Abstract
Aim: This study was designed to evaluate the synergistic activities of hydroalcoholic extracts of medicinal plants Origanum vulgare and Hypericum perforatum and their active components, carvacrol and hypericin against Staphylococcus aureus. Methods: The synergistic effects of the plants, as well as carvacrol and hypericin, were examined using a checkered method against S. aureus (ATCC 12600). Results: A fractional inhibitory concentration of 0.5 was obtained for combination of O. vulgare and H. perforatum and 0.49 for combination of the active ingredients carvacrol and hypericin, both of which indicated a synergistic effect. Conclusion: This preliminary evaluation demonstrated a synergistic property of O. vulgare and H. perforatum extracts in treating S. aureus infection. This study indicates that combination of the plants, as well as combination of carvacrol and hypericin, might be used as a new antibacterial strategy against S. aureus.
Lay abstract We studied and evaluated the synergistic activities of hydroalcoholic extracts of oregano and St John’s wort and their active components, carvacrol and hypericin, against Staphylococcus aureus. The results suggest that a combination of oregano and St John’s wort extracts, as well as a combination of hypericin and carvacrol, have potential for use as natural and effective combinations against S. aureus infections.
Graphical Abstract
Staphylococci are Gram-positive cocci that appear in clusters. Among the different species of Staphylococci, the three species Staphylococcus aureus, S. saprophyticus and S. epidermidis are the most important [Citation1]. S. aureus is one of the most important causes of nosocomial and community-acquired infections [Citation2]. Due to high pathogenicity and resistance to antimicrobial and antibacterial drugs, S. aureus has become one of the most important health problems in the world. S. aureus is identified by production of coagulase enzymes and is highly pathogenic due to several extracellular toxins and factors [Citation3]. This bacterium causes various virulent infections and food poisoning in humans [Citation4]. It is one of the main causes of surgical wound infections in hospitalized patients and medical device-related contaminations [Citation5]. Staphylococcus aureus is predominantly colonized on the surface of the skin and mucosa, and can also survive in all tissues of the body [Citation6]. Approximately 20–40% of healthy people can be healthy carriers of S. aureus at any time. In some people such as hospital staff, the likelihood of being a carrier is high [Citation7]. Approximately 30% of the population is a nasal carrier of S. aureus. In postoperative patients with potential S. aureus wound infection, microbial culture from the wound site is the most important factor for identification [Citation8].
Both the increasing incidence of resistance to antibiotics and the side effects of these drugs have been among the factors that have led to the expansion of research on medicinal plants in recent years [Citation9–13]. The excessive consumption of antibiotics has led to the emergence of methicillin-resistant S. aureus strains, which are currently one of the problems faced by hospitals [Citation14–16]. S. aureus is resistant to certain types of common antibiotics, including oxacillin antibiotics (oxacillin, methysilin and colloxacillin), as well as all beta-lactam antibiotics such as penicillin, amoxicillin and cephalosporins [Citation17]. Due to the comparatively fewer side effects of medicinal plants, their use for the treatment of various diseases has long attracted attention and has grown steadily in recent years. In the last century, the use of plant-based and natural medicinal sources as a subdiscipline of traditional medicine has played a decisive role in the prevention, control and treatment of diseases.
Considering these advantages, the tendency to use herbal drugs is increasing [Citation18]. Origanum vulgare has antibacterial and antifungal effects [Citation19,Citation20] as well as antioxidant properties [Citation21–24]. The main compound of O. vulgare is carvacrol [Citation25,Citation26]. Hypericum perforatum has antimicrobial effects [Citation27] and hypericin is one of the most important compounds of this plant [Citation28–31]. Plant-based antibiotics and their synergistic effects could be a useful and practical solution to prevent antibiotic resistance. Studies of synergistic effects of plant extracts are therefore necessary to identify new combinations with highly desirable efficacy. Despite the obtained valuable information about the medicinal plants O. vulgare and H. perforatum, and their active compounds such as carvacrol and hypericin, their synergistic effects have not yet been studied. The current study is a preliminary evaluation of antibacterial and synergistic activities of the extracts of medicinal plants O. vulgare and H. perforatum and their active components, carvacrol and hypericin, against S. aureus.
Methods
Preparation of hydroalcoholic extracts & active ingredients of medicinal plants
To prepare the hydroalcoholic extracts, the plants were first dried in laboratory conditions and then 200 g of the powder of each plant was mixed with ethanol 70% (Nasr Alcohol, Iran). The mixture was shaken for approximately 6 h and then left in the laboratory for 24 h. The mixture was then passed through a filter paper, and the solvent was separated from the extract using a distiller (IKA® RV10) in vacuum (rotary) conditions at 40°C and 150 r.p.m. The concentrated extract of the plant was poured into the plate to dry.
