Abstract
BNC105 is a tubulin targeting compound that selectively disrupts vasculature within solid tumors. The severe tumor hypoxia and necrosis that ensues translates to short term tumor growth inhibition. We sought to identify the molecular and cellular events activated following BNC105 treatment that drives tumor recovery. We investigated tumor adaptation to BNC105-induced hypoxia in animal models of breast and renal cancer. HIF-1α and GLUT-1 were found to be strongly upregulated by BNC105 as was the VEGF signaling axis. Phosphorylation of mTOR, 4E-BP-1 and elF2α were upregulated, consistent with increased protein synthesis and increased expression of VEGF-A. We sought to investigate the potential therapeutic utility of combining BNC105 with agents targeting VEGF and mTOR signaling. Bevacizumab and pazopanib target the VEGF axis and have been approved for first line use in renal cancer. Everolimus targets mTOR and is currently approved in second line therapy of renal and particular breast cancers. We combined these agents with BNC105 to explore the effects on tumor vasculature, tumor growth inhibition and animal survival. Bevacizumab hindered tumor vascular recovery following BNC105 treatment leading to greater tumor growth inhibition in a breast cancer model. Consistent with this, addition of BNC105 to pazopanib treatment resulted in a significant increase in survival in an orthotopic renal cancer model. Combination treatment of BNC105 with everolimus also increased tumor growth inhibition. BNC105 is currently being evaluated in a randomized phase II clinical trial in combination with everolimus in renal cancer.
Abbreviations
| HIF1α | = | hypoxia-inducible factor 1-alpha |
| GLUT-1 | = | glucose transporter 1 |
| mTOR | = | mammalian target of rapamycin |
| 4EBP1 | = | eukaryotic translation initiation factor 4E binding protein 1 |
| eIF2a | = | eukaryotic translation initiation factor 2a |
| VEGF | = | vascular endothelial growth factor |
| VDA | = | vascular disrupting agent |
| NSCLC | = | non-small-cell lung carcinoma |
| PERK | = | protein kinase-like endoplasmic reticulum kinase |
| PDGFR | = | platelet-derived growth factor receptor |
| TKI | = | tyrosine kinase inhibitor |
| PFS | = | progression free survival |
| IFNα | = | interferon α |
| H&E | = | hematoxylin and eosin |
Introduction
Vascular Disrupting Agents (VDAs) are distinct from angiogenic inhibitors in that they cause acute disruption of existing tumor blood vessels rather than suppress neovasculature.Citation1,2 VDAs cause the relatively fast growing and chaotic tumor vasculature to collapse, ceasing tumor blood perfusion with subsequent tumor ischemia, hypoxia and necrosis. VDAs achieve immediate vascular damage and are suited to acute administration regimens,Citation3 distinct from anti-angiogenic agents such as bevacizumab which require longer term administration.
Preclinical evaluations have demonstrated that VDAs cause tumor cavitation, while the rim of the tumor, which has access to normal vasculature surrounding the tumor, remains resistant to the effects of a VDA.Citation3,4 It is thought that the viable cells within the tumor rim activate a suite of adaptive and survival mechanisms implicit to many tumors and are responsible for driving the recovery of the tumor from the VDA treatment.Citation5,6,7 Given the propensity of tumors to recover from the effects of VDA action, clinical development has sought to investigate agents to use in combination with VDA treatment regimens.
Until now, VDAs have been primarily combined with traditional chemotherapeutics. Several preclinical publications and clinical trials have reported on combinations with paclitaxelCitation8 and carboplatinCitation9 in recurrent ovarian cancer (NCT01332656), NSCLC (NCT01263886) or advanced solid tumors (NCT00113438). Although these combinations were generally well tolerated in patients, the improvement to patient outcome was not sufficient for these combinations to gain clinical traction.Citation10 More recently, the potential complementarity of VDAs and anti-angiogenic drugs has been reportedCitation11 and is currently being evaluated in clinical trials (NCT01193595 and NCT01305213) in solid tumors and ovarian cancer. To date, these combinations have been shown to be well tolerated in patients with efficacy data not yet published.
