Abstract
Aim: Given the lack of data in the literature, we examined the impact of diabetes mellitus (DM) on melanoma survival and the impact of melanoma on glycemic control. Materials & methods: Patients with melanoma with and without DM were matched 1:1 (2005–2016). Kaplan–Meier analysis was used to estimate overall survival and progression-free survival (PFS). Mixed models compared hemoglobin A1c (HbA1c) and glucose measures over time. Results: Mean HbA1c during the year after cancer diagnosis was 6.7%. The 5-year PFS rate was 89% (95% CI: 81–99%) for patients with DM and 63% (95% CI: 51–79%) for patients without DM (p = 0.02). Conclusion: Melanoma did not adversely impact glycemic control. The DM did not adversely impact survival of patients with melanoma, although increased PFS for melanoma was seen in individuals with DM.
Lay abstract
The objective of this study was to identify the effect of diabetes mellitus (DM) on survival of patients with melanoma and to determine whether melanoma and its treatment affected glycemic control. We used an institutional cancer registry to identify 112 patients with melanoma and grouped them by the presence (n = 56) or absence (n = 56) of DM. Patients were matched by age and year of melanoma diagnosis. For individuals with melanoma, DM did not decrease survival rates, and the diagnosis of melanoma did not affect glycemic control.
Melanoma is the fifth most common cause of cancer in men and women [Citation1]. Its incidence continues to increase [Citation2]. Many risk factors for melanoma are well known, including ultraviolet light exposure, skin type, eye color, number of nevi, immunosuppression and family and personal history [Citation3]. A more recently recognized risk factor may be diabetes mellitus (DM), a very common disease [Citation4,Citation5]. A recent study reported a positive association between DM and ulcerated melanoma [Citation6]. Microvascular disease coupled with the proinflammatory environment of DM might account for this association. However, this possible relationship remains largely unstudied. Additionally, the impact of melanoma and its treatment on DM are unknown. For example, checkpoint inhibitor-induced DM has been reported [Citation7–11]. Likewise, the effects of DM and its treatment on melanoma are unknown.
There is a paucity of data about how DM and treatment of melanoma interact to affect each other’s outcomes. For instance, does the presence of DM affect survival in melanoma? Does melanoma and its treatment affect glycemic control in DM? Such information could better inform patients and their providers on what to expect when both diseases are managed concurrently. Previous reports from our institution have sought to examine these relationships for patients with solid-organ malignancies (e.g., breast, prostate, lung, pancreas), but data are lacking for cutaneous malignancies [Citation12–16]. Therefore, we aimed to determine the effect of melanoma on glycemic control and to evaluate the effect of DM on survival of patients with melanoma.
Methods & materials
This study was approved by the Mayo Clinic Institutional Review Board.
Case selection
Cases were selected by following protocols from our institution that were previously used to study patients with solid-organ malignancies and DM [Citation12–16]. Electronic medical records of patients with melanoma that was newly diagnosed from 1 January 2005, through to 31 December 2016, were obtained from the institutional cancer registry. The following data were collected: age at melanoma diagnosis, diagnosis date, race/ethnicity and stage of tumor.
All melanoma cases were cross-referenced against a list of all patients seen during the study period who also had a known diagnosis of DM (International Classification of Diseases, Ninth Revision, diagnostic code 250.00). We excluded patients who received full or partial treatment at another institution or who had another primary cancer. From this dataset, patients with melanoma and DM were matched to patients with melanoma and without DM at a 1:1 ratio by using a greedy algorithm [Citation17]. Variables included in the matching algorithm were age, sex and year of melanoma diagnosis. Year of melanoma diagnosis was used as a matching variable to ensure a similar duration of follow-up for patients with and without DM.
Data for glucose (all patients) and hemoglobin A1c (HbA1c; for DM patients only) were obtained from the laboratory information system. Electronic health records were reviewed for additional information that included type of melanoma treatment (surgery, chemotherapy, radiotherapy, targeted therapy) and any available data about DM (date of DM diagnosis, type of therapy, complications).