Staphylococcus aureus bacterium
Staphylococcus aureus strain (ATCC 12600) was purchased from Iranian Research Organization for Science and Technology.
Synergism protocol
In order to investigate the combined effects of hydroalcoholic extracts of O. vulgare and H. perforatum and their active ingredients hypericin and carvacrol, the following concentrates were prepared for each of the compounds according to the amount of minimum inhibitory concentration (MIC), which was previously separately measured (4MIC0, 2MIC0, MIC0, MIC0, MIC0/2 and MIC0/4).
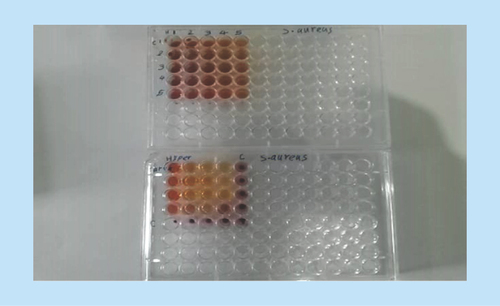
The combination effects of hydroalcoholic extracts of O. vulgare and H. perforatum against S. aureus were investigated using checkerboard test in a sterile 96-well plate. First, 50 μl of sterile Mueller–Hinton agar growth medium was added to all wells, then the plant extract samples were treated with different concentrations of the extract (20 μl of each extract). Then, 10 μl of microbial suspension with 0.5 McFarland standard turbidity (1.5 × 108 CFU/ml) was added to each wall. The plates were incubated at 37°C and 50% humidity for 24 h [Citation29].
Bacterial growth inhibition was measured by 2,3,5-triphenyltetrazolium chloride, in such a way that if the color of the wells turned purplish, the bacteria in the wells were considered living, and lack of the color was considered to indicate bacterial growth inhibition. The results were analyzed using the formula below and interpreted as follows:
FIC A = Combination effect/MIC A: The effect of MIC A alone.
FIC B = Combination effect/MIC B: The effect of MIC B alone.
Interpretation of the obtained results of proposed model by checkered method carried out according to Fratini et al. was as follows: if the results are less than 1 (FIC < 1), the effect is synergistic [Citation29]. If the results are equal to 1 (FIC = 1), then the effect is indifferent. If the results are greater than 1 (FIC > 1), this indicates an antagonistic effect, and if the results are greater than 2 (FIC > 2), one of the combination drugs is above its effective dose [Citation29].
Study of synergism by disc diffusion method
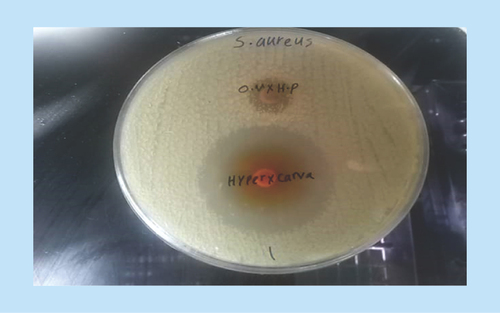
To investigate synergism using a disk diffusion method, the checkered method carried out according to Fratini et al. was used with a minor modification [Citation29]. In brief, a sterile Mueller–Hinton agar growth medium was divided into 10-cm plates, and using a sterile swab, a grass culture was prepared from 0.5 McFarland standard turbidity obtained from 24 h culture of S. aureus. Then, the disks were impregnated with 40 μl of 10,000 μg/ml of stock solution of the O. vulgare and H. perforatum extracts. They were also impregnated with 40 μl of 5000 μg/ml of stock solutions of hypericin and carvacrol and placed on each other as double in the Mueller–Hinton agar growth medium. Each of the above was used as a control in a separate experiment, then the plates were incubated for 24 h, and the diameter of the growth inhibition zone was measured.
Results
A fractional inhibitory concentration (FIC) of 0.5 was obtained for combination of hydroalcoholic extracts of O. vulgare and H. perforatum and 0.49 for combination of the active ingredients carvacrol and hypericin, indicating synergistic effects for both of them ( and & ). Additional information is shown in .
Table 1. Results of fractional inhibitory concentration and disk diffusion of the groups.
Discussion
The results of the present study, which examined the inhibitory effects of the extracts and their active ingredients on S. aureus, demonstrated synergistic effects of both combinations on the studied pathogen and strengthening of the antibacterial effects on this pathogen.