BNC105 is a tubulin targeting VDA which displays selective disruption of tumor blood vessels with a therapeutic margin considerably wider than that of other previously described tubulin targeting agents.Citation3 In preclinical models, BNC105 causes rapid occlusion of tumor vasculature, resulting in hypoxic stress, necrosis and a suppression of tumor growth.Citation3 A phase I trial in late stage cancer patients and a phase II trial in mesothelioma patients demonstrated that BNC105 is well tolerated at dose levels that cause maximal VDA effect in animal models without causing hematological adverse events.Citation12 A number of patients receiving BNC105 as monotherapy experienced disease stabilization with one case of durable partial response.Citation13 Clinical evaluation of BNC105 in combination with standard of care treatments is currently being explored in renal and ovarian cancer.
We have previously demonstrated preclinically that suppressed tumor growth is evident for up to 4 d following a single administration of BNC105P (the pro-drug form of BNC105), following this time interval the tumor growth rate returns to control levels.Citation3 We have sought to identify rational combinations with targeted agents that increase the potential therapeutic utility of BNC105 without compromising safety. Our investigations were based on the identification of proteins upregulated after BNC105P treatment and the subsequent evaluation of combination therapies that incorporate agents that target these proteins.
Results
Combination with a VEGF-A targeted therapy suppresses tumor vasculature recovery following BNC105 action
Mice bearing solid tumors derived from subcutaneous inoculation of MDA-MB-231 human breast cancer cells were treated with a single administration of BNC105P. An increase in secreted VEGF-A was seen 30 hours post-treatment (). Given the previously established role of VEGF-A as an initiator of angiogenesis this increased expression suggests that surviving cancer cells potentially assist revascularisation of the tumor mass through production of VEGF-A.
Figure 1. BNC105P and targeted therapy treatment suppresses vasculature recovery in MDA-MB-231 xenograft. Tumors were grown to an average of ∼360 mm3. On day 1 of treatment, animals (n = 10/group) were treated once with saline, bevacizumab, BNC105P or bevacizumab and BNC105P. (A) IHC of secreted VEGF-A 30hrs post-BNC105P in tumor sections. (B) H33342 composite fluorescent images in tumor sections post-BNC105P. (C) Percentage H33342 perfusion ± SEM. Significant (*P < 0 .05, **P < 0 .01, P < ***0.001) decrease in perfusion indicated.
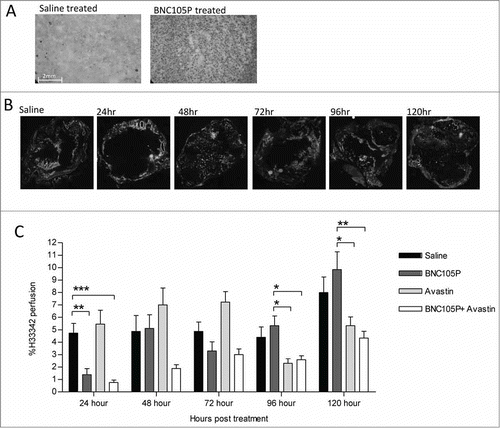
We investigated the timescale of tumor vascular disruption caused by a single administration of BNC105P over a 120 hour period. Animals were sacrificed every day and tumor vascular perfusion measured using the fluorescent stain, H33342. Animals treated with BNC105P alone displayed disruption of tumor vascular perfusion 24 hours post-treatment, with perfusion returning to pre-treatment levels by 48 hours (). To investigate the potential effects of inhibiting this VEGF-A signaling to suppress tumor vasculature recovery from BNC105 action, animals were also treated concurrently with a single dose of bevacizumab. We reasoned that concurrent treatment with bevacizumab would curtail VEGF-A signaling resulting in a more sustained BNC105 induced vascular disruption. Animals treated with bevacizumab alone did not display any effects on tumor perfusion at the early time points. Consistent with the anti-angiogenic effect of bevacizumab, a reduction in tumor perfusion was seen at the later time-points (). Combination treatment of BNC105P and bevacizumab caused a disruption in tumor perfusion that was evident at the earliest time point (24 hours) and persisted throughout the 120 hour period investigated. The combination of VEGF-A suppression with bevacizumab transformed the transient effect of BNC105 alone into one of significant sustained disruption of perfusion after 96 and 120 hours.