Statistical analysis
Statistical analyses were conducted as previously described [Citation12–16]. We compared patient characteristics and clinical variables for melanoma cases with and without DM. Continuous variables were compared by using paired t tests; categorical variables were compared with the McNemar test or Bowker test for symmetry. The HbA1c levels during the first year after the melanoma diagnosis were evaluated with a linear mixed model in DM cases only (HbA1c values were not obtained for most patients without DM). Time (in days) was considered a fixed effect, and an individual-specific random effect was included. A similar approach was used to model glucose values during the first year. Fixed effects included days, case or control designation, an interaction term (days × case–control designation) and patient-specific and matched pair-specific random effects. Optimal glycemic control was defined as a mean glucose value less than 126 mg/dl during the year after DM diagnosis [Citation18].
Overall survival (OS) was defined as the time from melanoma diagnosis until death from any cause. For OS, patients were considered censored at the last known follow-up date when death was not documented. Progression-free survival (PFS) was defined as the time from melanoma diagnosis until disease progression or death from any cause. Patients were considered censored at the last known date they were alive and disease progression had not occurred. The 5-year OS and PFS were estimated with the Kaplan–Meier method and compared between groups by using the log-rank test. A Cox proportional hazards regression model was used to assess DM effect on OS and PFS and included matched pairs as the stratum variable. Sample size was based on the number of available cases from 2005 through 2016 and provided 80% power to detect a difference in hazard ratio (HR) of 1.9 or greater for OS. The p-values < 0.05 were considered statistically significant. SAS version 9.4 (SAS Institute Inc, NC, USA) was used for analysis. Data for continuous variables were reported as mean (SD) and categorical variables as percentage.
Results
Patient characteristics
We analyzed 56 matched pairs (). Mean (SD) age at diagnosis was 69.8 (9.9) years, 111 (99%) were white and 78 (70%) had Stage I disease. No differences in race/ethnicity, tumor stage or Eastern Cooperative Oncology Group performance status were detected for patients with and without DM, although patients with DM had a higher BMI (mean [SD], 31.2 [6.3] vs 28.4 [6.8] kg/m2; p = 0.03).
Table 1. Characteristics of patients with melanoma.
Treatment information
The median (range) self-reported time since DM diagnosis was 11 (1–40) years (). The majority of patients (n = 29 [56%]) were receiving oral therapy for DM at the time of their melanoma diagnosis. Only four patients (8%) changed their DM therapy within 1 year after the melanoma diagnosis; 11 patients (21%) used insulin within 1 year after the cancer diagnosis. Complications from DM were documented for seven patients (13%) within 1 year after the melanoma diagnosis.
Table 2. Diabetes mellitus treatment for patients with melanoma (n = 52†).
Nonsurgical treatments for melanoma were required for only a few patients (). More patients in the non-DM group received immunotherapy (5 [8.9%] vs [0%]; p = 0.046). Among DM patients, four changed their DM therapy within 1 year of the melanoma diagnosis: one to oral therapy, one to oral plus insulin therapy and two to insulin therapy. Corticosteroids were taken by five patients with DM (9%) and two patients without DM (4%).
Melanoma effect on DM and metabolic control
The HbA1c data, measured within 1 year after the melanoma diagnosis, were available for 35 of the 56 patients (63%) with DM. Mean (SD) HbA1c was 6.7% (1.0%) and 12 (34%) had at least one HbA1c measurement of 7.0% or greater within 1 year of the melanoma diagnosis. The HbA1c did not significantly change during the 1 year after the melanoma diagnosis (p = 0.72).
Mean glucose values were significantly different between patients with DM (141 mg/dl) and patients without DM (103 mg/dl; p = 0.002). Neither group had a decline in glucose values during the 1 year after cancer diagnosis; we did not observe any significant interaction effect (p = 0.82) or time effect (p = 0.99).
DM effect on melanoma survival
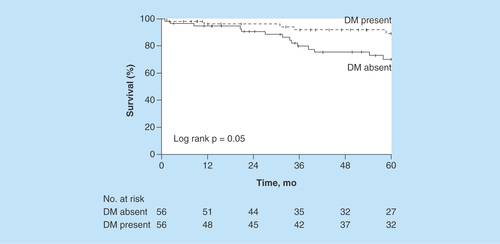
The estimated 5-year OS rate was 89% (95% CI: 80–99%) for patients with DM versus 70% (95% CI: 58–85%) for patients without DM (). The HR for matched pairs was 0.42 (95% CI: 0.15–1.18; p = 0.10). We observed no differences in OS among patients with and without DM on the basis of glucose control.