Interestingly, the results on the synergistic effects in the disk diffusion test also showed a direct correlation with the inhibitory effect, so that the growth inhibition zones in the combination test for both the active ingredients and the extracts were greater than those in the combination test for either alone. In these tests, the extracts and active ingredients showed synergistic effects, probably due to the presence of common active ingredients in the plants, namely carvacrol and hypericin. This was also confirmed in the test of active ingredients and their synergistic effects.
O. vulgare has antimicrobial effects on Gram-positive and Gram-negative bacteria [Citation33–37]. H. perforatum has a range of antimicrobial activities against bacteria, viruses, fungi and yeasts [Citation38–42]. Unlike antibiotics and chemotherapeutic agents, few studies have so far addressed the potential mechanisms for production of plant-derived products [Citation43]. Plant-based and natural compounds, as well as their active ingredients, may work through mediating metabolism by activating enzymes, inhibiting the function of inhibitors that affect nutrients in the environment, interfering with enzymatic processes at the nucleus or ribosome level, inducing changes in the membrane or even interfering with secondary metabolism [Citation44]. The synergistic compounds in this study are likely to exert their effects through one or more than one of these mechanisms.
This study confirmed the synergistic properties of hydroalcoholic O. vulgare and H. perforatum extracts against S. aureus. Combination of hydroalcoholic O. majorana extract and H. perforatum, as well as combination of carvacrol and hypericin will allow us to use a lower concentration of the extract or their active ingredients, hence reducing the possible toxic effects.
Fratini et al. (2017) showed that essential oil of O. vulgare L. and Leptospermum scoparium have synergistic effects against S. aureus [Citation29]. In general, herbal plants in the laminaceae family are known for their antimicrobial effects, which is due to high levels of phenol compounds such as carvacrol and thymol. It has been shown that the cardinal action mechanism of carvacrol on bacterial cells involves the decomposition of proton-motive force and the drainage of the ATP pool with blood cells [Citation45]. The only antibacterial principle isolated to date is a hypericin, hyperforin and tetraketone [Citation46].
In our study, the synergistic effect of carvacrol and hypericin was 0.49, and the synergistic effect of O. vulgare and H. perforatum was also 0.5. One of the reasons why the combination of carvacrol and hypericin has a better effect than O. vulgare and H. perforatum is the purity of effective compounds. Overall, O. vulgare and H. perforatum extracts and also carvacrol and hypericin may be an effective alternative to chemotherapeutic drugs in staphylococcal infections.
Conclusion & future perspective
The present study provided evidence of the antimicrobial and synergistic effects of the combination of hydroalcoholic O. vulgare and H. perforatum extracts, as well as combination of carvacrol and hypericin, on S. aureus infection. This suggests that, in the future, this combination could be used as a polyherbal antibiotic compound to control bacterial infections, especially of S. aureus. We did not examine the possible synergistic effects of the plant extract or their active ingredients with commercial antibacterial agents. If they possess synergistic activity with commercial antibiotics, this could have several beneficial effects for patients. This synergistic activity could enable the reduction of doses of commercial antibiotics, which in turn would reduce their toxic effects. Furthermore, infection is always associated with oxidative stress. Therefore, these plants with their antioxidant properties may reduce the injuries associated with these infections.
This experimental study was designed to evaluate the antibacterial and synergistic activities of hydroalcoholic extracts of herbs of Origanum vulgare and Hypericum perforatum and their active components, carvacrol and hypericin, against Staphylococcus aureus.
The synergistic effects of H. perforatum and O. vulgare, and carvacrol and hypericin, were examined using a Checkerboard test and AZDAST test against S. aureus.
A fractional inhibitory concentration of 0.5 was obtained for combination of O. vulgare and H. perforatum and 0.49 for combination of the active ingredients carvacrol and hypericin, both of which indicate a synergistic effect.
The results of this study indicate that combination of O. vulgare and H. perforatum, as well as combination of carvacrol and hypericin, might be used as a new strategy for antibacterials against S. aureus strain.
Author contributions
M Bahmani drafted the manuscript. B Ashrafi performed the statistical analysis. M Bahmani and B Ashrafi carried out the study. S Soroush, M Rafieian-Kopaei, M Khaksarian and M Rashidipour, M Nazer collected the data and revised the manuscript critically for important text and content. All authors read and approved the final manuscript.
Acknowledgments
This article was extracted from PhD thesis of M Bahmani. Also, we thank all the members of Razi Herbal Medicines Research Center such as S Abbaszadeh, R Mohammadrezaei-Khorramabadi.