Combination of BNC105 with the VEGF-A inhibitor bevacizumab results in increased tumor growth inhibition in a xenograft model of breast cancer
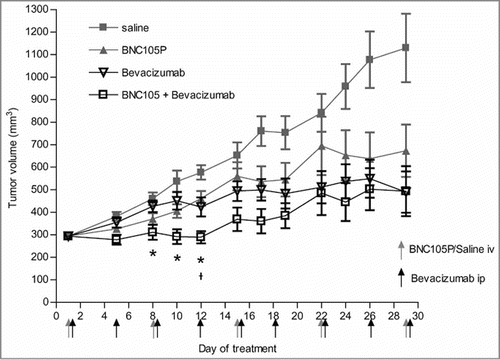
Given the observation that BNC105 and bevacizumab combine to cause sustained disruption of blood perfusion in tumors, we investigated whether this activity translated into increased tumor growth inhibition when these 2 drugs are combined. Mice bearing MDA-MB-231 solid tumors were treated with BNC105P, bevacizumab or the combination. The combination of BNC105P with bevacizumab was shown to be statistically significant during part of the treatment regimen compared to treatment with either of the monotherapies BNC105P alone (Day 12) or bevacizumab alone (Day 8–12) (). The effect however was temporal and significant differences between either of the monotherapies and the combination were not seen after day 12.
Figure 2. The combination of BNC105P with bevacizumab results in significant tumor growth inhibition of MDA-MB-231 xenografts in female Balb/c nude mice. Tumors were grown to approximately 300 mm3 prior to treatment. On day 1 of treatment, animals (n = 10/group) were treated with either saline, BNC105P, bevacizumab or BNC105P and bevacizumab. Mean ± SEM represented. Significant inhibition of tumor growth by the combination compared to BNC105 (*) and Bevacizumab (ᵼ) indicated.
Molecular markers associated with tumor recovery from BNC105 action in renal cancer
Given the demonstrated role of VEGF signaling in renal cancer and the recent success of VEGFR targeting agents in this cancer type, we expanded our investigation to renal cancer models. Initial studies were performed on mice bearing subcutaneous tumors arising from the human renal cancer cell line Caki-1 treated with a single dose of BNC105P. Tumors were recovered 24 hours post-treatment, sectioned and stained for a number of proteins known to be involved in adaptive responses of tumors to hypoxia. Clearly increased phosphorylation levels of the proteins mTOR and 4E-BP-1 were detectable in the tumor periphery (), suggesting increased mTOR signaling in this zone.
Figure 3. Molecular and cellular events associated with BNC105 induced vascular disruption and tumor recovery. (A) IHC showing DAB detection of ph-mTOR and ph-4E-BP-1 in the viable rim of Caki-1 tumors 24 hours post 32 mg/kg BNC105P. Upper panel of each indicates treatment with saline only. (B) H33342 fluorescent images of Renca orthotopic tumors (composite images) and metastatic lung lesions (Met.). Slides post-stained with H&E. (C) IHC showing Fast Red detection in representative viable tumor sections 24 hours post 32 mg/kg BNC105P or saline treatment. Control indicates detection antibody used only. (D) IHC showing Fast Red detection of secreted VEGF-A in Renca orthotopic tumors (composite image) 24 and 48 hours post 32 mg/kg BNC105P.