Figure 1. Overall survival.
Figure shows Kaplan–Meier survival curves after stratifying patients by DM status.
DM: Diabetes mellitus.
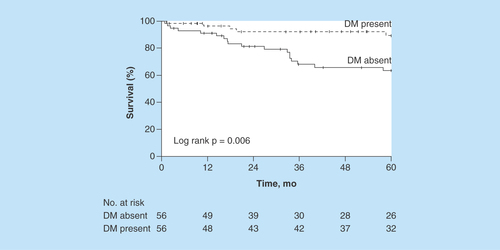
The estimated 5-year PFS rate was 89% (95% CI: 81–99%) for patients with DM and 63% (95% CI: 51–79%) for patients without DM (). The HR for matched pairs was 0.29 (95% CI: 0.11–0.80; p = 0.02).
Figure 2. Progression-free survival.
Figure shows Kaplan–Meier survival curves after stratifying patients by DM status.
DM: Diabetes mellitus.
Nine patients (DM, n = 2; non-DM, n = 7) received chemotherapy (n = 3), immunotherapy (n = 5) or targeted therapy (n = 4). In this subgroup, PFS was significantly decreased compared with patients who did not receive any of these therapies (p < 0.001). Median PFS for the treated patients was 16.3 (95% CI: 11.0–33.5) months; PFS was not estimated for patients not receiving therapy.
Discussion
Previous published studies examining the relationship between malignancies and outcomes in patients with DM and concomitant cancer have focused primarily on solid-organ tumors [Citation12–16]. Multiple studies from our institution have not shown a negative effect of DM across cancer types (e.g., breast, lung, colorectal, pancreas, prostate). The DM was not associated with an increased mortality rate for any of those cancer types and the concurrent malignancy did not affect glycemic control during a 1-year period. Due to the paucity of published data on individual-level outcomes for patients with cutaneous malignancies and DM, we performed a similar case–control analysis to evaluate the effects of DM on melanoma survival and the effects of melanoma on glycemic control. To our knowledge, this is the first report of an analysis exploring outcomes of patients with DM and melanoma.
Similar to findings reported with solid-organ malignancies, the results of the current study showed that DM also did not affect OS of patients with melanoma. Additionally, melanoma and its associated treatment did not adversely affect glycemic control during the 1 year that it was assessed. However, in contrast to the studies of solid-organ malignancies, we noted a significant and unanticipated improvement in the 5-year PFS in patients with DM.
No mechanism clearly explains why patients with DM had a superior 5-year PFS rate. We did have significantly more patients without DM receiving immunotherapy and the PFS rate was lower in those receiving immunotherapy, chemotherapy or targeted therapy; the therapeutic approach may have affected outcome. Additionally, a recent study of individuals with metastatic melanoma undergoing systemic chemotherapy reported that obese individuals had an improved PFS [Citation19] and obesity rates were higher in our subgroup of patients with DM. Other possible mechanisms that might link increased BMI with improved outcomes are increased circulating estrogens and alternative mechanisms of fatty acid metabolism [Citation20]. Studies in melanoma found benefit with the addition of tamoxifen to chemotherapy in obese males and obese postmenopausal females [Citation21]. Thus, estrogens may have a complex role in melanoma with regard to cell growth, differentiation and therapeutic resistance.
Angiogenesis is thought to have a critical role in melanoma progression and metastasis through the production of growth factors that affect the extracellular matrix proteins, matrix metalloproteases and integrins [Citation22]. Thus, reductions in growth factor production or activity may have inhibitory effects on melanoma; for example, bevacizumab, a monoclonal antibody to VEGF, improves the disease-free survival rate in patients with advanced melanoma [Citation23]. The microvascular disease seen in patients with DM likewise might affect PFS rates, with microvascular disease having an inherent antineovascularization effect in peripheral tissues such as the skin. Microvascular insufficiency in DM may alter the cytokine milieu to inhibit angiogenesis; its effects potentially can act synergistically with other therapies. However, angiogenesis in DM and obesity is complex, with tissue-specific paradoxes that include neovascularization leading to microvascular complications [Citation24].
