature. For instance, two additional studies did not find any relationship between DM and decreased survival [Citation12,Citation13].
Clearly, uncertainty remains regarding the effect of DM on survival for patients with colorectal cancer. The strength of our study is its case–control design. With a case–control study, a disease-based difference can be shown with confidence in two groups. In our study, there was no difference in survival for patients with colorectal cancer with or without DM.
Factors other than cancer may contribute to prognosis for patients with colorectal cancer and DM such as cardiovascular status, microvascular complications of DM, age, pulmonary status and cognitive status. Moreover, treatment of patients with colorectal cancer and DM may be less aggressive than treatment of patients without DM. Patients with DM may also be less able to tolerate chemotherapy. All of these factors may influence prognosis.
For patients and healthcare providers responsible for management of DM, it is encouraging that glycemic control did not worsen, at least over the year timeframe analyzed here. This observation is similar to that of our previous analyses of other solid organ malignancies [Citation15–18]. Although HbA1c was available for only about half of the DM patients, it did not increase over 12 months. Glucose levels actually declined in both case and control patients, and very few DM patients had to progress to insulin therapy.
There is also scant data about CEA for colorectal cancer patients with DM [Citation20–23]. One study showed a statistically significant association between increased CEA level and DM and a correlation between CEA serum level and HbA1c level. Another study [Citation21] found that serum levels of cancer antigen (CA) 19–9, CEA, CA 72–4, and neuron-specific enolase were increased in patients with diabetes and correlated with higher glucose levels for CA 19–9, CEA and CA 72–4. Yet another study [Citation22] found a decrease in tumor markers with regulation of glycemic control. Hasan and Mohieldein [Citation23] showed significant differences in CEA level for women with DM compared with control patients. We found that the CEA level was higher in patients with colorectal cancer and DM than in those without DM. CEA also decreased more quickly over time in patients with colorectal cancer and DM. Possibly the CEA level, which is routinely used in managing care of patients with colorectal cancer, is not accurate in patients with DM. This can have implications for treatment decisions and is an area worthy of future research.
This study is not without limitations. The study was properly powered; however, the sample size was small. Moreover, the study duration was short. Future studies should confirm our findings in a larger dataset over a longer time. In addition, the sample population was primarily white. Therefore, results from this study may not apply to other racial or ethnic groups or individuals in other socioeconomic strata. We also did not have official causes of death for patients in the study. Finally, the number and severity of microvascular or macrovascular complications of DM were not well documented, and it is feasible that survival results may change if controlled for these variables.
Despite the above limitations, this case–control study showed that DM and colorectal cancer did not negatively interact with one another to impact survival or affect glycemic control in those patients who had DM. Taken together with the authors’ previous analyses of breast, prostate and lung cancers, the findings here demonstrate a common observation about the interaction of solid organ tumors and DM. We did note differences between patients with and without DM, regarding CEA level, a finding that requires further study.
Future perspective
With the findings of this study, practitioners can be assured that DM does not affect survival of patients with colorectal cancer and that colorectal cancer does not negatively impact glycemic control in patients with DM.
The impact of colorectal cancer on diabetes mellitus (DM) and the impact of DM on survival of patients with colorectal cancer are unknown on an individual level.
The patients with DM had a higher BMI (p = 0.002).
Among those with DM, the mean hemoglobin A1C during the year following their cancer diagnosis was 6.8%.
The mean glucose level was significantly different between patients with (144 mg/dl) and without (108 mg/dl) DM (p < 0.001).
In both groups (patients with and without DM), glucose values decreased during the year following the cancer diagnosis (p < 0.001).
The 5-year overall survival rate was 56% (95% CI: 42–68%) for patients with DM versus 57% (95% CI: 43–69%) for patients without DM (p = 0.62).
Authors’ contributions
All authors contributed to data acquisition and interpretation, drafting and final review of the manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 66(1), 7–30 (2016).
- GBd mortality and causes of death collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385(9963), 117–171 (2015).
- Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J. Clin. 59(6), 366–378 (2009).
- Sharma A, Ng H, Kumar A et al. Colorectal cancer: histopathologic differences in tumor characteristics between patients with and without diabetes. Clin. Colorectal Cancer 13(1), 54–61 (2014).