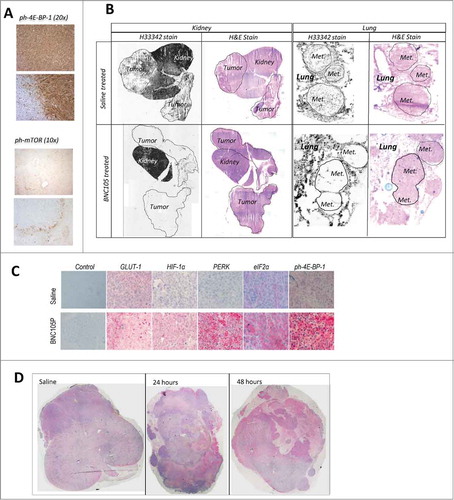
To further add strength to these observations we used the Renca orthotopic model, which closely simulates the human disease.Citation14 We also expanded the immunohistochemical analyses of markers to identify the molecular events underpinning tumor recovery following BNC105 induced vascular disruption. Renca cancer cells were implanted orthotopically under the kidney capsule of Balb/c mice giving rise to solid tumor growth and spontaneous metastasis to the lungs. A single treatment with BNC105P resulted in total disappearance of blood vessel perfusion within the tumor mass while sparing the corresponding perfusion in the normal areas of the kidney. A similar level of vascular disruption was observed in metastatic lesions in the lung (Fig. 3B). Importantly, despite the large vascular disruption caused by BNC105 within the tumor mass, blood perfusion within the normal regions of the kidney and lung remained intact.
Tumor bearing kidneys were recovered 24 hours post-BNC105P treatment and sections stained for proteins known to be involved in tumor adaptive response to hypoxia. PERK, GLUT-1, elF2α and HIF-1α displayed increased staining following a single BNC105P treatment. Very high levels of phosphorylated 4E-BP-1 expression were also seen (). This is consistent with increased protein translation and angiogenesis driving tumor recovery. Increased VEGF-A expression was prominent 24 hours post-BNC105P treatment and became more pronounced by 48 hours ().
Combination of BNC105 with pazopanib increases overall survival in an orthotopic model of renal cancer
Pazopanib, which targets VEGFR1, 2 and 3, PDGFR α/β and c-Kit, is one of a number of tyrosine kinase inhibitors (TKI) approved for the treatment of metastatic renal cell carcinoma. Given the evidence of increased VEGF axis signaling following BNC105 anti-tumor action we sought to investigate the potential benefit of combining BNC105 with pazopanib. In first line treatment, TKIs offer progression free survival (PFS) for renal cancer patients of approximately 11 monthsCitation15,16 compared to that of traditional IFNα treatment of 5 monthsCitation15 and their contribution to overall survival is approximately 29 months vs 21.8 months for IFNα.Citation17,18 We specifically set out to investigate the potential further benefit to overall survival by combining BNC105 with pazopanib. The Renca orthotopic renal cancer model being a close simulation of the human disease was used for this investigation.Citation14
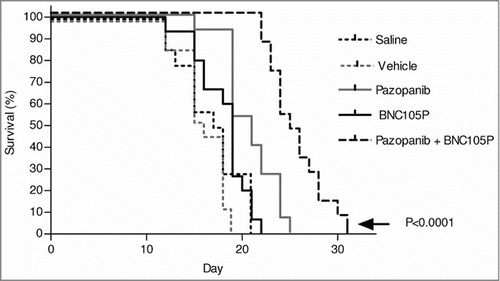
Following inoculation of Renca cells under the kidney capsule, tumors were allowed to form and grow for 11 d with treatment initiated on Day 12. Animals were treated with BNC105P, pazopanib or the combination. Pazopanib treatment was administered once daily for 21 Days. BNC105P was administered twice, one week apart on Days 2 and 9 of the treatment period.
The combination treatment was very well tolerated, indicated by lack of clinical signs and minimal changes in weight. A significant increase in animal overall survival was achieved by treatment with the combination (P < 0.0001) ().
Figure 4. The combination of BNC105 with pazopanib significantly increases overall survival in an orthotopic model of renal cancer. On day 1 of treatment, animals (n = 10/group) were treated with either saline, vehicle, pazopanib, BNC105P or pazopanib and BNC105P for 21 d. The combination of BNC105 with pazopanib significantly increased overall survival compared to monotherapies (P < 0.0001).
Combination of BNC105 with the mTOR inhibitor everolimus results in synergistic tumor growth inhibition in a xenograft model of renal cancer
The increased staining observed for phosphorylated mTOR and 4E-BP-1 in Caki-1 tumors, and increased eIF2α expression in the orthotopic renal model suggested that these tumor cells are heavily dependent on the activation of mTOR for survival and recovery following BNC105P treatment. This provided further support for investigating BNC105 in combination with everolimus.