Concomitant medications that are common in DM may improve survival. A recent cohort study of propranolol in invasive melanoma showed an 80% lower risk of recurrence [Citation25]. Additionally, diabetic medications may affect melanoma outcome by modulating the effects of systemic therapy. An active area of melanoma research is the use of diabetic medications such as phenformin, the precursor for metformin, with BRAF inhibitors. Phenformin can enhance the treatment benefit of BRAF inhibition in melanoma [Citation26] and enhance the efficacy of extracellular signal-regulated kinase inhibition in NF1-mutated melanoma [Citation27]. Finally, an ongoing clinical trial is examining the use of phenformin in combination with dabrafenib and trametinib in patients with BRAF-mutated melanoma (ClinicalTrials.gov identifier: NCT03026517).
We acknowledge limitations to our study, which was retrospectively conducted and included a relatively small sample of patients. Findings should be confirmed in a larger patient cohort that is treated for a longer period. Also, the cohort was mostly white and with early-stage melanoma and results may be less generalizable to patients of other racial/ethnic backgrounds or with more advanced melanoma. Finally, data on cause of death were not available.
Future perspective
This study showed that melanoma did not affect glycemic control during the 1-year period after the melanoma diagnosis, and DM did not increase mortality risk from melanoma. From this perspective, some degree of reassurance can be offered to the patient. The superior 5-year PFS rate in patients with DM is thought provoking and warrants further study.
The impact of melanoma on diabetes mellitus (DM) and the impact of DM on survival of patients with melanoma remain unknown on individual levels.
Patients with DM had a higher BMI (p = 0.03).
Among those with DM, the mean hemoglobin A1c during the year after their cancer diagnosis was 6.7%.
Mean glucose values were significantly different (p = 0.002) between patients with DM (141 mg/dl) and patients without DM (103 mg/dl).
The estimated 5-year overall survival rate was 89% (95% CI: 80–99%) for patients with DM versus 70% (95% CI: 58–85%) for patients without DM. The HR for matched pairs was 0.42 (95% CI: 0.15–1.18; p = 0.10).
The estimated 5-year progression-free survival rate was 89% (95% CI: 81–99%) for patients with DM and 63% (95% CI: 51–79%) for patients without DM. The HR for matched pairs was 0.29 (95% CI: 0.11–0.80; p = 0.02).
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Author contributions
All authors conceived of the study, collected data, performed the analysis and assisted with manuscript writing.
Acknowledgments
The abstract has been accepted for oral presentation and online e-Poster at the 2019 American Academy of Dermatology Annual Meeting, Washington, DC, USA, 1–5 March 2019.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 68(1), 7–30 (2018).
- Cronin KA, Lake AJ, Scott S et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer 124(13), 2785–2800 (2018).
- Johnson MM, Leachman SA, Aspinwall LG et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 4(1), 13–37 (2017).
- Tseng HW, Shiue YL, Tsai KW, Huang WC, Tang PL, Lam HC. Risk of skin cancer in patients with diabetes mellitus: a nationwide retrospective cohort study in Taiwan. Medicine (Baltimore) 95(26), e4070 (2016).
- Qi L, Qi X, Xiong H et al. Type 2 diabetes mellitus and risk of malignant melanoma: a systematic review and meta-analysis of cohort studies. Iran J. Public Health 43(7), 857–866 (2014).
- von Schuckmann LA, Smith D, Hughes MCB et al. Associations of statins and diabetes with iagnosis of ulcerated cutaneous melanoma. J. Invest. Dermatol. 137(12), 2599–2605 (2017).
- Cheema A, Makadia B, Karwadia T, Bajwa R, Hossain M. Autoimmune diabetes associated with pembrolizumab: a review of published case reports. World J. Oncol. 9(1), 1–4 (2018).
