Abstract
microRNAs (miRNAs) are non coding RNAs with different biological functions and pathological implications. Given their role as post-transcriptional gene expression regulators, they are involved in several important physiological processes like development, cell differentiation and cell signaling. miRNAs act as modulators of gene expression programs in different diseases, particularly in cancer, where they act through the repression of genes which are critical for carcinogenesis. The expression level of mature miRNAs is the result of a fine mechanism of biogenesis, carried out by different enzymatic complexes that exert their function at transcriptional and post-transcriptional levels. In this review, we will focus our discussion on the alterations in the miRNA biogenesis machinery, and its impact on the establishment and development of cancer programs.
Keywords:
Abbreviations
| Ago2 | = | Argonaute 2 protein |
| Ars2 | = | Arsenic Resistance protein 2 |
| circRNA | = | circular RNA |
| miRNAs | = | microRNAs |
| hnRNPs | = | heterogeneous nuclear ribonucleoproteins |
| DGCR8 | = | DiGeorge syndrome Critical Region 8 protein |
| TRBP | = | TAR RNA binding protein |
| PACT | = | kinase R–activating protein |
| RISC | = | RNA-induced silencing complex |
| PABP | = | poly(A)-binding protein |
| EMT | = | epithelial–mesenchymal transition |
| PRC2 | = | Polycomb repressor complex |
| MK2 | = | MAPK-activated protein kinase 2 |
| KSRP | = | KH-type splicing regulatory protein |
| XPO5 | = | exportin 5 |
| TUT4 | = | terminal uridine transferase-4 |
miRNAs as Cancer Modulators
For over a decade, different studies pointed out the relevance of miRNAs biology in cancer, indicating that they can act as cancer genes, either as tumor suppressors, negatively regulating protein-coding oncogenes, or as oncomiRs, repressing known tumor suppressors.Citation1,2 Functional studies have demonstrated that miRNAs can affect cancer phenotypes, and several reports have identified miRNA expression profiles that provide information about tumor origin, prognosis or risk prediction, even better than other expression profiles like mRNA signaturesCitation3,4 (A more detailed overview is discussed in ref 5). Furthermore, understanding the physiological and pathological miRNA biogenesis mechanisms is important to gain knowledge on the role of this process in carcinogenesis, a situation that will result in the development and improvement of tools for diagnosis, risk evaluation and follow up of cancer patients.
From the Beginning: MiRNA Biogenesis
miRNAs sequences are distributed all throughout the genome, being localized in exonic or intronic regions, as well as intergenic locations.Citation6 The biogenesis of miRNAs starts with their transcription by RNA polymerase II,Citation7 although some other miRNAs are transcribed by RNA polymerase III,Citation7,8 resulting in a primary transcript known as pri-miRNA which contains a 33bp hairpin stem, a terminal loop and a flanking single stranded sequence of hundreds of bases or even several kilobases. In general, pri-miRNAs are capped at the 5´end and polyadenylated at the 3´end.Citation7,9 After transcription, the RNase III Drosha processes the pri-miRNA by cleaving it 11bp away from the hairpin stem (SD junction).Citation10 During miRNA biogenesis, Drosha might create 2 different complexes to facilitate pri-miRNA cleavage. One is composed by the RNA helicases, p68 and p72, and the heterogeneous nuclear ribonucleoproteins (hnRNPs). The other complex, known as the microprocessor, is composed by Drosha and the DiGeorge syndrome Critical Region 8 protein (DGCR8), a dsRNA-binding protein that stabilizes Drosha through interaction with its C-terminal domain.Citation11,12 DGCR8, also serves as a molecular ruler, directing the cleavage of Drosha to the SD junction.Citation13 Drosha digestion can occur co-transcriptionally or before splicing,Citation14 and the product of this digestion is an intermediary RNA molecule known as pre-miRNA, which has ∼22 nt in the stem and ∼48nt in the terminal loop.Citation15,16
Alternatively, some non-canonical biogenesis pathways may occur during mRNA splicing, giving rise to “miRtrons”. MiRtrons are in fact, the spliced-out introns of mRNAs, which constitute functional pre-miRNAs. Therefore, production of miRtrons is independent of Drosha digestionCitation17 ().
Figure 1. Biogenesis of miRNAs (A) Production of miRNAs starts in the nucleus with the polimerization of the primary hairpin miRNA transcript (pri-miRNA) by RNA polymerase II or III, followed by the cleavage and digestion of the pri-miRNA by the microprocessor complex (Drosha–DGCR8). The resulting transcript is the pre-miRNA, which is exported to the cytoplasm by Exportin-5–Ran-GTP. Once in the cytoplasm, Dicer, TRBP and Paz proteins cleave the pre-miRNA hairpin and digest it to produce a mature duplex miRNA. Then, one of the strands is loaded onto the RISC complex and finally this guides the miRNA to its mRNA target to silence it by direct degradation or by translational repression. (B) Mechanism of post-transcriptional regulation of mRNA target by miRNA i) Regulation by translation repression. ii) Regulation by repression of translation initiation. iii) Regulation by mRNA degradation. iv) Regulation by degradation or storage of mRNA targets in P bodies.
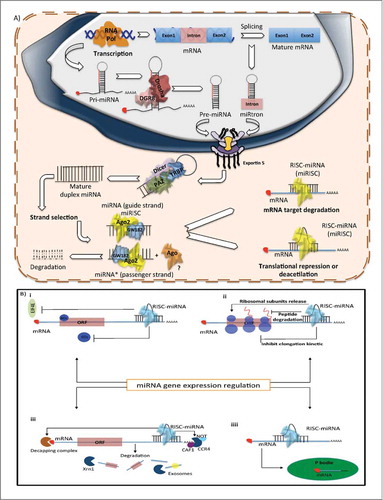
Following pre-miRNA generation, Exportin-5, a Ran-GTP-dependent dsRNA-binding protein, transports the pre-miRNAs to the cytoplasm in a GTP dependent process.Citation18 Exportin-5 can also protect pre-miRNAs against nuclear degradation.Citation19 Once in the cytoplasm, Dicer, another RNase III, digests the pre-miRNA into a 22nt mature duplex miRNA (miRNA:miRNA*, where miRNA* is called as the passenger strand).Citation20,21 During this process, Dicer is associated with other proteins like TAR RNA binding protein (TRBP) and kinase R–activating protein (PACT) to increase its stability and its processing activity.Citation22,23 Dicer is an essential protein of miRNA maturation and its down-regulation decreases the mature miRNA levels. In fact, under certain conditions the absence of Dicer is lethalCitation24,25 ().
After generation of the miRNA duplex, the strands are unwound in an ATP-independent process (it is not clear how this process occurs). One strand (the miRNA-guide strand) is loaded onto the RNA-induced silencing complex (RISC), formed by the association of Dicer, TRBP, PACT, most commonly the Argonaute 2 protein (Ago2)Citation22,26 and GW182, which promotes Argonaute stability.Citation27 The resultant complex between mature miRNA and RISC is denominated miRSC. In mammals, selection of the guide strand is dictated by thermodynamic stability, the less stable strand at the 5´end has more probability of being incorporated into the RISC; the remaining strand (miRNA*-passenger strand) is excluded and generally degraded.Citation28,29 However, recent miRNA sequencing data, as well as results from our laboratory, demonstrate that a large number of miRNA* are not degraded and are expressed in similar concentrations to their corresponding guide strand.Citation30 These observations suggest that the passenger strand might also be incorporated into the RISC complex. Consequently, one miRNA sequence can produce 2 different mature miRNAs, each one having different targets and, therefore, different biological functionsCitation31 ().