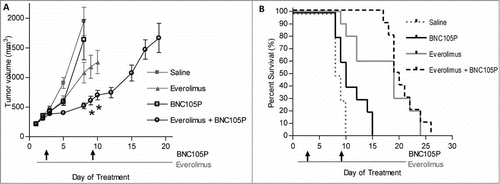
Animals bearing subcutaneous solid tumors of the Renca murine renal cancer cell line were treated with BNC105P, everolimus or the combination. A synergistic effect was observed (Robs > Radd), with animals treated with the combination experiencing greater tumor growth inhibition (73%, Robs = 0.73) compared to animals treated with BNC105 or everolimus as monotherapies (46% or 18% respectively, Radd = 0.56) at Day 8 (). Furthermore, animals treated with BNC105P in combination with everolimus experienced increased PFS while on therapy, although this survival advantage disappeared following the cessation of treatment ().
Figure 5. Inhibition of mTOR with everolimus results in synergistic tumor growth inhibition. (A) Tumors were grown to approximately 120–180 mm3. On day 1 of treatment, animals (n = 10/group) were treated with either saline, everolimus, BNC105P or everolimus and BNC105 for 21 d A significant reduction in tumor size compared to everolimus alone was observed Day 9 and 10 (p < 0.05). (B) Overall survival.
Discussion
The supply of oxygenated blood is physiologically fundamental to the growth of tumors and disease progression. Targeting tumor blood vessels has been an important and effective strategy in curtailing cancer progression. This is evidenced by the development, approval and clinical use of agents which inhibit tumor angiogenesis such as bevacizumab or TKIs such as pazopanib. The acute disruption of tumor blood vasculature through the use of VDAs has undergone clinical evaluation in several cancer types in combination with a number of different agents.Citation19 Experience gained to date suggests that this strategy can yield acute anti-tumor effects, which however do not translate to long lasting tumor suppression.Citation19,20 Clinical experience has been consistent with these preclinical observations. Response rates observed clinically to date with VDAs have been mediocre and there have been only a few instances where patients have experienced partial or complete responses. The majority of patients that experienced benefit from VDA treatment occurred in the form of disease stabilization, potentially attributable to the anti-proliferative effects that tubulin-binding agents also have on tumor parenchymal cells.Citation3,12,13 Phase II trials involving the combination of a VDA with platinum or taxane-based regimens have provided some encouraging dataCitation9,21 but follow-on phase III trials did not support further development.Citation10
We have explored the notion that a way to harness the action of VDA agents is through combination with targeted therapeutics which exploit the adaptive responses of the tumors to a sudden depletion of vasculature. Consistent with this thinking, we focused on the role of hypoxia as an integral component of the adaptive responses that take place within the tumor microenvironment. Under normal conditions, rapid cellular proliferation causes tumors to outgrow their own blood supply resulting in areas of chronic hypoxia. Additionally, aberrant shut-down of tumor blood vessels by BNC105 also results in areas of acute hypoxia. Tumors utilize a number of cellular and metabolic changes to withstand hypoxia and support further growth.Citation22 We reasoned that such adaptive responses may be in operation within the tumor following the widespread hypoxia caused by BNC105 action. Despite the normally oxygenated outer layer of tumor cells escaping hypoxia and only being subjected to the anti-proliferative effects of VDA treatment, the use of therapeutics targeted to the adaptive responses of the tumor microenvironment to hypoxia potentially allows for a greater stabilization or inhibition of the disease. In preclinical models the minimum dose of BNC105P causing maximum vascular disruption (>95%) is 10 mg/kg.Citation3 Further increases in the concentration of BNC105P used does not increase the vascular disruption effect. Due to the wide therapeutic window offered by BNC105, escalation to 32 mg/kg has been possible. Although maximum disruptive effect is seen at 10 mg/kg, dose escalation to 32 mg/kg sees a considerable increase in tumor growth inhibition. A possible explanation for this may be because both the vascular disruptive and anti-proliferative mechanism of BNC105 are in action.
