Abstract
Recent reports suggest a potentially beneficial increase in abiraterone exposure when abiraterone acetate plus prednisone is administered with food. We evaluated the basis for current dosing recommendations under modified fasted conditions for patients with metastatic castration-resistant prostate cancer with a PubMed search of studies assessing the impact of food on abiraterone pharmacokinetics. Studies show that abiraterone exposure increases with administration in a fed versus fasted state and with high-fat versus low-fat meals. Food effect is substantially attenuated in metastatic castration-resistant prostate cancer patients compared with healthy subjects. The potential variability in absorption, unproven clinical benefits and increased toxicity with abiraterone acetate plus prednisone administration in the fed state underscore the importance of adhering to current dosing recommendations.
Despite castrate levels of testosterone from androgen deprivation therapy through surgical castration or gonadotropin-releasing hormone analogs, metastatic castration-resistant prostate cancer (mCRPC) progresses due to continued androgen synthesis in the adrenals and within the prostate tumor tissue and, subsequently, persistent androgen receptor activation [Citation1]. Abiraterone acetate (AA) is the prodrug of abiraterone, which suppresses extragonadal androgen production via selective inhibition of cytochrome P450 C17 (CYP17) [Citation2]. When administered in combination with prednisone/prednisolone, AA improves survival of patients with mCRPC who had not received prior chemotherapy and of those who progressed after previous docetaxel therapy [Citation3,Citation4].
Multiple factors may influence drug absorption, exposure and bioavailability after oral administration of anticancer agents, including food intake [Citation5]. Consequently, pharmacokinetic studies were essential in providing scientific basis for optimal conditions for AA plus prednisone administration for patients with mCRPC. The US FDA-approved product label specifies that AA plus prednisone “must be taken on an empty stomach”, with no food to be consumed for at least 2 h before and 1 h after drug administration (i.e., modified fasted state) [Citation6]. Similarly, the European Medicines Agency summary of product characteristics specifies that AA plus prednisone “must not be taken with food”, but should be taken at least 2 h after eating, with no food to be consumed for at least 1 h after dosing [Citation7].
A number of recent clinical studies report a broad spectrum of results that seemingly conflict with the recommendation for administration of AA plus prednisone in a fasted or modified fasted state. The results range from improved clinical responses attributed to increased abiraterone exposure when AA plus prednisone is taken with food to no clinical differences when AA plus prednisone is taken in the fed versus fasted state. A deeper understanding of the potential food effects for AA is important, because it could provide the basis for more rational pharmacologic dosing and monitoring and, in turn, improved treatment for patients with mCRPC.
The objectives of this article are to present a comprehensive review of relevant data on the impact of food intake on the pharmacokinetics of abiraterone and to provide a rational basis for a recommendation on the optimal administration of AA plus prednisone in patients with mCRPC.
Methods
We conducted a literature search on PubMed.gov using the search terms ‘abiraterone acetate’, ‘food effect’, ‘fed’, ‘pharmacokinetics’ and ‘prostate cancer’ without filtering for dates. We selected published literature covering the following topics: initial pharmacokinetic studies that defined the standard dose and dosing conditions for AA in patients with mCRPC; preclinical and early clinical trials that evaluated the effect of food and the timing of food intake on abiraterone exposure in healthy subjects; studies that assessed possible connections between abiraterone exposure due to food conditions and efficacy outcomes, serum androgen levels and safety in patients with mCRPC; randomized Phase III studies that served as the basis for approval of AA plus prednisone under fasted conditions in patients with mCRPC; studies evaluating interpatient variability in abiraterone exposure under various food conditions and its potential causes; population pharmacokinetic analyses that considered food effects; and, finally, clinical studies assessing low-dose versus standard-dose AA plus prednisone under defined food conditions allowing conclusions on any association between abiraterone exposure/pharmacokinetics versus efficacy in patients with mCRPC. In addition to published studies, we included data from recent congress presentations if they related to these topics. Sixteen original manuscripts and three congress publications were included in this comprehensive review.
Pharmacokinetics of abiraterone & dosing recommendations
Following oral administration, AA is fully hydrolyzed to its active moiety, abiraterone, followed by sulfate conjugation and N-oxidation to form its main circulating metabolites [Citation8,Citation9]. Abiraterone plasma concentrations increase rapidly, reaching their maximum (Cmax) in approximately 2 h when a 1000-mg dose of AA is administered in the fasted state [Citation8–10]. In a Phase I clinical study in patients with mCRPC, systemic exposure to abiraterone generally increased proportionally with increasing AA doses over the range from 250 to 1000 mg daily [Citation6,Citation10]. The mean terminal half-life of abiraterone in plasma in patients with mCRPC is approximately 12 h [Citation6,Citation10]. When a single oral dose of 14C-AA was administered to healthy male subjects under fasted conditions, approximately 88% of the radioactive dose was excreted in the feces and 5% was excreted in the urine; the major compounds recovered in feces were unchanged AA and abiraterone, accounting for approximately 55 and 22% of the administered dose, respectively [Citation8]. The data suggest that abiraterone has low bioavailability following oral administration as AA.