Finally, the miRISC functions as a guide to recognize the mRNA targets, based on complementarity rules, to negatively regulate mRNAs. During this process, Ago2, a protein with RNA cleavage activity, together with GW182, which interacts with the cytoplasmic poly(A)-binding protein (PABP) and the PAN2–PAN3 and CCR4–NOT deadenylaseCitation27 plays a central role in miRNA-mediated mRNA silencing.Citation32 There are at least 3 possible mechanisms by which miRNA mediate repression of gene expression: (1) mRNA target hybridization and degradation, (2) translation inhibition during the initiation or elongation phases and (3) mRNA decay by its recruitment to P bodiesCitation33,34 ().
miRNA Biogenesis Defects and Their Biological Consequences in Cancer Transcriptional Regulation, a Transcription Factor Network
In cancer, numerous transcription factors, some of them well-characterized tumor suppressors or oncogenes, regulate miRNA transcription. Nucleosome positioning methods and ChIP-on-ChIP or ChIP-seq analysis suggest that a set of transcription factors promotes or inhibits miRNA transcription, many of them overlapping with well-known transcription factors of coding genes like Myc and p53, as well as cell type–specific transcription factors such as MEF2, PU.1, and REST.Citation35,36 Furthermore, cellular context triggers pri-miRNA transcription in response to growth factor stimuli such as PDGF, TGF-β and BDNF.Citation37-Citation39
Recent evidence indicates that the oncogenic transcription factor Myc acts as a miRNA transcriptional regulator, promoting the transcription of some oncogenic miRNAs as well as the transcriptional inhibition of tumor suppressor miRNAs.Citation40,41 One of the first documented oncogenic miRNA clusters promoted by Myc is miR-17–92, which is activated when Myc binds to the E-box in the miR-17–92 coding sequence.Citation42,43 The miR-17–92 cluster is frequently over-expressed in a variety of tumors like B-cell lymphomas, breast, colon, lung, pancreas, prostate, and stomach cancers.Citation44,45 Some other tumorigenic miRNAs induced by Myc are miR-19a/b, implicated in cancer metabolism and cancer cell survival,Citation46 miR-18a which contributes to angiogenesisCitation47 and miR-9 which modulates the expression of mediators of metastasis.Citation48
Myc can also actively repress the transcription of numerous miRNAs, including some members of the let-7 and miR-29 families, as well as miR-15a/16–1, miR-26a and miR-34a.Citation41 These miRNAs have been related to antiproliferative, proapoptotic and antitumorigenic activities in different tumors.Citation49,50 In fact, miRNAs regulated by Myc can silence some Myc regulators, in a coordinated negative feedback loop.Citation51 Myc not only regulates miRNA activity during transcription, it also blocks the maturation of certain miRNAs through its cooperative relationship with some other binding proteins like Lin28, which acts as negative regulator of let-7.Citation52
Epigenetic Alterations at MiRNA loci
Epigenetic mechanisms are also important for miRNA transcriptional regulation. Different approaches have shown that DNA methylation and histone deacetylase inhibitors can modify the expression of several miRNAs.Citation53,54 The characterization of CpG island content in genomic regions harboring miRNAs, reveals that such regions share a similar DNA and chromatin context, for example, the promotion of a closed chromatin configuration defined by CpG island hypermethylation and covalent histone modifications.Citation55,56
The identification of miRNAs undergoing DNA methylation in a broad set of tumors, pointed out the importance of this process in miRNA downregulation and in the establishment of cancer programs. miR-124 and miR-34, well defined tumor suppressors, are subject to epigenetic silencing by aberrant DNA hypermethylation affecting cell cycle pathways in tumorsCitation54,57,58 ; while down-regulation of miR-34 affects the Notch pathway involved in cell invasion and apoptosis.Citation59 Furthermore, DNA methylation profiles in miRNA promoter regions can be useful as a diagnostic and prognostic marker. For example, miR-23b, a miRNA with tumor suppressor activity in prostate cancer, is down-regulated through DNA hypermethylation of its promoter region and its expression level is correlated with overall survival and recurrence-free survival.Citation60 A more comprehensive list of hypermethylated miRNAs in cancer is included in .
Table 1. Methylated miRNAs and their role in cancer
Deregulated expression of miRNAs in cancer is also a consequence of alteration in histone marks, which occur primarily due to the aberrant action of histone deacetylases and the Polycomb repressor complex (PRC2). For example, over expression of PRC2 in prostate cancer contributes to the repression of miR-101 and miR-205 by increasing the levels of H3K27me3 at their promoters. These alterations result in an increased rate of cell proliferation. In colorectal cancer, chromatin at promoter regions of tumor-suppressor miRNAs show a closed configuration, producing a repressed transcriptional state.Citation67 Moreover, BRCA1, a well-known tumor suppressor, in addition to its canonical function, can also epigenetically repress the oncomiR miR-155 via its association with HDAC2, which deacetylates histones H2A and H3 on the miR-155 promoter.Citation68
CTCF, another epigenetic factor, acts as a border that delimits the propagation of DNA methylation and histone repressive marks over different regulatory regions controlling gene expression. In different cancers, CTCF is lost, promoting repressive epigenetic mechanisms.Citation69 Recent studies have shown that CTCF regulates miRNAs such as the tumor suppressor miR-125b1 and the oncomiR miR-375 in breast cancer cells.Citation70
Post-transcriptional Regulation
Editing miRNA hairpin base pairs
Another potential regulatory mechanism for miRNA biogenesis and activity, is the post-transcriptional editing carried out by the catalyzing enzymes ADAR1 and ADAR2. In this process, adenosine residues are replaced by inosine (A-to-I), therefore, producing an A–U base pair instead of an I–G base pair.Citation71 Consequently, miRNA edition may influence the transition from pri-miRNA to pre-miRNA. Furthermore, it may also affect miRNA-target specificity by modifying the seed region (a necessary sequence for mRNA and miRNA hybridization).Citation72 It has been demonstrated that the miRNA-editing process is affected in gliomas, resulting in the production of unedited forms of miR-376*, which lacks the ability to target its natural mRNAs targets, like AMFR. This alteration promotes a higher invasive capacity in the glioma cells.Citation73
Drosha processing and alterations in cancer
Immunoprecipitation analyses reveal that the RNA helicases p68 (DDX5) and p72 (DDX17) are associated with the microprocessor complex,Citation74 modulating the association of Drosha and the pri-miRNA. Additionally, p68 and p72 might interact with some other RNA processing enzymes or transcription factors, modifying Drosha processivity.Citation75,76 For example, p68/72 interacts with Smad, p53, and the estrogen receptor, which also regulate miRNA processing. The Smad proteins, act as signal transducers promoting the expression of at least 20 human miRNAs by increasing Drosha cleavage activity upon their target pri-miRNAs.Citation77 Although there is no clear idea about the mechanisms that determine the set of miRNAs undergoing this type of regulation, sequencing data show that the majority of miRNAs regulated by Smad contain a consensus sequence within the stem region of the corresponding pri-miRNA, to which Smads bind directly.Citation78 In breast cancer, especially in invasive tumors, TGF-β promotes the expression of the oncomiR miR-155 through Smad4 activity. Moreover, there is a positive co-regulated interaction between miR-155 and TGF-β, as miR-155 negatively regulates RHO A, which in turn silences TGF- β, favoring EMT, cell migration and invasionCitation79 ().
Figure 2. Post-transcriptional regulation of miRNA biogenesis in response to cellular signals. (A) RNA helicase (promotes the structural remodeling), TGF-β stimulation, DNA damage (p53), Smads and BRCA promote miRNA processing enhancing pre-miRNA production. Conversely, DR5 and ADAR1 prevent the transition between pri-miRNA to pre-miRNA of a subset of miRNAs. (B) Hormone receptor stimulation or negative regulation over miRNA biogenesis. Androgen receptor (AR) promotes the transcription of the miR-23a/27a/24-a cluster. Moreover, AR enhances the progression from pri-miRNA to pre-miRNA of this cluster. Furthermore, when E2 and ER-α bind the pri-miRNA of the miR-23a/27a/24-a cluster it reduces its processing by Drosha. Additionally, ER-β prevents the biogenesis of the pri-miR-30a through its direct association with Drosha.
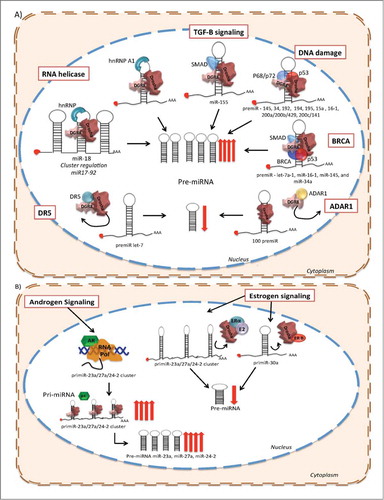
In a different cellular context, the tumor suppressor p53 is related with the biological activity of miRNAs, not only because p53 is, in itself, targeted by miRNAs, but also because it regulates miRNAs expression at different levels. Immunoprecipitation experiments have demonstrated that p53 might enhance the cleavage processivity of Drosha.Citation80,81 Thus, p53 can promote the processing of specific pri-miRNAs to pre-miRNAs such as miR-145 and miR‑34, which regulate the cell cycle,Citation82 miR-192, miR-194, miR-195, miR-15a and miR16–1,Citation83 and miR‑200a/200b/429, miR-200c/141 that antagonize EMT.Citation84 Recent data showed that p53 plays another regulatory role in miRNA maturation by influencing the accessibility to miRNA targets through the recruitment of RNA-binding proteins, which compete against miRNAs for binding to the 3ʹ UTRs on mRNAsCitation85 ().