- Gonzalez N, Prieto I, Del Puerto-Nevado L et al. 2017 update on the relationship between diabetes and colorectal cancer: epidemiology, potential molecular mechanisms and therapeutic implications. Oncotarget 8(11), 18456–18485 (2017).
- Rosato V, Tavani A, Gracia-Lavedan E et al. Type 2 diabetes, antidiabetic medications, and colorectal cancer risk: two case–control studies from Italy and Spain. Front. Oncol. 6, 210 (2016).
- de Kort S, Simons CC, van den Brandt PA et al. Diabetes mellitus Type 2 and subsite-specific colorectal cancer risk in men and women: results from the Netherlands Cohort Study on diet and cancer. Eur. J. Gastroenterol. Hepatol. 28(8), 896–903 (2016).
- de Kort S, Masclee AAM, Sanduleanu S et al. Higher risk of colorectal cancer in patients with newly diagnosed diabetes mellitus before the age of colorectal cancer screening initiation. Sci. Rep. 7, 46527 (2017).
- Zhu B, Wu X, Wu B, Pei D, Zhang L, Wei L. The relationship between diabetes and colorectal cancer prognosis: a meta-analysis based on the cohort studies. PLoS ONE 12(4), e0176068 (2017).
- Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G. Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Dis. Colon Rectum 56(11), 1304–1319 (2013).
- Luo J, Lin HC, He K, Hendryx M. Diabetes and prognosis in older persons with colorectal cancer. Br. J. Cancer 110(7), 1847–1854 (2014).
- Jullumstro E, Kollind M, Lydersen S, Edna TH. Diabetes mellitus and outcomes of colorectal cancer. Acta Oncol. 48(3), 361–367 (2009).
- Noh GY, Hwang DY, Choi YH, Lee YY. Effect of diabetes mellitus on outcomes of colorectal cancer. J. Korean Soc. Coloproctol. 26(6), 424–428 (2010).
- Karlin NJ, Dueck AC, Cook CB. Cancer with diabetes: prevalence, metabolic control and survival in an academic oncology practice. Endocr. Pract. 18(6), 898–905 (2012).
- Karlin NJ, Dueck AC, Nagi Reddy SK, Verona PM, Cook CB. Implications of breast cancer with diabetes mellitus on patient outcomes and care. Diabetes Manag. (Lond.) 4(5), 411–419 (2014).
- Karlin NJ, Amin SB, Verona PM, Kosiorek HE, Cook CB. Co-existing prostate cancer and diabetes mellitus: implications for patient outcomes and care. Endocr. Pract. 23(7), 816–821 (2017).
- Karlin NJ, Amin SB, Buras MR, Kosiorek HE, Verona PM, Cook CB. Patient outcomes from lung cancer and diabetes mellitus: a matched case–control study. Future Sci. OA 4(1), FSO248 (2018).
- Karlin NJ, Amin SB, Kosiorek HE, Buras MR, Verona PM, Cook CB. Survival and glycemic control outcomes among patients with coexisting pancreatic cancer and diabetes mellitus. Future Sci. OA 4(4), FSO291 (2018).
- Faries DE, Haro JM, Leon AC. Analysis of Observational Healthcare Data Using SAS. SAS, Cary, NC, USA (2010).
- Zayed AA, Beano AM, Amer FN et al. Serum levels of carcinoembryonic antigen in patients with Type 2 diabetes. Endocr. Pract. 22(11), 1310–1318 (2016).
- Shang X, Song C, Du X, Shao H, Xu D, Wang X. The serum levels of tumor marker CA19-9, CEA, CA72-4, and NSE in type 2 diabetes without malignancy and the relations to the metabolic control. Saudi Med. J. 38(2), 204–208 (2017).
- Ata N, Dal K, Kucukazman M et al. The effect of glycemic control on CEA, CA 19-9, amylase and lipase levels. Open Med. (Wars.) 10(1), 8–13 (2015).
- Hasan M, Mohieldein A. Association between serum carcinoembryonic antigen level and oxidative stress parameters among diabetic females. Int. J. Clin. Exp. Med. 8(4), 6489–6494 (2015).