During clinical development, the selection of a 1000 mg daily dose in the fasted state was justified based on pharmacokinetic and pharmacodynamic data, as well as clinical responses and safety. The recommended Phase II dose of 1000 mg was selected based on a dose-ranging (250–2000 mg) study in 21 fasted patients with chemotherapy-naive CRPC [Citation11]. The area under the abiraterone concentration–time curve (AUC) and the Cmax increased with dose [Citation11], although increasing the dose from 1000 to 2000 mg only increases abiraterone exposure by approximately 8% [Citation6]. Moreover, abiraterone markedly increased steroid precursor levels upstream of CYP17, including deoxycorticosterone and corticosterone, with a plateau reached at doses from 750 to 2000 mg. At disease progression, no increases in steroids downstream of CYP17 were observed, suggesting that the enzyme inhibition was durable and irreversible in this dose range. The current food condition recommendations for administration of AA plus prednisone were made based on the abundance of clinical experience with the 1000 mg dose in a fasted state compared with the fed state. Moreover, although the safety of long-term AA plus prednisone administration in the fasted state was established in clinical investigations and the pivotal Phase III trials [Citation3,Citation4,Citation12,Citation13], long-term safety data for AA plus prednisone in the fed state have not been clearly defined. Low-dose prednisone is combined with AA to diminish adrenocorticotropic hormone release and ameliorate the effects of the mineralocorticoid excess. Prednisone can be administered as monotherapy without food [Citation14] and is not expected to impact the pharmacokinetics of abiraterone.
Clinical assessment of abiraterone exposure under various food conditions
The effect of food has been studied extensively using various AA formulations, which have been found to be bioequivalent, from capsules to tablets to the final marketed formulation (). Initially, a drug-in-capsule dosage form was used in Phase I/II studies [Citation10] that consisted of 250 mg of AA in hard gelatin capsules with no additional excipients. A preliminary 250-mg tablet formulation was subsequently introduced to support additional Phase I/II clinical trials [Citation11]. This preliminary tablet formulation was formulated with AA, lactose monohydrate, microcrystalline cellulose, povidone (K29/K32), croscarmellose sodium, sodium lauryl sulfate, colloidal silicon dioxide and magnesium stearate. The same qualitative formulation was used in the final formulation for Phase III [Citation3,Citation13], with a slight increase in the quantity of magnesium stearate to eliminate intermittent tablet sticking during tablet compression. The Phase III formulation is the final marketed formulation and was used to support several key pharmacokinetic studies, as outlined in .
Table 1. Overview of clinical studies evaluating food effect on abiraterone pharmacokinetics.
The potential effect of food on abiraterone exposure was first observed in the aforementioned Phase I study conducted in 21 men with chemotherapy-naive CRPC [Citation11]. Patients assigned to the 1000 mg and 2000 mg dose cohorts were randomized to receive single doses of AA administered 5 days apart on Days -7 and -3 before the start of continuous daily dosing. One dose was administered with a high fat content meal, and the other was given after an overnight fast. The study was conducted using the capsule formulation. When AA was administered with the high fat content meal, exposure to abiraterone was significantly increased, by 3.6-fold at the 1000 mg dose (n = 9, p = 0.004) and 4.4-fold at the 2000 mg dose (n = 3, p = 0.049), compared with administration in a fasted state. The variability between fed patients was comparable to that observed between fasted patients. Prolonged gastrointestinal transit time under fed state was thought to have contributed to the observed increase in abiraterone exposure [Citation15].
A subsequent Phase I study that evaluated the pharmacokinetics of abiraterone in the fasted versus fed states in patients with chemotherapy-naive progressive CRPC demonstrated that food increased abiraterone absorption in a dose-dependent manner [Citation10]. A total of 33 patients received single doses of a preliminary tablet formulation of AA for pharmacokinetic analysis over the dose range of 250 mg to 1000 mg either after an overnight fast or 30 min after an 800- to 1000-calorie, high-fat (50% of total caloric content) meal. Daily dosing began 7 days later. Food did not affect abiraterone exposure at the 250 mg dose. However, the administration of AA with food compared with fasting increased the mean abiraterone AUC by 2.2-fold at the 500 mg dose, 5.6-fold at the 750 mg dose and 4.1-fold at the 1000 mg dose. Mean Cmax was higher in the fed than fasted state at all dose levels. For the 1000 mg dose of AA, Cmax was reached in a mean of 4 h in the fed state compared with 1.8 h in the fasted state.