BRCA is also a post-transcriptional modulator of miRNA biogenesis. The tumor-suppressor BRCA1 binds directly to the pri-miRNA sequences of let-7a-1, miR-16–1, miR-145, and miR-34a (all of them tumor suppressors), and increases the pre-miRNA levels of this subset of miRNAs through the interaction with Drosha, Smad and p53. This regulatory mechanism expands the potential consequences of BRCA disruption in cancer and its possible impact in genomic stabilityCitation86 ().
The increased proliferation rate of cancer cells is reflected in many genomic and biochemical processes, which also have an important impact on miRNA biogenesis. For instance, under physiological conditions Arsenic Resistance protein 2 (Ars2) is required for cell proliferation,Citation87 furthermore, ARS2 contributes to microRNA biogenesis under cell proliferation signaling. Ars2 depletion represses the biogenesis of a subset of miRNAs that are important in cancer, including let-7 and miR-21Citation88 (). Experimental evidence suggests that Ars2 either binds directly to pri-miRNA transcripts and recruits the Drosha microprocessor or acts as a cofactor for Drosha's enzymatic activity.Citation89 Other examples involve apoptotic modulators, like DR5 (TRAIL-R2) which also inhibits miRNA maturation of let-7 through direct interaction with Drosha and DGCR8 in pancreatic cancer cell lines, promoting proliferation of cancer cells. Moreover, the expression level of DR5 in pancreatic tumor samples is correlated with poor outcomeCitation90 ().
In addition to its previously mentioned editing function, ADAR1, can form a complex along with DGCR8, preventing the association between DGCR8 and Drosha during pri-miRNA processing. Moreover, it seems that ADAR1 can control the expression of more than 100 miRNAs which are positive regulators of metastatic programs in melanomaCitation91().
The participation of several signal transduction pathways in the maturation process of miRNAs has also been described. Recent work has demonstrated that upon activation, the kinase MAPK-activated protein kinase 2 (MK2) phosphorylates p68, enhancing its nuclear localization and incorporation into the microprocessor complex. In breast cancer cell lines, the inhibition of MK2 signaling promotes cell proliferation by enhancing the expression of c-Myc through the suppression of pri-miRNA processing of miR-145, which targets c-MycCitation92().
Apart from their activity as transcription factors, hormone receptors could affect the maturation of miRNAs by preventing the pri-miRNA to pre-miRNA conversion. In breast cancer, ERβ down-regulates miR-30a inhibiting pri-miRNA polymerization, while ERα, but not ERβ, shows inhibitory effects over the maturation of the pri-miRNA cluster miR-23b/27b/24–1 through its direct binding to the p68/p72 Drosha microprocessor complex, which can be activated by E2.Citation93 A Recent study reported that E2 negatively regulates the expression of miR-30c in endometrial cancer cells, likely through prevention of miRNA maturation.Citation94 Moreover, the androgen receptor, an important tumorigenic player in prostate cancer, induces the transcription of miR-23a, miR-27a and miR-24–2, but more significantly accelerates primiR-23a/27a/24–2 cluster processing. The evidence indicates that pri-miR-23/27/24 cluster is regulated by hormone signaling in different cancers, which highlights its potential implication in the therapeutic area as a new drug targetCitation95 ().
More than a Loop, the Architecture of Pri-miRNA and its Regulatory Role
An important aspect in miRNA genomic organization is that a set of miRNAs can be located within the same transcription unit in the same manner as a polycistronic transcript. These miRNAs clustered inside the same transcriptional unit may be subject to independent regulation. There are few examples of miRNAs located in the same cluster, which are processed independently from each other. Some studies indicate that the hnRNP A1 binds to the loop region of miR-18, contained in the cluster mir17–92, generating a structural rearrangement in the hairpin that promotes Drosha cleavage, favoring the independent and unique processing of miR-18.Citation96 The loop region of miR-18a is evolutionarily conserved, suggesting that some other well-conserved loop regions can be modulated by this mechanism (). Some other studies have pointed out the importance of the loop region in pri-miRNA processing regulation, and have described the action of KH-type splicing regulatory protein (KSRP) which directly interacts with G-rich regions present in the loop of some pri-miRNAs, like let-7a and miR-206, promoting Drosha and Dicer processingCitation97 ().
Figure 3. Several post-transcriptional mechanisms of miRNA biogenesis regulation. (A) Lin28 prevents the association of Drosha to the pri-miRNA let-7. (B) KSRP binds to the loop region and promotes Drosha processing. (C) Lin28 prevents the association of Dicer to the pre-miRNA let-7. (D) Lin28 promotes the association of TUT4 with the pre-miRNA let-7, enhancing the 3′ uridinylation of the pre-miRNA, and consequently its degradation. (E) KSRP binds to the loop region and promotes Dicer processing. (F) MAPK/ERK signaling modulates the expression or activity of Dicer, by promoting phosphorylation of TRBP. (G) The recognition of the 5′ monophosphate of the pre-miRNAs by Dicer is disrupted by the RNA-methyltransferase BCDIN3D, which phospho-dimethylates the pre-miR-145, and decreases miRNA processing by Dicer. (H) EGFR inhibits the processing of pre-miRNA through phosphorylation of AGO2-Y393, which attenuates the processing of pre-miRNAs to mature miRNAs under hypoxic conditions.
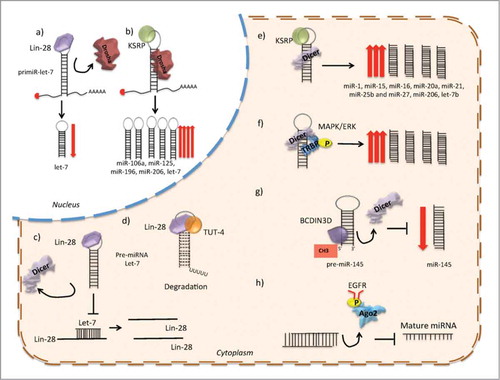
Another well-described mechanism is the link between Lin28 and let-7. Lin28 proteins are oncogenes activated in cancer which function through the repression of the let-7 miRNA family.Citation98 It has been described that Lin28 blocks let-7 processing at the pri- and pre-miRNA steps, inhibiting the association of the microprocessor or Dicer complexes. This inhibitory mechanism can be the result of the strong interaction between Lin28 and Drosha/DGCR8. Alternatively, the interaction of Lin28 with the loop region might rearrange the secondary structure of the hairpin and inhibit Drosha cleavage.Citation99 Lin28A and Lin28B are in fact targets of let-7, indicating that Lin28/let-7 regulation involves a double-negative feedback loop, which under physiological conditions serves as a developmental switchCitation100 ().
In cancer, germline mutations play an important role in the establishment of tumor pathways. In this context, the effect of germline mutations on the regulation of let-7/Lin28 loop might have a huge impact, in particular in breast cancer. The Lin28 rs3811463 (T/C) SNP, located near the let-7 binding site, might disrupt the loop of Lin28/let-7. Specifically, the C allele induced the repression of let-7 by Lin28, resulting in an increased expression of Lin28 and the consequent downregulation of mature let-7.Citation101
Exportin 5: Defects in Pre-miRNA Transportat to the Cytoplasm
The miRNA biogenesis pathway can also be affected by mutations in the conveyor exportin 5 (XPO5). Some tumors have mutations that generate pre-miRNA accumulation in the nucleus, reducing miRNA processing and diminishing mature miRNA expression.Citation102 The mutant exportin protein lacks a C-terminal region that prevents its association and the export of the pre-miRNA to the cytoplasm, inducing pre-miRNA degradation in the nucleus.Citation103
Cytoplasmatic Regulation
Role of Dicer cleavage and expression in cancer
Studies in murine models show that partial depletion of Dicer and Drosha accelerates cellular transformation and tumorigenesis.Citation104 Furthermore, the complete depletion of Dicer causes miRNA silencing, tumor development and lethality. Heterozygous germ-line mutations in Dicer1 have been described in the pleuropulmonary blastoma-inherited cancer syndrome,Citation105 and somatic missense mutations have been detected in ovarian tumors.Citation106 In addition, mutations in other proteins can also alter the expression of Dicer and consequently, its function. For example, truncating mutations in TARBP2, a stabilizer of Dicer 1 protein, down-regulates miRNA global expression in sporadic and hereditary colon carcinomas with microsatellite instability.Citation107
Lin28 can also be transported between the nucleus and cytoplasm, although it is enriched in the cytoplasm, suggesting that this is its primary compartment. Lin28 over-expression results in the reduction of Dicer association with let-7 pre-miRNA and, therefore, reduces mature duplex miRNA levels. One possible explanation, is that Lin28 competes with Dicer for recognition of the let-7 pre-miRNA sequence.Citation108 Another mechanism involves the 3′-polyuridylation of pre-let-7, accomplished through cooperative activity of Lin28 with terminal uridine transferase-4 (TUT4).Citation99 Sequencing data revealed that the loop of pre-miRNA let-7 has a tetra-nucleotide sequence required for Lin28 binding and consequently Lin28/TUT4 uridylation.Citation109 Furthermore, knockdown of TUT4 and Lin28 in cancer cells decreased the level of stem cell markers, suggesting that they are required for the maintenance of a tumoral stem cell phenotype ().