Financial & competing interests disclosure
This study was funded by the Research and Technology Deputy of Lorestan University of Medical Sciences, Khorramabad, Iran. No. A-10-1379-1. The authors would like to express their gratitude for financial support of the Research and Technology Deputy of Lorestan University of Medical Sciences, Khorramabad, Iran. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Xia J, Gao J, Kokudo N, Hasegawa K, Tang W. Methicillin-resistant Staphylococcus aureus antibiotic resistance and virulence. Biosci. Trends 7(3), 113–121 (2013).
- Sahm DF, Critchley IA, Kelly LJ et al. Evaluation of current activities of fluoroquinolones against Gram-negative bacilli using centralized in vitro testing and electronic surveillance. Antimicrob. Agents Chemother. 45(1), 267–274 (2001).
- Shaffer RK. The challenge of antibiotic-resistant Staphylococcus: lessons from hospital nurseries in the mid-20th century. Yale. J. Biol. Med. 86(2), 261–270 (2013).
- Tiwari HK, Sapkota D, Gaur A, Mathuria JP, Singh A, Sen MR. Molecular typing of clinical Staphylococcus aureus isolates from northern India using coagulase gene PCR-RFLP. Southeast Asian J. Trop. Med. Public Health 39(3), 467–473 (2008).
- Wisal ME, Hamid MA, Hadia EA, Jalii IM, Ali AS. Coagulase gene polymorphism of Staphylococcus aureus strains isolated from human, animals and enviroment. Pak. J. Biol. Sci. 8(2), 278–280 (2005).
- Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin. Microbiol. Rev. 18(3), 521–540 (2015).
- Waldvogel FA. Staphylococcus aureus. In: Principles and Practice of Infectious Diseases (7th Edition). Mandell GL, Bennett JE, Dolin R ( Eds). Churchill Livingstone Press, Philadelphia, PA, USA, 2543–2578 (2009).
- Paule SM, Hacek DM, Kufner B et al. Performance of the BD Geneohm methicillin-resistant Staphylococcus aureus test before and during high-volume clinical use. J. Clin. Microbiol. 45, 2993–2998 (2002).
- Vannuffel P, Gigi J, Ezzedine H et al. Specific detection of methicillin-resistant Staphylococcus species by multiplex PCR. J. Clin. Microbiol. 33(11), 2864–2867 (1995).
- Orret FA, Land M. Meteicillin-resistant Staphylococcus aureus prevalence: current susceptibility patterns in Trinidad. BMC Infect. Dis. 6, 83 (2006).
- Nimmo GR, Cooms GW, Pearson JC et al. Meticillin resistant Staphylococcus aureus in the Australian community: an evoling epidemic. Med. J. Aust. 184(8), 374–375 (2006).
- Anwar MS, Jaffery G, Rehman Bhatti KU, Tayyib M, Bokhari SR. Staphylococcus aureus and MRSA nasal carriage in general population. J. Coll. Physicians Surg. Pak. 14(11), 661–664 (2004).
- Edoh V, Gadou D, Tia H, Gnonsahe D. Epidemiology and prevention of Staphylococcus aureus nasal carriage in patients and staff at the Cococy Hemodialysis Center in Abidjan, Ivory Coast. Med. Trop. 63(6), 590–592 (2013).
- Center for Food Security and Public Health (2006). www.cfsphiastate.edu.MethicillinresistantStaphylococcusaureus.
- Cohen Mitchell L. Staphylococcus aureus: biology, mechanisms of virulence, epidemiology. J. Pediatr. 108(5), 796–799 (1986).
- Jevons MP. Celbenin-resistant staphylococci. Br. Med. J. 1, 124–125 (1961).
- Fenga C, Foti M, Daidone A et al. Prevalence of Staphylococcus aureus methicillin-resistant (MRSA) among health care workers. G. Ital. Med. Lav. Ergon. 29, 416–417 (2007).
- Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12, 564–582 (1999).
- Soylu EM, Soylu S, Kurt S. Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans. Mycopathologia 161, 119–128 (2006).
- Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 10, 813–829 (2003).
- Gouladis M, Tzakoy O, Verykokidoy E, Harvala C. Screening of some Greek aromatic plants for antioxidant activity. Phytother. Res. 17, 194–195 (2003).
- Hazzit M, Baaliouamer A, Faleiro ML, Miguel MG. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food Chem. 54, 6314–6321 (2006).
- El Babili F, Bouajila J, Souchard JP et al. Oregano: chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. J. Food Sci. 76(3), C512–C518 (2011).
- Ocaña-Fuentes A, Arranz-Gutiérrez E, Señorans FJ, Reglero G. Supercritical fluid extraction of oregano (Origanum vulgare) essentials oils: anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food Chem. Toxicol. 48(6), 1568–1575 (2010).