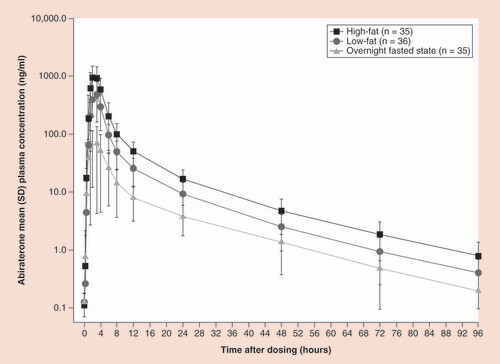
When the final Phase III formulation (i.e., the current marketed ZYTIGA® tablet) became available, a pharmacokinetic study of abiraterone was designed specifically to assess food effects that not only definitively demonstrated that the abiraterone pharmacokinetics depended on food state (fasted versus fed) but also that the fat composition of the meal has a significant effect. In a Phase I randomized, open-label, single-dose, three-period crossover study, 36 healthy male subjects were randomized to one of six treatment sequences, and received a single 1000-mg dose of AA after a high-fat meal, after a low-fat meal or while fasting [Citation16]. In each treatment period, the subjects fasted overnight; those who took AA with a meal did so approximately 30 min after eating. The high-fat meal contained 826 calories (56.5% from fat), and the low-fat meal contained 299 calories (7.3% from fat). Each treatment was separated by a 7-day washout period. Mean abiraterone plasma concentrations increased rapidly after AA dosing under the three food conditions, suggesting no significant food-associated impairment of metabolic activation from AA to abiraterone. Food did not substantially change time to Cmax (Tmax) (range, 2–3 h) or terminal half-life (range, 15.7–17.9 h), suggesting there was no significant food effect on drug elimination. AA administration under fed conditions increased abiraterone exposure compared with the fasted state, with the geometric mean Cmax and AUC from time 0 to infinity (AUC0-∞) increasing seven- and five-fold, respectively, with a low-fat meal, and 17- and ten-fold, respectively, with a high-fat meal (). The 90% confidence intervals (CIs) for abiraterone AUC0-∞ ratio estimates were all outside the 80–125% bioequivalence range (90% CIs: 816–1152% and 388–549% for high-fat meal/fasted and low-fat meal/fasted, respectively).
Mean (standard deviation) abiraterone plasma concentration–time curves (log-linear scale) following a single 1000-mg dose of abiraterone acetate in a fasted state, with a low-fat meal, or with a high-fat meal in healthy subjects.
A population pharmacokinetics analysis based on multiple studies, including the Phase III studies in mCRPC patients and pharmacokinetic studies in healthy subjects, confirmed that food affects the relative bioavailability of abiraterone [Citation17]. In Phase III studies, AA plus prednisone was taken at least 1 h before a meal or at least 2 h after a meal. This analysis was designed to evaluate the similarities and differences in abiraterone pharmacokinetics between mCRPC patients who had or had not received prior chemotherapy and between patients and healthy subjects. Food intake and health status were identified as significant covariates in the population pharmacokinetics model whereas age, body weight, concomitant treatment therapy and prior lines of therapy were not found to have significant effect on abiraterone PK. Although the extent of the food effect on abiraterone pharmacokinetics at the very least depended on health status (healthy vs mCRPC), it was not found to depend on whether mCRPC patients had or had not received prior chemotherapy. The analysis also revealed a 33% reduction in apparent oral clearance (CL/F) in patients compared with healthy subjects. The difference in CL/F may be attributed to the different food intake conditions between studies with patients (modified fasted state) versus those with healthy subjects (controlled overnight fasting). Abiraterone pharmacokinetics were characterized by a high between- and within-subject variability (i.e., between-subject coefficient of variation [CV%] for relative bioavailability for the modified fasting state was 61.1% and the CV% for within-subject variability was 71.3%).
Based on the aforementioned findings, the significance of timing of food intake on abiraterone pharmacokinetics was further investigated in a randomized, open-label, single-dose four-period crossover study [Citation18]. A total of 51 healthy male subjects, including 24 Caucasian and 27 Japanese subjects, were randomized to one of four treatment sequences and, in each treatment period, received a single 1000-mg dose of the final tablet formulation AA after an overnight fast. The timing of a standard medium-fat meal (412 calories; 12 g of fat) in each treatment period was based on the assigned sequence, and included 4 h post-dose (overnight fasted state); 1 h and 4 h post-dose; 2 h pre-dose and 4 h post-dose; or 2 h pre-dose and 2 h post-dose. Plasma abiraterone exposure increased markedly when AA was administered 2 h after a meal compared with the fasted state (). The increase in abiraterone plasma concentrations was approximately 7- to 7.5-fold when the meal was 4 h post-dose and approximately 4.4- to 4.8-fold when the meal was 1 and 4 h post-dose, compared with fasted conditions. Exposure to abiraterone increased by approximately 57% with meal administration at 1 and 4 h post-dose compared with at 4 h post-dose. The 90% CIs for abiraterone AUC0-∞ ratios were above the 80–125% bioequivalence range (90% CIs: 383–511% and 413–550% for 2 h pre-dose and 4 h post-dose/1 h and 4 h post-dose and 2 h pre-dose and 2 h post-dose/1 h and 4 h post-dose, respectively). The median Tmax of abiraterone was similar across all treatment groups and consistent with previous studies (1.5–2.0 h). These results suggest that the labeled modified fasted state may not be adequate to eliminate the food effect with AA.
Effect of food timing on the mean (standard deviation) plasma concentration–time profiles of abiraterone.