p53 mutations are common alterations in the majority of tumors with a variety of consequences. In this regard, it has been shown that mutant p53 can down-regulate Dicer expression through different inhibitory mechanism such as the direct association of TAp63 (a pro-apoptotitc p53 family member) to the DICER promoter in a transcriptional regulatory manner.Citation110 In murine models, the relationship between mutant p53, TAp63, and Dicer might contribute to the metastatic process promoting cell invasionCitation111 ().
In several cancer programs, the pleiotropic activity of miRNAs constitutes a mean that provides a wide range of modulatory factors which can considerably modify the malignant phenotype of cancer cells. Particularly, in cell reprograming, miRNAs have been proved to work as factors that may accelerate or suppress the reprogramming process. Recent work in colo-rectal cancer demonstrated that Dicer1-deficient cells showed a reduced number of reprogrammed cells than wild type cells, suggesting that the miRNAs biogenesis machinery can also impact the reprogramming process and tumor phenotype.Citation112
The expression of Dicer may also be regulated by cofactors such as TRBP and PACT. Depletion of either of these cofactors decreases the basal levels of Dicer protein.Citation23 Furthermore, TRBP mutations have been described in cancer and are associated with decreased miRNA levels and with the destabilization of Dicer. Moreover, the overexpression of TRBP contributes to the malignant phenotype of cancer cells.Citation113 It has been shown that cellular signaling pathways like MAPK/ERK can also modulate the expression or activity of Dicer by promoting the phosphorylation of TRBP, which enhances miRNA production by increasing the stability of DicerCitation113().
Finally, the recognition of the 5′ monophosphate in pre-miRNAs by Dicer has been reported to be an important mechanism to achieve effective miRNA biogenesis. Recently, the RNA-methyltransferase BCDIN3D, has been identified as a negative regulator for miRNA maturation. In breast cancer, BCDIN3D phospho-dimethylates the tumor suppressor pre-miR-145 causing a reduction in its processing by DicerCitation114().
Clinical Value of miRNA Biogenesis Machinery Profiles
Consistent with the functional consequences of alterations in the expression of proteins involved in miRNA biogenesis, different studies have highlighted the clinical relevance of defining markers for tumor prognosis and aggressiveness based on the status of the miRNA biogenesis machinery. For example, differences in the expression levels and cellular localization of Dicer between different tumor types and clinical outcomes suggest that Dicer and Drosha might have more than one function among cancer cells with different phenotypes. summarizes some of the studies that report the relationship of Dicer and Drosha expression with clinical outcomes in different cancers.
Table 2. Relation between Drosha and Dicer with clinical parameters
Argonaute, a Multi-role Protein in Cancer
During the final steps of miRNA biogenesis, the expression of Ago2 is not only critical for the formation of the miRISC, but also for the amount of mature miRNAs. Ectopic expression of Ago proteins results in an increase in mature miRNA levels.Citation128 This correlation between Ago and mature miRNA levels, suggests that Ago must be expressed in a controlled and limited fashion in the cell to maintain miRNA homeostasis under physiological conditions. Interestingly, the altered expression of Ago2 is also associated with a transformed phenotype in breast cancer cells.Citation129
Hypoxia contributes to altered gene expression in tumors. In addition to affecting the activity of coding genes, increased levels of hypoxia in cancer cells also affects miRNA maturation and stability. Hypoxia leads to an increase in the expression of the enzyme prolyl hydroxylation, which hydroxilates Ago2. Thus, hydroxylation of Ago2 is required for miRNA loading onto the RISC; more hydroxilated Ago2 protein results in an increase level of mature miRNAs. These data show how a posttranslational modification of Ago2 via hypoxia might mediate the miRNA biogenesis pathway.Citation130
Stress responses in tumor cells are also important for the modulation of miRNA biogenesis. EGFR, a well-known oncogene, suppresses the maturation of specific miRNAs in response to hypoxic stress. The association between EGFR and Ago2 promotes the Ago2-Y393 phosphorylation, which in turn inhibits miRNA processing by impairing the proper formation of the RISC-Ago2 complex. Strikingly, high levels of phosphorylated-Y393-Ago2 have been correlated with poor survival in breast cancer.Citation131
The role of Ago2 in the function of mature miRNAs extends beyond its activity as member of the RISC complex. In fact, down-regulation of Ago2 reduces the half-lives of multiple miRNAs. Argonaute proteins post-transcriptionally regulate mature miRNA levels via increasing miRNA stability.Citation132 Ago2 may also have a role as translational repressor of the miRNA-mRNA duplex. All this data highlights the versatility of Ago2 as modulator of miRNA gene expression and function.Citation133
Even though Ago2 is the only catalytic argonaute protein in mammals, all 4 human argonaute proteins bind to miRNAs. Genomic approaches have shown that some miRNA subpopulations preferentially bind to a certain argonaute protein (Ago1, Ago3, Ago4 - which might be redundant- and Ago2) in a context dependent manner with different implications in carcinogenesis.Citation134,27 Conversely, miRNA incorporation to the RISC complex might be regulated and influenced by different factors; one of them is incorporation of ago 3, which enhances the incorporation of the passenger strand of the tumor suppressor let-7a (let7-a*) to the RISC complex, and by consequence, promotes its biological activity in cancer cells. It seems that Ago3 modulates the ratio between microRNA guide and passenger strands.Citation135
Conclusion - New Insights
The advent of new analytical methods, such as RNA-seq and Chip-seq, has allowed us to gain insight into the versatility of factors controlling gene expression and how the disturbance of such factors might operate to determine the altered phenotypes of cancer cells. Since their discovery, miRNAs have been the subject of intense research. During this time, we have gained considerable understanding about the biogenesis pathways and mechanisms of action of miRNAs. However, one thing we have learned is that there are many ways in which the processes of miRNA production, stability and maturation can be orchestrated. Moreover, new mechanisms for miRNA biogenesis have been described and they might play important roles as cancer drivers (). It is possible that as we increase our power to unveil novel factors involved in miRNA biogenesis, we will also find new disruption mechanisms that alter the proper function of these molecules in the cancer scenario, giving us the opportunity to explore new veins for biomarker discovery and development of new targeted drugs.
Table 3. Novel mechanisms of miRNA biogenesis and their possible impact on cancer
Disclosure of Potential Conflicts of Interests
No potential conflicts of interest were disclosed.
References
- Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med 2009; 60:167-79; PMID: 19630570; http://dx.doi.org/10.1146/annurev.med.59.053006.104707
- Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 2006; 6:259-69; PMID:16557279; http://dx.doi.org/10.1038/nrc1840
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 2006; 103:2257-61; PMID: 16461460; http://dx.doi.org/10.1073/pnas.0510565103
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature 2005; 435:834-8; PMID: 15944708; http://dx.doi.org/10.1038/nature03702.
- Lujambio A, Lowe SW. The microcosmos of cancer. Nature 2012; 482:347-55; PMID:22337054; http://dx.doi.org/10.1038/nature10888
- Altuvia Y, Landgraf P, Lithwick G, Elefant N, Pfeffer S, Aravin A, Brownstein MJ, Tuschl T, Margalit H. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res 2005; 33:2697-706; PMID:15891114; http://dx.doi.org/10.1093/nar/gki567
- Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. Embo J 2004; 23:4051-60; PMID: 15372072; http://dx.doi.org/10.1038/sj.emboj.7600385
- Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol 2006; 13:1097-101.
- Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. Rna 2004; 10:1957-66; PMID:15525708; http://dx.doi.org/10.1261/rna.7135204
- Blaszczyk J, Tropea JE, Bubunenko M, Routzahn KM, Waugh DS, Court DL, Ji X. Crystallographic and modeling studies of RNase III suggest a mechanism for double-stranded RNA cleavage. Structure 2001; 9:1225-36; PMID:11738048; http://dx.doi.org/10.1016/S0969-2126(01)00685-2
- Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev 2004; 18:3016-27; PMID:15574589; http://dx.doi.org/10.1101/gad.1262504
- Han J, Pedersen JS, Kwon SC, Belair CD, Kim YK, Yeom KH, Yang WY, Haussler D, Blelloch R, Kim VN. Posttranscriptional crossregulation between Drosha and DGCR8. Cell 2009; 136:75-84
- Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, Sohn SY, Cho Y, Zhang BT, Kim VN. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 2006; 125:887-901; PMID:16751099; http://dx.doi.org/10.1016/j.cell.2006.03.043
- Morlando M, Ballarino M, Gromak N, Pagano F, Bozzoni I, Proudfoot NJ. Primary microRNA transcripts are processed co-transcriptionally. Nat Struct Mol Biol 2008; 15:902-9; PMID:19172742; http://dx.doi.org/10.1038/nsmb.1475
- Zeng Y, Cullen BR. Sequence requirements for micro RNA processing and function in human cells. Rna 2003; 9:112-23; PMID:12554881; http://dx.doi.org/10.1261/rna.2780503
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003; 425:415-9.
- Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Mol Cell 2007; 28:328-36; PMID:17964270; http://dx.doi.org/10.1016/j.molcel.2007.09.028
- Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev 2003; 17:3011-6; PMID:14681208; http://dx.doi.org/10.1101/gad.1158803
- Zeng Y, Cullen BR. Structural requirements for pre-microRNA binding and nuclear export by Exportin 5. Nucleic Acids Res 2004; 32:4776-85; PMID: 15356295; http://dx.doi.org/10.1093/nar/gkh824
- Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 2005; 123:631-40; PMID:16271387; http://dx.doi.org/10.1016/j.cell.2005.10.022
- Feng Y, Zhang X, Graves P, Zeng Y. A comprehensive analysis of precursor microRNA cleavage by human Dicer. Rna 2012; 18:2083-92; PMID: 22984192; http://dx.doi.org/10.1261/rna.033688.112
- Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 2005; 436:740-4; PMID:15973356; http://dx.doi.org/10.1038/nature03868
- Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. Embo J 2006; 25:522-32; PMID:16424907; http://dx.doi.org/10.1038/sj.emboj.7600942
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet 2003; 35:215-7.
- Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci 2008; 28:4322-30; PMID: 18434510; http://dx.doi.org/10.1523/JNEUROSCI.4815-07.2008
- Maniataki E, Mourelatos Z. A human, ATP-independent, RISC assembly machine fueled by pre-miRNA. Genes Dev 2005; 19:2979-90; PMID: 16357216; http://dx.doi.org/10.1101/gad.1384005
- Daschkey S, Rottgers S, Giri A, Bradtke J, Teigler-Schlegel A, Meister G, Borkhardt A, Landgraf P. MicroRNAs distinguish cytogenetic subgroups in pediatric AML and contribute to complex regulatory networks in AML-relevant pathways. PloS One 2013; 8:e56334; PMID:23418555
- Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell 2003; 115:199-208; PMID:14567917; http://dx.doi.org/10.1016/S0092-8674(03)00759-1
- Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell 2003; 115:209-16; PMID:14567918; http://dx.doi.org/10.1016/S0092-8674(03)00801-8
- Romero-Cordoba S, Rodriguez-Cuevas S, Rebollar-Vega R, Quintanar-Jurado V, Maffuz-Aziz A, Jimenez-Sanchez G, Bautista-Pina V, Arellano-Llamas R, Hidalgo-Miranda A. Identification and pathway analysis of microRNAs with no previous involvement in breast cancer. PLoS One 2012; 7:e31904.
- Marco A, Macpherson JI, Ronshaugen M, Griffiths-Jones S. MicroRNAs from the same precursor have different targeting properties. Silence 2012; 3:8; PMID:23016695; http://dx.doi.org/10.1186/1758-907X-3-8
- Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol 2008; 9:22-32; PMID:18073770; http://dx.doi.org/10.1038/nrm2321
- Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell 2008; 132:9-14; PMID:18191211; http://dx.doi.org/10.1016/j.cell.2007.12.024
- Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol 2005; 7:719-23.
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al. A microRNA component of the p53 tumour suppressor network. Nature 2007; 447:1130-4; PMID: 17554337; http://dx.doi.org/10.1038/nature05939
- Davis BN, Hata A. Regulation of MicroRNA Biogenesis: A miRiad of mechanisms. Cell Commun Signal 2009; 7:18; PMID:19664273; http://dx.doi.org/10.1186/1478-811X-7-18
- Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem 2009; 284:3728-38; PMID:19088079; http://dx.doi.org/10.1074/jbc.M808788200
- Shao M, Rossi S, Chelladurai B, Shimizu M, Ntukogu O, Ivan M, Calin GA, Matei D. PDGF induced microRNA alterations in cancer cells. Nucleic Acids Res 2011; 39:4035-47; PMID: 21266476; http://dx.doi.org/10.1093/nar/gkq1305
- Butz H, Racz K, Hunyady L, Patocs A. Crosstalk between TGF-beta signaling and the microRNA machinery. Trends Pharmacol Sci 2012; 33:382-93; PMID:22613783; http://dx.doi.org/10.1016/j.tips.2012.04.003
- Bui TV, Mendell JT. Myc: Maestro of MicroRNAs. Genes Cancer 2010; 1:568-75; PMID: 20882107; http://dx.doi.org/10.1177/1947601910377491
- Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 2008; 40:43-50; PMID:18066065; http://dx.doi.org/10.1038/ng.2007.30
- O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005; 435:839-43; PMID: 15944709; http://dx.doi.org/10.1038/nature03677
- Loven J, Zinin N, Wahlstrom T, Muller I, Brodin P, Fredlund E, Ribacke U, Pivarcsi A, Pahlman S, Henriksson M. MYCN-regulated microRNAs repress estrogen receptor-alpha (ESR1) expression and neuronal differentiation in human neuroblastoma. Proc Natl Acad Sci U S A 2010; 107:1553-8; PMID:20080637; http://dx.doi.org/10.1073/pnas.0913517107
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature 2005; 435:828-33; PMID:15944707
- Yu G, Tang JQ, Tian ML, Li H, Wang X, Wu T, Zhu J, Huang SJ, Wan YL. Prognostic values of the miR-17-92 cluster and its paralogs in colon cancer. J Surg Oncol 2012; 106:232-7; PMID:22065543
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, Henderson JM, Kutok JL, Rajewsky K. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol 2008; 9:405-14; PMID: 18327259
- Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet 2006; 38:1060-5; PMID:16878133; http://dx.doi.org/10.1038/ng1855
- Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 2010; 12:247-56.
- Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T, Ambesi-Impiombato A, Califano A, Migliazza A, Bhagat G, et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell 2010; 17:28-40; PMID:20060366
- Linsley PS, Schelter J, Burchard J, Kibukawa M, Martin MM, Bartz SR, Johnson JM, Cummins JM, Raymond CK, Dai H, et al. Transcripts targeted by the microRNA-16 family cooperatively regulate cell cycle progression. Mol Cell Biol 2007; 27:2240-52.
- Cannell IG, Kong YW, Johnston SJ, Chen ML, Collins HM, Dobbyn HC, Elia A, Kress TR, Dickens M, Clemens MJ, et al. p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. Proc Natl Acad Sci U S A 2010; 107:5375-80.