- Nostro A, Blanco AR, Cannatelli MA et al. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Letters 230, 191–195 (2004).
- Chorianopoulos N, Kalpoutzakis E, Aligiannis N, Mitaku S, Nychas GJ, Haroutounian SA. Essential oils of Satureja, Origanum, and Thymus species: chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 52, 8261–8267 (2004).
- Glisic S, Popadic S, Skala D. St. John’s Wort (Hypericum perforatum L.) – Supercritical extraction, antimicrobial and antidepressant activity of extract and their components. Chem. Industry 60, 61–72 (2006).
- Radusienea J, Judzentieneb A, Bernotieneb G. Essential oil composition and variability of Hypericum perforatum L. growing in Lithuania. Biochem. Syst. Ecol. 55, 113–124 (2005).
- Fratini F, Mancini S, Turchi B et al. A novel interpretation of the fractional inhibitory concentration index: the case Origanum vulgare L. and Leptospermum scoparium JR et G. Forst essential oils against Staphylococcus aureus strains. Microbiol. Res. 1, 195 11–17 (2017).
- Sy CL, Huang TS, Chen CS et al. Synergy of β-lactams with vancomycin against methicillin-resistant Staphylococcus aureus: correlation of disk diffusion and checkerboard methods. J. Clin. Microbiol. 54(3), 565–568 (2016).
- Eliopoulos GM. Synergism and antagonism. Infect. Dis. Clin. North Am. 3, 399–406 (1989).
- Bahmani M. Phytochemical profiles and antibacterial activities of hydroalcoholic extracts of Origanum vulgare, Hypericum perforatum, carvacrol and hypericin as a promising anti-Staphylococcus aureus. Ph.D thesis- project code “A-10-1379-1” and with the title “Evaluating the anti-bacterial and reconstructive effect of titanium dioxide nanoprojects synthesized with Origanum vulgare, Hypericum perforatum, carvacrol and hypericin loaded in alginate scaffolds in wounds infected with Staphylococcus aureus in diabetic rats” (2017). http://pajouhesh.lums.ac.ir/pass_req.php?sid=1&slc_lang=fa.
- Chaves-López C, Martin-Sánchez AM, Fuentes-Zaragoza E et al. Role of Oregano (Origanum vulgare) essential oil as a surface fungus inhibitor on fermented sausages: evaluation of its effect on microbial and physicochemical characteristics. J. Food Prot. 75(1), 104–111 (2012).
- Horosova K, Bujnakova D, Kmet V. Effect of oregano essential oil on chicken lactobacilli and E. coli. Fol. Microbiol. 51, 278–280 (2006).
- Aureli P, Costantini A, Zolea S. Antimicrobial activity of some essential oils against Listeria monocytogenes. J. Food Protec. 55, 344–348 (1992).
- Biondi D, Cianci P, Geraci C, Ruberto G, Piattelli M. Antimicrobial activity and chemical composition of essential oils from Sicilian aromatic plants. Flavour Fragrance J. 8, 331–337 (1993).
- Muller RF, Berger B, Yegen O. Chemical composition and fungitoxic properties to phytopathogenic fungi of essential oils of selected aromatic plants growing wild in Turkey. J. Agric. Food Chem. 43, 2262–2266 (1995).
- Crockett S. Essential oil and volatile components of the genus Hypericum (Hypericaceae). Nat. Prod. Commun. 5(9), 1493–1506 (2010).
- Burt S. Essential oils: their antibacterial properties and potential application in foods- a review. J. Food Micro. 94, 223–253 (2004).
- Bertoli A, Cirak C, Teixeira Da Silva JA. Hypericum species as sources of valuable essential oil. Med. Aromat. Plant Sci. Biotechnol. 5(1), 29–47 (2011).
- Larypoor M, Akhavansepahy A, Rahimifard N, Rashedi H. Antidermatophyte activity of the essential oil of Hypericum perforatum of North of Iran. J. Med. Plants 8(31), 110–117 (2009).
- Leite AM, Lima EO, Souza EL, Diniz FFM, Trajano VN, Medeiros IL. Inhibitory effect of -pinene, α -pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. RBCF 43, 58052–58230 (2007).
- Koech KR, Wachira FN, Ngure RM et al. Antimicrobial, synergistic and antioxidant activities of tea polyphenols. Microbial pathogens and strategies for combating them. Sci. Technol. Edu. 971–981 (2013).
- Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Revi. 12, 564–582 (1999).
- Thormar H. Lipids and essential oils as antimicrobial agents. John Wiley & Sons Ltd, Chichester, UK (2010).
- Saddiqe Z, Naeem I, Maimoona A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 5, 131(3), 511–521 (2005).