Adapted with permission from [Citation18] © Springer (2015).
![Figure 2. Food timing impacts abiraterone exposure. Effect of food timing on the mean (standard deviation) plasma concentration–time profiles of abiraterone.Adapted with permission from [Citation18] © Springer (2015).](/cms/asset/6d62c0d6-77ea-4796-9bbd-139a45c92b6c/iipk_a_12364612_f0002.jpg)
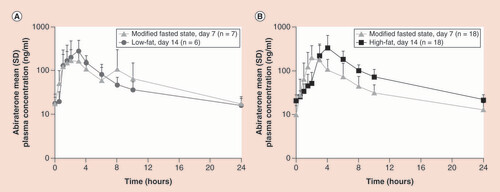
Despite the increase in abiraterone exposure, short-term dosing of AA plus prednisone with food did not alter its safety profile in a Phase II, open-label multicenter study involving 25 patients with mCRPC [Citation16]. The primary end point was the proportion of patients with grade ≥3 adverse events of special interest or grade ≥3 serious adverse events. Patients received the final tablet formulation of AA 1000 mg once daily and prednisone 5 mg twice daily on Days 1 to 7 in the modified fasted state, and then within 30 min of a low-fat meal (two slices of white bread with jam or jelly and 8 oz of 1% milk) or high-fat meal (McDonald’s Big Breakfast® and 8 oz whole milk) on Days 8 to 14. From Day 15 onward, patients continued to receive AA plus prednisone treatment in the modified fasted state until disease progression. No patient had a grade ≥3 adverse event related to study treatment during the observation period. Unexpectedly, the pharmacokinetic analysis found a minimal change in abiraterone exposure when treatment was administered with a low-fat meal compared with the modified fasted state; the geometric mean Cmax and AUC from time 0 to 24 h (AUC0–24) values increased by 35% (geometric mean ratio [GMR] = 135%; 90% CI: 96–191%) and 7% (GMR = 107%; 90% CI: 66–171%), respectively. With the high-fat meal, the geometric mean Cmax and AUC0–24 values increased by 74% (GMR = 174%; 90% CI: 121–251%) and 105% (GMR = 205%; 90% CI: 162–259%), respectively, compared with the fasted state (). Median Tmax was reached in 2 h under the modified fasted state, compared with 2.5 and 4 h with the low-fat and high-fat meals, respectively. Based on the observations in this study and previous aforementioned studies, food effects had a significantly smaller impact on abiraterone pharmacokinetics in patients with mCRPC after repeated dose compared with that in healthy subjects after single dose.
(A) Arithmetic mean (standard deviation) abiraterone plasma concentration–time profile (log-linear scale) under Day 7 modified fasted conditions or Day 14 low-fat meal. (B) Arithmetic mean (standard deviation) abiraterone plasma concentration–time profile (log-linear scale) under Day 7 modified fasted conditions or Day 14 high-fat meal.
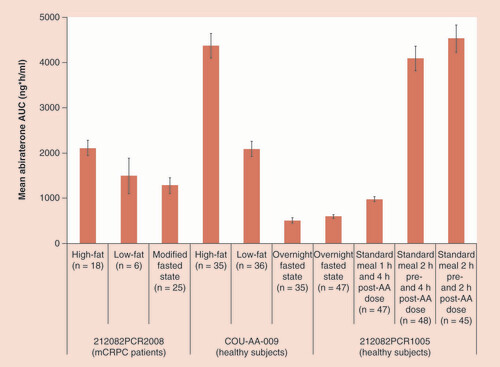
A summary of the effect of fat content of food and food timing on the exposure of abiraterone observed in abiraterone food studies is depicted in .
The values are reported as the arithmetic mean ± standard error of the mean. A standard meal was given 4 h post-AA dose in all studies.
Mechanisms of food effects on abiraterone pharmacokinetics
The mechanism responsible for the food effect on abiraterone bioavailability appears to depend on changes in drug solubility and absorption process. In a relative bioavailability study (COU-AA-010) [Citation19], systemic exposure to abiraterone following 1000 mg of AA as an oral liquid olive oil formulation given under fasted conditions was approximately 4.5-fold higher relative to a tablet formulation. These results suggest that the food effect on abiraterone is mainly attributed to its poor solubility and a solubilization effect produced by the lipid content in the food.
When incubated with fasted-state human intestinal fluid in vitro, AA is rapidly hydrolyzed to generate abiraterone at concentrations largely exceeding its apparent solubility, which suggests the existence of intestinal supersaturation [Citation20]. This finding was also observed after oral administration of a 250-mg dose of AA in fasted healthy male subjects with subsequent intraluminal sampling of duodenal fluids [Citation20]. In turn, the generation of abiraterone supersaturation provides a strong driving force for the absorption of abiraterone.