Immunohistochemical analysis of tumors extracted from mice treated with a single dose of BNC105P revealed upregulation of proteins previously shown to be involved in response to hypoxia. As expected, BNC105 induced an increased presence of HIF-1α, consistent with literature demonstrating HIF-1α stabilization in an acutely hypoxic environment where oxygen dependent hydroxylation is inhibited.Citation23,24 Several genes regulating angiogenesis, proliferation and metabolism are targets of HIF binding and transcriptional activation. Consistent with this was the observed upregulation of GLUT-1 and VEGF-A following BNC105 action. GLUT-1 is a glucose transporter that is controlled by HIF-1α under hypoxic conditions and in conjunction with other glycolytic proteins contributes to suppression of apoptosis.Citation25 The return of tumor perfusion 48 hours post-BNC105P treatment is associated with an array of tumor microenvironment responses of which VEGF-A secretion, visualized at 30 hours, is very prominent. Blockade of VEGF-A signaling with bevacizumab was able to delay perfusion returning to pre-treatment levels and achieved a modest tumor growth inhibition.
Metastatic renal cancer has proven sensitive to inhibitors of the VEGF signaling pathway with bevacizumab and a number of VEGFR TKIs becoming first line standard of care in recent years. These agents have shifted the bar significantly in this disease setting with patients experiencing more than double the benefit in terms of PFS (11 months on average in first line therapyCitation15,16 compared to 5 months PFS experienced by patients before the advent of these VEGF/VEGFR axis targeting agentsCitation15). However, there has not been a corresponding doubling in overall survival offered by these agents with increased benefit offered by VEGFR inhibitors being comparatively moderate (21.8 months (IFNα) to 28.4 months (pazopanib)Citation17,18 ).
We specifically sought to investigate the potential improvement in overall survival offered through a combination of BNC105 with the TKI pazopanib. We demonstrated a clear advantage of treating animals bearing orthotopic kidney tumors which lived longer when treated with BNC105P in combination with pazopanib compared to animals treated with the respective monotherapies. This is a novel finding which warrants exploration of BNC105 in combination with pazopanib in clinical trials.
Increased phosphorylation of mTOR and phosphorylation of 4E-BP-1 was prominent in the viable rim, with phosphorylated 4E-BP-1 demarcating islands of viable tumor cells 48 hours post-BNC105P treatment. Consistent with increased 4E-BP-1 phosphorylation was the increase in expression of elF2α. 4E-BP-1 binds elF2α preventing protein translation. Upon phosphorylation, 4E-BP-1 dissociates from elF2α releasing it to initiate protein translation.Citation26 These protein expression changes provide support for the notion that tumor cells following VDA action phosphorylate mTOR which signals 4E-BP-1 phosphorylation resulting in the release/activation of eIF2α which in turn drives protein translation/synthesis, leading to increased levels of VEGF-A. Increased VEGF-A expression was prominent 24 hours post-BNC105P treatment and became more pronounced by 48 hours.
This observation led us to hypothesize that concurrent treatment with BNC105P and everolimus may also produce greater efficacy as was observed with the bevacizumab and BNC105 combination. Further support for this combination comes from prior observations that a VDA in combination with an mTOR inhibition regimen is more efficacious than the respective monotherapies in reducing endothelial cell sprouting, causing tumor vascular damage and reducing tumor blood volume.Citation27 Our results provide further support for an everolimus and BNC105 combination treatment. In animal models, the combination treatment displayed synergy in reducing the growth of Renca tumors and increased PFS while on treatment compared to instances where each of these agents were used as monotherapies.
Everolimus is currently approved for use in renal cancer patients that have progressed on tyrosine kinase inhibitor (TKI) therapies. Renal cancer patients treated with everolimus experience PFS of 4.9 months.Citation28 A Phase I/II randomized clinical trial comparing the efficacy of everolimus versus everolimus in combination with BNC105 is currently underway.