- Chang TC, Zeitels LR, Hwang HW, Chivukula RR, Wentzel EA, Dews M, Jung J, Gao P, Dang CV, Beer MA, et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci U S A 2009; 106:3384-9; PMID:19211792; http://dx.doi.org/10.1073/pnas.0808300106
- Lujambio A, Calin GA, Villanueva A, Ropero S, Sanchez-Cespedes M, Blanco D, Montuenga LM, Rossi S, Nicoloso MS, Faller WJ, et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci U S A 2008; 105:13556-61; PMID:18768788; http://dx.doi.org/10.1073/pnas.0803055105
- Lujambio A, Ropero S, Ballestar E, Fraga MF, Cerrato C, Setien F, Casado S, Suarez-Gauthier A, Sanchez-Cespedes M, Git A, et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res 2007; 67:1424-9; PMID: 17308079; http://dx.doi.org/10.1158/0008-5472.CAN-06-4218
- Lujambio A, Portela A, Liz J, Melo SA, Rossi S, Spizzo R, Croce CM, Calin GA, Esteller M. CpG island hypermethylation-associated silencing of non-coding RNAs transcribed from ultraconserved regions in human cancer. Oncogene 2010; 29:6390-401; PMID:20802525; http://dx.doi.org/10.1038/onc.2010.361
- Urdinguio RG, Fernandez AF, Lopez-Nieva P, Rossi S, Huertas D, Kulis M, Liu CG, Croce CM, Calin GA, Esteller M. Disrupted microRNA expression caused by Mecp2 loss in a mouse model of Rett syndrome. Epigenetics 2010; 5:656-63; PMID: 20716963; http://dx.doi.org/10.4161/epi.5.7.13055
- Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M, Vandenberg SR, Ginzinger DG, James CD, Costello JF, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med 2008; 6:14; PMID:18577219; http://dx.doi.org/10.1186/1741-7015-6-14
- Agirre X, Vilas-Zornoza A, Jimenez-Velasco A, Martin-Subero JI, Cordeu L, Garate L, San Jose-Eneriz E, Abizanda G, Rodriguez-Otero P, Fortes P, et al. Epigenetic silencing of the tumor suppressor microRNA Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia. Cancer Res 2009; 69:4443-53; PMID: 19435910; http://dx.doi.org/10.1158/0008-5472.CAN-08-4025
- Pang RT, Leung CO, Ye TM, Liu W, Chiu PC, Lam KK, Lee KF, Yeung WS. MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinogenesis 2010; 31:1037-44; PMID:20351093; http://dx.doi.org/10.1093/carcin/bgq066
- Majid S, Dar AA, Saini S, Arora S, Shahryari V, Zaman MS, Chang I, Yamamura S, Tanaka Y, Deng G, et al. MicroRNA-23b represses proto-oncogene Src kinase, functions as a methylation-silenced tumor suppressor with diagnostic and prognostic significance in prostate cancer. Cancer Res 2012; 72:6435-46; PMID:23074286
- Suh SO, Chen Y, Zaman MS, Hirata H, Yamamura S, Shahryari V, Liu J, Tabatabai ZL, Kakar S, Deng G, et al. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis 2011; 32:772-8; PMID:21349819; http://dx.doi.org/10.1093/carcin/bgr036
- Gao XN, Lin J, Li YH, Gao L, Wang XR, Wang W, Kang HY, Yan GT, Wang LL, Yu L. MicroRNA-193a represses c-kit expression and functions as a methylation-silenced tumor suppressor in acute myeloid leukemia. Oncogene 2011; 30:3416-28; PMID: 21399664; http://dx.doi.org/10.1038/onc.2011.62
- Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol 2010; 11:136-46; PMID:20022810; http://dx.doi.org/10.1016/S1470-2045(09)70343-2
- Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 2008; 451:147-52.
- Chen WS, Leung CM, Pan HW, Hu LY, Li SC, Ho MR, Tsai KW. Silencing of miR-1-1 and miR-133a-2 cluster expression by DNA hypermethylation in colorectal cancer. Oncol Rep 2012; 28:1069-76; PMID:22766685
- Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, Carneiro F, Esteller M. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene 2012; 31:2062-74; PMID:21874049; http://dx.doi.org/10.1038/onc.2011.383
- Goel A, Boland CR. Epigenetics of Colorectal Cancer. Gastroenterology 2012; 143; 1442-60; PMID: 23000599 http://dx.doi.org/10.1053/j.gastro.2012.09.032
- Chang S, Wang RH, Akagi K, Kim KA, Martin BK, Cavallone L, Haines DC, Basik M, Mai P, Poggi E, et al. Tumor suppressor BRCA1 epigenetically controls oncogenic microRNA-155. Nat Med 2011; 17:1275-82; PMID:21946536; http://dx.doi.org/10.1038/nm.2459
- Soto-Reyes E, Gonzalez-Barrios R, Cisneros-Soberanis F, Herrera-Goepfert R, Perez V, Cantu D, Prada D, Castro C, Recillas-Targa F, Herrera LA. Disruption of CTCF at the miR-125b1 locus in gynecological cancers. BMC Cancer 2012; 12:40; PMID: 22277129; http://dx.doi.org/10.1186/1471-2407-12-40
- Zhang Y, Yan LX, Wu QN, Du ZM, Chen J, Liao DZ, Huang MY, Hou JH, Wu QL, Zeng MS, et al. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res 2011; 71:3552-62; PMID:21444677; http://dx.doi.org/10.1158/0008-5472.CAN-10-2435
- Siomi H, Siomi MC. Posttranscriptional regulation of microRNA biogenesis in animals. Mol Cell 2010; 38:323-32; PMID:20471939; http://dx.doi.org/10.1016/j.molcel.2010.03.013
- Kawahara Y, Zinshteyn B, Chendrimada TP, Shiekhattar R, Nishikura K. RNA editing of the microRNA-151 precursor blocks cleavage by the Dicer-TRBP complex. EMBO Rep 2007; 8:763-9; PMID: 17599088; http://dx.doi.org/10.1038/sj.embor.7401011
- Choudhury Y, Tay FC, Lam DH, Sandanaraj E, Tang C, Ang BT, Wang S. Attenuated adenosine-to-inosine editing of microRNA-376a* promotes invasiveness of glioblastoma cells. J Clin Invest 2012; 122:4059-76; PMID:23093778; http://dx.doi.org/10.1172/JCI62925
- Shiohama A, Sasaki T, Noda S, Minoshima S, Shimizu N. Nucleolar localization of DGCR8 and identification of eleven DGCR8-associated proteins. Exp Cell Res 2007; 313:4196-207; PMID:17765891; http://dx.doi.org/10.1016/j.yexcr.2007.07.020
- Fuller-Pace FV, Ali S. The DEAD box RNA helicases p68 (Ddx5) and p72 (Ddx17): novel transcriptional co-regulators. Biochem Soc Trans 2008; 36:609-12; PMID:18631126; http://dx.doi.org/10.1042/BST0360609
- Fuller-Pace FV. DExD/H box RNA helicases: multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res 2006; 34:4206-15; PMID:16935882; http://dx.doi.org/10.1093/nar/gkl460
- Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 2008; 454:56-61; PMID:18548003; http://dx.doi.org/10.1038/nature07086
- Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell 2010; 39:373-84; PMID:20705240; http://dx.doi.org/10.1016/j.molcel.2010.07.011
- Kong W, Yang H, He L, Zhao JJ, Coppola D, Dalton WS, Cheng JQ. MicroRNA-155 is regulated by the transforming growth factor β/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol 2008; 28:6773-84; PMID:18794355; http://dx.doi.org/10.1128/MCB.00941-08
- Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature 2009; 460:529-33; PMID:19626115; http://dx.doi.org/10.1038/nature08199
- Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer 2012; 12:613-26; PMID:22898542; http://dx.doi.org/10.1038/nrc3318
- Zhu H, Dougherty U, Robinson V, Mustafi R, Pekow J, Kupfer S, Li YC, Hart J, Goss K, Fichera A, et al. EGFR signals downregulate tumor suppressors miR-143 and miR-145 in Western diet-promoted murine colon cancer: role of G1 regulators. Mol Cancer Res 2011; 9:960-75; PMID:21653642; http://dx.doi.org/10.1158/1541-7786.MCR-10-0531
- Pichiorri F, Suh SS, Rocci A, De Luca L, Taccioli C, Santhanam R, Zhou W, Benson DM, Jr., Hofmainster C, Alder H, et al. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell 2010; 18:367-81.
- Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, et al. p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med 2011; 208:875-83; PMID:21518799; http://dx.doi.org/10.1084/jem.20110235
- Leveille N, Elkon R, Davalos V, Manoharan V, Hollingworth D, Oude Vrielink J, le Sage C, Melo CA, Horlings HM, Wesseling J, et al. Selective inhibition of microRNA accessibility by RBM38 is required for p53 activity. Nat Commun 2011; 2:513; PMID:22027593; http://dx.doi.org/10.1038/ncomms1519
- Kawai S, Amano A. BRCA1 regulates microRNA biogenesis via the DROSHA microprocessor complex. J Cell Biol 2012; 197:201-8; PMID:22492723; http://dx.doi.org/10.1083/jcb.201110008
- Wilson MD, Wang D, Wagner R, Breyssens H, Gertsenstein M, Lobe C, Lu X, Nagy A, Burke RD, Koop BF, et al. ARS2 is a conserved eukaryotic gene essential for early mammalian development. Mol Cell Biol 2008; 28:1503-14; PMID:18086880; http://dx.doi.org/10.1128/MCB.01565-07
- Gruber JJ, Zatechka DS, Sabin LR, Yong J, Lum JJ, Kong M, Zong WX, Zhang Z, Lau CK, Rawlings J, et al. Ars2 links the nuclear cap-binding complex to RNA interference and cell proliferation. Cell 2009; 138:328-39; PMID:19632182; http://dx.doi.org/10.1016/j.cell.2009.04.046
- Sabin LR, Zhou R, Gruber JJ, Lukinova N, Bambina S, Berman A, Lau CK, Thompson CB, Cherry S. Ars2 regulates both miRNA- and siRNA- dependent silencing and suppresses RNA virus infection in Drosophila. Cell 2009; 138:340-51.
- Haselmann V, Kurz A, Bertsch U, Hubner S, Olempska-Muller M, Fritsch J, Hasler R, Pickl A, Fritsche H, Annewanter F, et al. Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology 2014; 146:278-90; PMID:24120475; http://dx.doi.org/10.1053/j.gastro.2013.10.009
- Nemlich Y, Greenberg E, Ortenberg R, Besser MJ, Barshack I, Jacob-Hirsch J, Jacoby E, Eyal E, Rivkin L, Prieto VG, et al. MicroRNA-mediated loss of ADAR1 in metastatic melanoma promotes tumor growth. J Clin Invest 2013; 123:2703-18; PMID:23728176; http://dx.doi.org/10.1172/JCI62980
- Hong S, Noh H, Chen H, Padia R, Pan ZK, Su SB, Jing Q, Ding HF, Huang S. Signaling by p38 MAPK stimulates nuclear localization of the microprocessor component p68 for processing of selected primary microRNAs. Sci Signaling 2013; 6:ra16; PMID:23482664
- Paris O, Ferraro L, Grober OM, Ravo M, De Filippo MR, Giurato G, Nassa G, Tarallo R, Cantarella C, Rizzo F, et al. Direct regulation of microRNA biogenesis and expression by estrogen receptor beta in hormone-responsive breast cancer. Oncogene 2012; 31:4196-206.
- Kong X, Xu X, Yan Y, Guo F, Li J, Hu Y, Zhou H, Xun Q. Estrogen Regulates the Tumour Suppressor MiRNA-30c and Its Target Gene, MTA-1, in Endometrial Cancer. PloS One 2014; 9:e90810; PMID:24595016
- Fletcher CE, Dart DA, Sita-Lumsden A, Cheng H, Rennie PS, Bevan CL. Androgen-regulated processing of the oncomir miR-27a, which targets Prohibitin in prostate cancer. Hum Mol Genet 2012; 21:3112-27.
- Michlewski G, Guil S, Semple CA, Caceres JF. Posttranscriptional regulation of miRNAs harboring conserved terminal loops. Mol Cell 2008; 32:383-93; PMID:18995836; http://dx.doi.org/10.1016/j.molcel.2008.10.013
- Trabucchi M, Briata P, Garcia-Mayoral M, Haase AD, Filipowicz W, Ramos A, Gherzi R, Rosenfeld MG. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 2009; 459:1010-4.
- Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O'Sullivan M, Lu J, Phillips LA, Lockhart VL, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet 2009; 41:843-8.
- Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho J, Yeom KH, Han J, Kim VN. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 2009; 138:696-708; PMID:19703396; http://dx.doi.org/10.1016/j.cell.2009.08.002
- Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, Wulczyn FG. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol 2008; 10:987-93; PMID:18604195; http://dx.doi.org/10.1038/ncb1759
- Chen AX, Yu KD, Fan L, Li JY, Yang C, Huang AJ, Shao ZM. Germline genetic variants disturbing the Let-7/LIN28 double-negative feedback loop alter breast cancer susceptibility. PLoS Genet 2011; 7:e189818992259; PMID:21912531
- Melo SA, Moutinho C, Ropero S, Calin GA, Rossi S, Spizzo R, Fernandez AF, Davalos V, Villanueva A, Montoya G, et al. A genetic defect in exportin-5 traps precursor microRNAs in the nucleus of cancer cells. Cancer Cell 2010; 18:303-15; PMID:20951941; http://dx.doi.org/10.1016/j.ccr.2010.09.007
- Melo SA, Esteller M. A precursor microRNA in a cancer cell nucleus: get me out of here! Cell Cycle 2011; 10:922-5; PMID:21346411
- Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol Chem 2007; 282:14328-36; PMID:17363372; http://dx.doi.org/10.1074/jbc.M611393200
- Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, Jarzembowski JA, Wikenheiser-Brokamp KA, Suarez BK, Whelan AJ, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science 2009; 325:965.
- Davalos V, Esteller M. Rolling the dice to discover the role of DICER in tumorigenesis. Cancer Cell 2012; 21:717-9; PMID:22698397; http://dx.doi.org/10.1016/j.ccr.2012.05.030
- Melo SA, Ropero S, Moutinho C, Aaltonen LA, Yamamoto H, Calin GA, Rossi S, Fernandez AF, Carneiro F, Oliveira C, et al. A TARBP2 mutation in human cancer impairs microRNA processing and DICER1 function. Nat Genet 2009; 41:365-70; PMID:19219043; http://dx.doi.org/10.1038/ng.317
- Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. Rna 2008; 14:1539-49.
- Heo I, Ha M, Lim J, Yoon MJ, Park JE, Kwon SC, Chang H, Kim VN. Mono-uridylation of pre-microRNA as a key step in the biogenesis of group II let-7 microRNAs. Cell 2012; 151:521-32; PMID:23063654; http://dx.doi.org/10.1016/j.cell.2012.09.022
- Su X, Chakravarti D, Cho MS, Liu L, Gi YJ, Lin YL, Leung ML, El-Naggar A, Creighton CJ, Suraokar MB, et al. TAp63 suppresses metastasis through coordinate regulation of dicer and miRNAs. Nature 2010; 467:986-90.
- Muller PA, Trinidad AG, Caswell PT, Norman JC, Vousden KH. Mutant p53 regulates Dicer through p63-dependent and -independent mechanisms to promote an invasive phenotype. J Biol Chem 2014; 289:122-32; PMID:24220032; http://dx.doi.org/10.1074/jbc.M113.502138
- Dewi DL, Ishii H, Haraguchi N, Nishikawa S, Kano Y, Fukusumi T, Ozaki M, Saito T, Sakai D, Satoh T, et al. Dicer 1, ribonuclease type III modulates a reprogramming effect in colorectal cancer cells. Int J Mol Med 2012; 29:1060-4; PMID:22446887
- Daniels SM, Gatignol A. The multiple functions of TRBP, at the hub of cell responses to viruses, stress, and cancer. Microbiol Mol Biol Rev 2012; 76:652-66; PMID:22933564; http://dx.doi.org/10.1128/MMBR.00012-12
- Xhemalce B, Robson SC, Kouzarides T. Human RNA methyltransferase BCDIN3D regulates microRNA processing. Cell 2012; 151:278-88.
- Jafarnejad SM, Sjoestroem C, Martinka M, Li G. Expression of the RNase III enzyme DROSHA is reduced during progression of human cutaneous melanoma. Mod Pathol: Official JU S Canada Acad Pathol, Inc 2013; 26:902-10; PMID:23370771
- Diaz-Garcia CV, Agudo-Lopez A, Perez C, Lopez-Martin JA, Rodriguez-Peralto JL, de Castro J, Cortijo A, Martinez-Villanueva M, Iglesias L, Garcia-Carbonero R, et al. DICER1, DROSHA and miRNAs in patients with non-small cell lung cancer: implications for outcomes and histologic classification. Carcinogenesis 2013; 34:1031-8; PMID:23349018; http://dx.doi.org/10.1093/carcin/bgt022
- Karube Y, Tanaka H, Osada H, Tomida S, Tatematsu Y, Yanagisawa K, Yatabe Y, Takamizawa J, Miyoshi S, Mitsudomi T, et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci 2005; 96:111-5; PMID:15723655; http://dx.doi.org/10.1111/j.1349-7006.2005.00015.x
- Caffrey E, Ingoldsby H, Wall D, Webber M, Dinneen K, Murillo LS, Inderhaug C, Newell J, Gupta S, Callagy G. Prognostic significance of deregulated dicer expression in breast cancer. PloS One 2013; 8:e83724.