The food effect was subsequently evaluated using a similar methodology: healthy male subjects received a 250-mg dose of AA in the fasted state and at 20 min after consuming 400 ml of Ensure Plus Vanilla® (which simulates a standard meal) [Citation21]. The abiraterone plasma concentration–time profile confirmed the positive food effect. However, in the duodenal samples, abiraterone was the major species in solution in the fasted state, whereas AA and abiraterone concentrations were more comparable in the postprandial state. This difference suggests that AA is more extensively degraded to abiraterone in the fasted state compared with the fed state. A series of in vitro studies was then performed to investigate why duodenal drug concentrations do not fully reflect the positive food effect seen with plasma drug concentrations. The results of the in vitro studies suggested that gastric processing of the AA formulation strongly influences drug concentrations measured in the duodenum, and that the superior solubility of fed state fluids becomes more apparent in the small intestine and likely dictates abiraterone absorption leading to the observed food effect [Citation21].
Clinical assessment of abiraterone exposure manipulation with food intake
Because clinical studies have demonstrated a positive food effect on abiraterone pharmacokinetics, increased focus has been placed on using food intake as a mechanism for increasing abiraterone exposure in the hope of achieving improved therapeutic outcomes. One rationale for the attempt to achieve higher abiraterone exposure with food may be the potential to inhibit secondary targets within prostate tumors, or alternatively to increase the concentrations of clinically active AA metabolites.
There is some evidence that 3β-hydroxysteroid dehydrogenase (3β-HSD), an enzyme in the androgen biosynthesis pathway that is upregulated at CRPC progression, is a potential secondary target of abiraterone at high concentrations of the agent. Using recombinant enzymes, abiraterone inhibited 3β-HSD1 and 3β-HSD2 with competitive Ki values of 2.1 and 8.8 μM, respectively [Citation22]. Abiraterone also inhibited 3β-HSD activity in prostate cancer cell lines, including conversion of dehydroepiandrosterone to Δ(4)-androstenedione, translocation of androgen receptors to the nucleus and expression of androgen-responsive genes [Citation22]. No clinical studies have been conducted to evaluate targeting of 3β-HSD1 as a therapeutic strategy. However, exploitation of secondary targets, or ‘off-targets’, is generally associated with a high risk of increased toxicity [Citation23,Citation24].
The enzyme 3β-HSD also converts abiraterone into a Δ(4)-abiraterone (D4A) metabolite, which has been reported to have CYP17 inhibitory activity comparable to that of abiraterone, 3βHSD inhibitory activity more potent than that of abiraterone and androgen receptor antagonist activity similar to that of enzalutamide in prostate cancer cell lines [Citation25,Citation26]. In a prostate cancer xenograft model, D4A significantly delayed tumor growth compared with abiraterone (p = 0.011) [Citation26]. However, concentrations of D4A in peripheral tissues of patients with mCRPC have not yet been determined, nor has it been confirmed whether D4A contributes to the clinical activity of abiraterone [Citation26].
The use of food to increase PK exposure to abiraterone in attempt to improve the therapeutic effect of AA was explored in a retrospective chart review of 19 patients with mCRPC who had experienced increasing prostate-specific antigen (PSA) levels while receiving AA with prednisone (5 mg twice daily) without food [Citation27]. After a switch to AA taken with food, PSA declines from the prefood baseline levels were observed in three patients (16%) without any significant increase in toxicity. One patient had a PSA decline of 62%, whereas the PSA declines in the other two patients were small (12 and 6%). Each of these three men had responded initially to AA when taken without food. The authors of the study concluded that a subset of patients with mCRPC may have improvements in PSA levels after taking AA plus prednisone with food. It should be noted, however, that PSA responses were only measured within 3 months of the switch, and the patients were not naive to AA plus prednisone therapy. In addition, there were a number of confounding factors, including the presence of ARv7 and prior therapy with another androgen receptor signaling–directed therapy (enzalutamide), which may impact the efficacy of AA plus prednisone at the standard dose. No definite conclusion on effect of food on exposure could be drawn from this retrospective study as no associated pharmacokinetics analysis of higher doses was conducted. The study also did not definitively evaluate the food timing or fat content of the meal. Most importantly, data collection and analyses were retrospective and may have been impacted by selection bias, incomplete data reporting, inconsistent use of the response criteria and other unknown variables. In the aforementioned food effect study, differences in PSA declines were not noted between the fasted and fed patient cohorts [Citation10], which conflicts with results reported by Stover et al. [Citation27].
A trial to determine response to AA dose escalation from 1000 mg/day to 1000 mg twice daily (without the use of food) plus prednisone at clinical progression is currently under way (NCT01503229). The US prescribing information for AA indicates the use of AA dose adjustment to 1000 mg twice daily to increase PK exposure of abiraterone when AA plus prednisone is co-administered with a strong CYP3A4 inducer [Citation6].
Recent studies have attempted to replicate the abiraterone exposure observed with a standard AA dose (i.e., 1000 mg/day) with prednisone under fasting conditions by administering lower doses (250 or 500 mg/day) with prednisone under fed conditions. The data show high variability in pharmacokinetic parameters associated with a potentially comparable exposure to abiraterone.