- Takahashi A, Tsutsumida A, Namikawa K, Yamazaki N. Fulminant Type 1 diabetes associated with nivolumab in a patient with metastatic melanoma. Melanoma Res. 28(2), 159–160 (2018).
- Okamoto M, Okamoto M, Gotoh K et al. Fulminant Type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J. Diabetes Investig. 7(6), 915–918 (2016).
- Shiba M, Inaba H, Ariyasu H et al. A case of Fulminant Type 1 diabetes mellitus accompanied by positive conversion of anti-insulin antibody after the administration of anti-CTLA-4 antibody following the discontinuation of anti-PD-1 antibody. Intern. Med. 57(14), 2029–2034 (2018).
- Chokr N, Farooq H, Guadalupe E. Fulminant diabetes in a patient with advanced melanoma on nivolumab. Case. Rep. Oncol. Med. 2018, 8981375 (2018).
- Karlin NJ, Amin SB, Buras MR, Kosiorek HE, Verona PM, Cook CB. Patient outcomes from lung cancer and diabetes mellitus: a matched case-control study. Future Sci. OA 4(1), FSO248 (2018).
- Karlin NJ, Amin SB, Verona PM, Kosiorek HE, Cook CB. Co-existing prostate cancer and diabetes mellitus: implications for patient outcomes and care. Endocr. Pract. 23(7), 816–821 (2017).
- Karlin NJ, Dueck AC, Cook CB. Cancer with diabetes: prevalence, metabolic control, and survival in an academic oncology practice. Endocr. Pract. 18(6), 898–905 (2012).
- Karlin NJ, Amin SB, Kosiorek HE, Buras MR, Verona PM, Cook CB. Survival and glycemic control outcomes among patients with coexisting pancreatic cancer and diabetes mellitus. Future Sci. OA 4(4), FSO291 (2018).
- Karlin NJ, Dueck AC, Reddy S, Verona PM, Cook CB. Implications of breast cancer with diabetes mellitus on patient outcomes and care. Diabetes Manag. 4(5), 411–419 (2014).
- Statistical Analysis System. Analysis of observational healthcare data using SAS. Faries DE, Leon AC, Haro JM, Obenchain RL ( Eds). SAS Publishing, NC, USA (2013). https://support.sas.com/content/dam/SAS/support/en/books/analysis-of-observational-health-care-data-using-sas/61876_excerpt.pdf.
- Centers for Disease Control and Prevention. National diabetes statistics report (2017). www.cdc.gov/diabetes/data/statistics/statistics-report.html.
- McQuade JL, Daniel CR, Hess KR et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 19(3), 310–322 (2018).
- Albiges L, Hakimi AA, Xie W et al. Body mass index and metastatic renal cell carcinoma: clinical and biological correlations. J. Clin. Oncol. 34(30), 3655–3663 (2016).
- Cocconi G, Bella M, Calabresi F et al. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. N. Engl. J. Med. 327(8), 516–523 (1992).
- Mahabeleshwar GH, Byzova TV. Angiogenesis in melanoma. Semin. Oncol. 34(6), 555–565 (2007).
- Corrie PG, Marshall A, Dunn JA et al. Adjuvant bevacizumab in patients with melanoma at high risk of recurrence (AVAST-M): preplanned interim results from a multicentre, open-label, randomised controlled Phase III study. Lancet Oncol. 15(6), 620–630 (2014).
- Cheng R, Ma JX. Angiogenesis in diabetes and obesity. Rev. Endocr. Metab. Disord. 16(1), 67–75 (2015).
- Livingstone E, Hollestein LM, van Herk-Sukel MP et al. Statin use and its effect on all-cause mortality of melanoma patients: a population-based Dutch cohort study. Cancer Med. 3(5), 1284–1293 (2014).
- Yuan P, Ito K, Perez-Lorenzo R et al. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc. Natl Acad. Sci. USA 110(45), 18226–18231 (2013).
- Trousil S, Chen S, Mu C et al. Phenformin enhances the efficacy of ERK inhibition in NF1-mutant melanoma. J. Invest. Dermatol. 137(5), 1135–1143 (2017).