- Kawahara K, Nakayama H, Nagata M, Yoshida R, Hirosue A, Tanaka T, Nakagawa Y, Matsuoka Y, Kojima T, Takamune Y, et al. A low dicer expression is associated with resistance to 5-FU-based chemoradiotherapy and a shorter overall survival in patients with oral squamous cell carcinoma. J Oral Pathol Med: Official Publ Int Assoc Oral Pathologists Am Acad Oral Pathol 2013; 43:350-6; PMID:24325353; http://dx.doi.org/10.1111/jop.12140
- Vincenzi B, Zoccoli A, Schiavon G, Iuliani M, Pantano F, Dell'aquila E, Ratta R, Muda AO, Perrone G, Brunelli C, et al. Dicer and Drosha expression and response to bevacizumab-based therapy in advanced colorectal cancer patients. Eur J Cancer 2013; 49:1501-8; PMID:23266047; http://dx.doi.org/10.1016/j.ejca.2012.11.014
- Papachristou DJ, Rao UN, Korpetinou A, Giannopoulou E, Sklirou E, Kontogeorgakos V, Kalofonos HP. Prognostic significance of Dicer cellular levels in soft tissue sarcomas. Cancer Invest 2012; 30:172-9; PMID:22149178; http://dx.doi.org/10.3109/07357907.2011.633293
- Zhu DX, Fan L, Lu RN, Fang C, Shen WY, Zou ZJ, Wang YH, Zhu HY, Miao KR, Liu P, et al. Downregulated Dicer expression predicts poor prognosis in chronic lymphocytic leukemia. Cancer Sci 2012; 103:875-81.
- Merritt WM, Lin YG, Han LY, Kamat AA, Spannuth WA, Schmandt R, Urbauer D, Pennacchio LA, Cheng JF, Nick AM, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med 2008; 359:2641-50.
- Shu GS, Yang ZL, Liu DC. Immunohistochemical study of Dicer and Drosha expression in the benign and malignant lesions of gallbladder and their clinicopathological significances. Pathol Res Pract 2012; 208:392-7; PMID:22658478; http://dx.doi.org/10.1016/j.prp.2012.05.001
- Passon N, Gerometta A, Puppin C, Lavarone E, Puglisi F, Tell G, Di Loreto C, Damante G. Expression of Dicer and Drosha in triple-negative breast cancer. J Clin Pathol 2012; 65:320-6; PMID:22259182; http://dx.doi.org/10.1136/jclinpath-2011-200496
- Guo X, Liao Q, Chen P, Li X, Xiong W, Ma J, Luo Z, Tang H, Deng M, Zheng Y, et al. The microRNA-processing enzymes: Drosha and Dicer can predict prognosis of nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2012; 138:49-56; PMID:21953080
- Li X, Tian X, Zhang B, Chen J. Polymorphisms in microRNA-related genes are associated with survival of patients with T-Cell lymphoma. Oncologist 2014; 19:243-9; PMID:24563077; http://dx.doi.org/10.1634/theoncologist.2013-0370
- Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell 2007; 131:1097-108; PMID:18083100; http://dx.doi.org/10.1016/j.cell.2007.10.032
- Adams BD, Claffey KP, White BA. Argonaute-2 expression is regulated by epidermal growth factor receptor and mitogen-activated protein kinase signaling and correlates with a transformed phenotype in breast cancer cells. Endocrinology 2009; 150:14-23; PMID:18787018; http://dx.doi.org/10.1210/en.2008-0984
- Wu C, So J, Davis-Dusenbery BN, Qi HH, Bloch DB, Shi Y, Lagna G, Hata A. Hypoxia potentiates microRNA-mediated gene silencing through posttranslational modification of Argonaute2. Mol Cell Biol 2011; 31:4760-74; PMID:21969601; http://dx.doi.org/10.1128/MCB.05776-11
- Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, Lim SO, Du Y, Wang Y, Chang WC, Chen CH, et al. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature 2013; 497:383-7; PMID:23636329; http://dx.doi.org/10.1038/nature12080
- Winter J, Diederichs S. Argonaute proteins regulate microRNA stability: Increased microRNA abundance by Argonaute proteins is due to microRNA stabilization. RNA Biol 2011; 8:1149-57; PMID:21941127; http://dx.doi.org/10.4161/rna.8.6.17665
- Janas MM, Wang B, Harris AS, Aguiar M, Shaffer JM, Subrahmanyam YV, Behlke MA, Wucherpfennig KW, Gygi SP, Gagnon E, et al. Alternative RISC assembly: binding and repression of microRNA-mRNA duplexes by human Ago proteins. Rna 2012; 18:2041-55.
- Dueck A, Ziegler C, Eichner A, Berezikov E, Meister G. microRNAs associated with the different human Argonaute proteins. Nucleic Acids Res 2012; 40:9850-62; PMID:22844086; http://dx.doi.org/10.1093/nar/gks705
- Winter J, Diederichs S. Argonaute-3 activates the let-7a passenger strand microRNA. RNA Biology 2013; 10:1631-43; PMID:24100239
- Plante I, Ple H, Landry P, Gunaratne PH, Provost P. Modulation of microRNA activity by semi-microRNAs. Front Genet 2012; 3:99.
- Zisoulis DG, Kai ZS, Chang RK, Pasquinelli AE. Autoregulation of microRNA biogenesis by let-7 and Argonaute. Nature 2012; 486:541-4; PMID:22722835
- Juvvuna PK, Khandelia P, Lee LM, Makeyev EV. Argonaute identity defines the length of mature mammalian microRNAs. Nucleic Acids Res 2012; 40:6808-20; PMID:22505576; http://dx.doi.org/10.1093/nar/gks293
- Wang T, Zang WQ, Li M, Wang N, Zheng YL, Zhao GQ. Effect of miR-451 on the biological behavior of the esophageal carcinoma cell line EC9706. Dig Dis Sci 2012; 58:706-14; PMID:23053883
- Tian Y, Nan Y, Han L, Zhang A, Wang G, Jia Z, Hao J, Pu P, Zhong Y, Kang C. MicroRNA miR-451 downregulates the PI3K/AKT pathway through CAB39 in human glioma. Int J Oncol 2012; 40:1105-12; PMID:22179124
- Bitarte N, Bandres E, Boni V, Zarate R, Rodriguez J, Gonzalez-Huarriz M, Lopez I, Javier Sola J, Alonso MM, Fortes P, et al. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells 2011; 29:1661-71; PMID:21948564; http://dx.doi.org/10.1002/stem.741
- Yang JS, Maurin T, Lai EC. Functional parameters of Dicer-independent microRNA biogenesis. Rna 2012; 18:945-57; PMID:22461413; http://dx.doi.org/10.1261/rna.032938.112
- Winter J, Link S, Witzigmann D, Hildenbrand C, Previti C, Diederichs S. Loop-miRs: active microRNAs generated from single-stranded loop regions. Nucleic Acids Res 2013; 41:5503-12; PMID:23580554; http://dx.doi.org/10.1093/nar/gkt251
- Cloonan N, Wani S, Xu Q, Gu J, Lea K, Heater S, Barbacioru C, Steptoe AL, Martin HC, Nourbakhsh E, et al. MicroRNAs and their isomiRs function cooperatively to target common biological pathways. Genome Biol 2011; 12:R126; PMID:22208850
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature 2013; 495:384-8; PMID:23446346; http://dx.doi.org/10.1038/nature11993
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013; 495:333-8; PMID:23446348; http://dx.doi.org/10.1038/nature11928
- Li ZF, Liang YM, Lau PN, Shen W, Wang DK, Cheung WT, Xue CJ, Poon LM, Lam YW. Dynamic localisation of mature microRNAs in Human nucleoli is influenced by exogenous genetic materials. PloS One 2013; 8:e70869; PMID:23940654
- Redfern AD, Colley SM, Beveridge DJ, Ikeda N, Epis MR, Li X, Foulds CE, Stuart LM, Barker A, Russell VJ, et al. RNA-induced silencing complex (RISC) Proteins PACT, TRBP, and Dicer are SRA binding nuclear receptor coregulators. Proc Natl Acad Sci U S A 2013; 110:6536-41; PMID:23550157; http://dx.doi.org/10.1073/pnas.1301620110