In one study, 15 men with mCRPC who had progressed on docetaxel were randomly assigned to receive AA at 250 mg/day with a standard meal, 250 mg/day with a high-fat meal or 1000 mg/day with prednisone (5 mg twice daily) under fasting conditions [Citation28]. After 1 week of treatment, the geometric mean AUC of abiraterone was somewhat lower with the low-dose regimens under fed conditions (405 and 372 ng·h/ml) than with the standard dose under fasted conditions (656 ng·h/ml). Progression-free survival in the three groups was 8.5, 6.3 and 4.9 months, respectively (p = 0.53). It is important to stress that these data are preliminary and involved only a limited number of patients.
In a single-center retrospective study, data from 90 men treated with standard-dose AA with prednisone (5 mg daily) in a fasted state and 21 men treated with low-dose AA (250 or 500 mg/day) with prednisone (5 mg daily) in the fed state were reviewed [Citation29]. Of these patients, 34 and 16, respectively, were chemotherapy-naive. At this center, low-dose AA in the fed state has been prescribed to men who otherwise cannot access the drug due to funding constraints, particularly in the prechemotherapy setting. PSA response rates (confirmed PSA decrease ≥50%) did not differ between the standard-dose and low-dose groups in the entire cohort (43 vs 32%; p = 0.36), but exhibited a trend favoring the standard dose under fasting conditions in the chemotherapy-naive cohort (53 vs 27%; p = 0.09). Median progression-free survival was generally comparable between groups in the entire cohort (4.4 vs 5.6 months; p = 0.3) and the chemotherapy-naive cohort (5.5 vs 4.6 months; p = 0.9). No significant differences in overall survival were observed.
In a third study, the effects of food on abiraterone pharmacokinetics and pharmacodynamics were examined in 72 patients with progressive CRPC randomized to standard (1000 mg in the fasted state) or low dose (250 mg with low-fat breakfast) AA, with PSA assessed monthly and PK samples (at 2 h post-dose only) collected up to 4 months [Citation30]. A noninferiority of low dose AA was reported based on changes in PSA from baseline at 12 weeks (50% PSA decline, 58.3 and 50% for low dose and standard dose AA, respectively) and median PSA PFS (≈14 months for both arms; p = 0.53). No differences in tolerability and safety between the two arms were noted. A lower Cmax was noted for the low dose AA arm compared with the standard dose arm. However, trough concentration (or Cmin), a pharmacokinetic parameter that was shown to be associated with the efficacy of AA [Citation31], was not reported.
Although these studies were analyzed statistically, the small sample sizes preclude interpretation of whether using low-dose AA under fed conditions provides outcomes comparable to those achieved with standard doses under fasted conditions. The clinical consequence of lower pharmacokinetic exposure to abiraterone with low-dose AA administered under fed conditions remains uncertain. Until large prospective studies evaluate the use of low-dose AA under fed conditions instead of standard dose AA under fasted conditions, and the pharmacokinetic/pharmacodynamic relationship for low-dose AA under fed conditions is appropriately characterized, this strategy should not be considered.
Considerations for recommendation on abiraterone acetate administration
Certain patient populations or individual patients are more likely to consume a high-fat diet than others, raising the possibility that they may be overdosed during long-term therapy with AA and prednisone [Citation32]. Although it has been suggested that labeling and prescribing anticancer agents based on food state is a wasteful practice [Citation5], it may be essential when prescribing AA plus prednisone in order to better regulate abiraterone exposure as well as the drug’s therapeutic index. It remains to be determined whether even moderate changes in abiraterone pharmacokinetics during long-term treatment will improve outcome or increase toxicity or both, and this warrants further investigation [Citation32]. When AA plus prednisone is taken without food, the pharmacokinetics are more predictable and more easily understood, and patients are more likely to adhere to the treatment regimen. Some literature reviews have concluded that food restrictions for oral anticancer drugs are burdensome, confusing and associated with reduced adherence, but these characteristics applied primarily to agents that must be taken with food [Citation33].
The dose selected for development of abiraterone acetate (1000 mg/day plus prednisone [5 mg twice daily]), as well as its administration without food, were determined after careful consideration of such factors. The fasted condition was the state in which the efficacy and benefit of AA plus prednisone were demonstrated conclusively in pivotal Phase III studies, providing the basis for the approval of AA plus prednisone for use in patients with mCRPC [Citation12,Citation13]. It has been shown that, in patients with mCRPC, abiraterone exposure was increased by two-fold only when AA plus prednisone was administered with a high-fat meal, while no appreciable changes were observed with a low-fat meal [Citation16]. It is apparent that using food as an off-label means for increasing abiraterone exposure or dose manipulation is impractical (and unethical) due to the need to take the agent with a high-fat meal on a long-term basis. Noncompliance with this meal requirement will lead to sub-optimal abiraterone exposure and potentially increase the risk of therapeutic failure.
Conclusion
The available data provide evidence on how food impacts abiraterone exposure (), although much is still not understood regarding its clinical implications. The food effect on abiraterone pharmacokinetics is evident with daily dosing, and is potentially due to changes in intestinal absorption of drug between the fasted and fed states. The food effect depends on the fat content of meals, with greater abiraterone exposure occurring after a high-fat meal than after a low-fat meal, and it also depends on the timing of food intake. Importantly, the food effect in patients with mCRPC appears to be reduced greatly compared with healthy subjects; the reason remains unknown. In total, AA administration has been safe and well tolerated under all food conditions evaluated. On the basis of available data and clinical evidence, it is prudent to continue to recommend that AA be taken in a fasted or modified fasted state, consistent with the pivotal Phase III trials [Citation12,Citation13] and product labeling [Citation6].
Future perspective
Further study is warranted to elucidate the underlying mechanism behind the differences in food effect on the pharmacokinetics of abiraterone between healthy subjects and cancer patients. Moreover, the observation that there is a difference between healthy subjects and actual target (cancer) patients indicates a need to further assess food effect in the target population in addition to routine studies in healthy subjects. Empty stomach has long been defined as no food intake for 2 h before or 1 h after dosing. This definition appears to be inadequate to eliminate the food effect observed with AA based on results from the food timing study 212082PCR1005 [Citation18]. In the future, clinical evaluation of the relationship between various food timing and pharmacokinetics should be considered for new drugs that show profound food effect to better guide optimal treatment administration.
Pharmacokinetics of abiraterone & dosing recommendations
The efficacy and safety of abiraterone acetate (AA) (1000 mg daily) plus prednisone were demonstrated in pivotal Phase III trials in which treatment was administered in a modified fasted state (no food for at least 2 h before and 1 h after dosing).
Clinical assessment of abiraterone exposure under various food conditions
A food effect exists for abiraterone, with increased drug exposure following AA administration in the fed state compared with the fasted state.
This food effect is more pronounced with a high-fat meal than a low-fat meal, and in healthy male subjects compared with patients with metastatic castration-resistant prostate cancer.
Clinical assessment of abiraterone exposure manipulation with food intake
The use of food to improve the therapeutic effect of AA or to allow use of low doses has been explored in small studies; results are preliminary, and should not be used to promote this approach in routine clinical practice.
Considerations for recommendation on abiraterone acetate administration
AA should continue to be administered at its standard dose (1000 mg once daily) under fasting conditions; this approach is important for controlling day-to-day variations in dietary intake and minimizing variability in exposure to abiraterone.
Financial & competing interests disclosure
This work was supported by Janssen Research & Development. C Chien, M Smith and P De Porre are employees of Janssen Research & Development and own stock in Johnson & Johnson. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing assistance was provided by Shala Thomas, PhD, of PAREXEL, and was funded by Janssen Global Services, LLC.
Additional information
Funding
References
- Auchus RJ , YuMK , NguyenS , MundleSD . Use of prednisone with abiraterone acetate in metastatic castration-resistant prostate cancer . Oncologist19 ( 12 ), 1231 – 1240 ( 2014 ).
- Massard C , FizaziK . Targeting continued androgen receptor signaling in prostate cancer . Clin. Cancer Res.17 ( 12 ), 3876 – 3883 ( 2011 ).
- Fizazi K , ScherHI , MolinaAet al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled Phase III study . Lancet Oncol.13 ( 10 ), 983 – 992 ( 2012 ).
- Ryan CJ , SmithMR , FizaziKet al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled Phase III study . Lancet Oncol.16 ( 2 ), 152 – 160 ( 2015 ).
- Ratain MJ . Importance of food effects for oral oncology drugs . Clin Adv. Hematol. Oncol.10 ( 6 ), 397 – 398 ( 2012 ).
- ZYTIGA® (abiraterone acetate) [US prescribing information] . Horsham, PA : Janssen Biotech Inc. ( 2017 ).
- ZYTIGA® (abiraterone acetate) [Europe product information] . European Medicines Agency ( 2016 ).
- Acharya M , GonzalezM , MannensGet al. A Phase I, open-label, single-dose, mass balance study of 14C-labeled abiraterone acetate in healthy male subjects . Xenobiotica43 ( 4 ), 379 – 389 ( 2013 ).
- Bernard A , VaccaroN , AcharyaMet al. Impact on abiraterone pharmacokinetics and safety: open-label drug-drug interaction studies with ketoconazole and rifampicin . Clin. Pharmacol. Drug Dev.4 ( 1 ), 63 – 73 ( 2015 ).
- Ryan CJ , SmithMR , FongLet al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy . J. Clin. Oncol.28 ( 9 ), 1481 – 1488 ( 2010 ).
- Attard G , ReidAH , YapTAet al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven . J. Clin. Oncol.26 ( 28 ), 4563 – 4571 ( 2008 ).
- de Bono JS , LogothetisCJ , MolinaAet al. Abiraterone and increased survival in metastatic prostate cancer . N. Engl. J Med.364 ( 21 ), 1995 – 2005 ( 2011 ).
- Ryan CJ , SmithMR , de BonoJSet al. Abiraterone in metastatic prostate cancer without previous chemotherapy . N. Engl. J Med.368 ( 2 ), 138 – 148 ( 2013 ).
- Gambertoglio JG , AmendWJJr , BenetLZ . Pharmacokinetics and bioavailability of prednisone and prednisolone in healthy volunteers and patients: a review . J Pharmacokinet. Biopharm.8 ( 1 ), 1 – 52 ( 1980 ).
- Singh BN , MalhotraBK . Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy . Clin. Pharmacokinet.43 ( 15 ), 1127 – 1156 ( 2004 ).
- Chi KN , SpratlinJ , KollmannsbergerCet al. Food effects on abiraterone pharmacokinetics in healthy subjects and patients with metastatic castration-resistant prostate cancer . J. Clin. Pharmacol.55 ( 12 ), 1406 – 1414 ( 2015 ).
- Stuyckens K , SaadF , XuXSet al. Population pharmacokinetic analysis of abiraterone in chemotherapy-naive and docetaxel-treated patients with metastatic castration-resistant prostate cancer . Clin. Pharmacokinet.53 ( 12 ), 1149 – 1160 ( 2014 ).
- Inoue K , ShishidoA , VaccaroNet al. Pharmacokinetics of abiraterone in healthy Japanese men: dose-proportionality and effect of food timing . Cancer Chemother. Pharmacol.75 ( 1 ), 49 – 58 ( 2015 ).
- Food and Drug Administration Center for Drug Evaluation and Research . Clinical pharmacology and biopharmaceutics review(s): Zytiga . US Food and Drug Administration . www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202379Orig1s000ClinPharmR.pdf .
- Stappaerts J , GeboersS , SnoeysJet al. Rapid conversion of the ester prodrug abiraterone acetate results in intestinal supersaturation and enhanced absorption of abiraterone: in vitro, rat in situ and human in vivo studies . Eur. J Pharm. Biopharm.90 , 1 – 7 ( 2015 ).
- Geboers S , StappaertsJ , MolsRet al. The effect of food on the intraluminal behavior of abiraterone acetate in man . J. Pharm. Sci.105 ( 9 ), 2974 – 2981 ( 2016 ).
- Li R , EvaulK , SharmaKKet al. Abiraterone inhibits 3beta-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancer . Clin. Cancer Res.18 ( 13 ), 3571 – 3579 ( 2012 ).
- Dy GK , AdjeiAA . Understanding, recognizing, and managing toxicities of targeted anticancer therapies . CA Cancer J. Clin.63 ( 4 ), 249 – 279 ( 2013 ).
- Liu S , KurzrockR . Understanding toxicities of targeted agents: implications for anti-tumor activity and management . Semin. Oncol.42 ( 6 ), 863 – 875 ( 2015 ).
- Emamekhoo H , LiZ , SharifiN . Clinical significance of D4A in prostate cancer therapy with abiraterone . Cell Cycle14 ( 20 ), 3213 – 3214 ( 2015 ).
- Li Z , BishopAC , AlyamaniMet al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer . Nature523 ( 7560 ), 347 – 351 ( 2015 ).
- Stover JT , MooreRA , DavisK , HarrisonMR , ArmstrongAJ . Reversal of PSA progression on abiraterone acetate through the administration with food in men with metastatic castration-resistant prostate cancer . Prostate Cancer Prostatic. Dis.18 ( 2 ), 161 – 166 ( 2015 ).
- Espinosa M , FalconA , GutierrezAet al. Pharmacokinetic food-effect study of abiraterone acetate (AA) in patients with metastatic castration resistant prostate cancer (mCRPC): the ABIFOOD trial . J. Clin. Oncol.34 ( Suppl. 2S ), Abstract 227 ( 2016 ).
- Leibowitz-Amit R , AtenafuEG , SeahJet al. Low-dose abiraterone (abi) with food in men with metastatic castration-resistant prostate cancer (mCRPC): the Princess Margaret Cancer Centre experience . J. Clin. Oncol.32 ( Suppl. 5S ), Abstract 5077 ( 2014 ).
- Szmulewitz RZ , IbraheemAF , PeerCJet al. A prospective international randomized Phase II study evaluating the food effect on the pharmacokinetics (PK) and pharmacodynamics (PD) of abiraterone acetate (AA) in men with castrate resistant prostate cancer (CRPC) . Presented at : American Society of Clinical Oncology Genitourinary Cancers Symposium . Orlando, FL , 16–18 February 2017 ( Poster A176 ).
- Xu XS , RyanCJ , StuyckensKet al. Modeling the relationship between exposure to abiraterone and prostate-specific antigen dynamics in patients with metastatic castration-resistant prostate cancer . Clin. Pharmacokinet.56 ( 1 ), 55 – 63 ( 2017 ).
- Todd M , MeyersML , CharnasR , AcharyaM , MolinaA . Fast and flawed or scientifically sound: the argument for administering oral oncology drugs during fasting . J. Clin. Oncol.30 ( 8 ), 888 – 889 ( 2012 ).
- Lin HM , SuriA , WebbIJ , AggarwalS . Relationships between food effects, patient adherence to treatment, and pharmcokinetics of oral anticancer drugs . J. Clin. Oncol.32 ( Suppl. ), Abstract e17614 ( 2014 ).