In summary, BNC105 is a tumor-selective VDA displaying a wider therapeutic window and greater selectivity than previously described VDAs. The challenges associated with use of any VDA as a monotherapy however are widely acknowledged. Through examination of the cellular and molecular changes that assist tumor recovery post-treatment with BNC105 we have been able to identify potential combination regimens with clinically relevant targeted therapeutics suppressing VEGF and mTOR signaling pathways that warrant further investigation in clinical trials.
Materials and Methods
Reagents
BNC105P compound, BNC105P clinical solution, bevacizumab (Avastin®) (Roche) and everolimus (Selleck Chemicals) were prepared to the required dose for each study with saline (0.9% sodium chloride solution). BNC105P is the pro-drug form of BNC105, 7-hydroxy-6-methoxy-2-methyl-3-(3,4,5-trimethoxybenzoyl)benzo[b]furan (used for i.v. administration).Citation3,29 Pazopanib (Votrient®) was generously provided by GlaxoSmithKline (USA) and prepared in 0.5% hydroxypropylmethylcellulose (HPMC) plus 0.1% Tween80 (both purchased from Sigma-Aldrich) in water.
A vascular disruption dose response was previously established with maximal disruption occurring at ≥10 mg/kg. The repeat maximum tolerated dose for BNC105P was determined to be 32 mg/kg, when dosed on day 1 and day 8 of a 21 day cycle in mice.Citation3 When compounds were administered in combination for tumor growth inhibition and survival studies 10 or 16 mg/kg BNC105P was used as indicated.
Cell lines and culture
The murine renal cell carcinoma (Renca) cell line and human renal cell carcinoma cell line (Caki-1) were purchased from the American Type Culture Collection. The human breast cancer cell line (MDA-MB-231) was a kind gift from the Women's and Children's Hospital, Adelaide, Australia. Cell lines were cultured in RPMI1640 supplemented with HEPES, Penicillin, Streptomycin, Glutamine (PSG), non-essential amino acids (NEAA), Sodium Pyruvate and 10% Fetal Bovine Serum (FBS) (Renca), RPMI1640 supplemented with HEPES, PSG and 10% FBS (MDA-MB-231), or McCoy's 5a supplemented with PSG and 10% FBS (Caki-1). All cancer cells were cultured in a humidified incubator at 37ºC with 5% CO2 in air. Media and supplements were purchased from Life Technologies. MDA-MB-231 cells were authenticated by STR profiling at completion of the studies. ATCC derived cell lines were stored at low passage in liquid nitrogen and used within 2 months of resuscitation.
In vivo models
Procedures involving the care and use of animals in this study were reviewed and approved by the SA Pathology/CHN Animal Ethics Committee, and conducted according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 7th edition, 2004 (National Health and Medical Research Council). All animals were purchased from Laboratory Animal Services, University of Adelaide, South Australia or the Animal Resource Center, Western Australia and housed under pathogen-free conditions.
Subcutaneous tumor models
Female Balb/c nude mice at 6 to 8 weeks old were subcutaneously inoculated with cancer cell lines derived from breast (MDA-MB-231) or kidney (Caki-1, Renca) cancers. MDA-MB-231 tumors were treated with saline (i.v.), 5 mg/kg bevacizumab (i.p.), 10 mg/kg BNC105P (i.v.) or a combination of BNC105P and bevacizumab. BNC105P was administered once per week and bevacizumab was administered twice per week. Renca tumors were treated with saline (i.v./p.o.), 2.5 mg/kg everolimus (p.o.), 16 mg/kg BNC105P (i.v.) or a combination of BNC105P and everolimus. BNC105P was administered on day 1 and 8 and everolimus was administered daily for 21 d. Tumor measurements were taken with digital calipers and tumor volume was calculated as volume (mm3) = length (mm) × width (mm) × height (mm). Animal health was monitored daily, animal weight and tumor measurements were taken 3 times per week. Animals were euthanized when tumor volume reached ethical limits.
Vascular disruption study
The vascular disruption assay was performed as previously described.Citation3 Briefly, female Balb/c nude mice bearing MDA-MB-231 xenografts were administered saline (i.v.), 5 mg/kg bevacizumab (i.p.), 10 mg/kg BNC105P (i.v.) or a combination of BNC105P and bevacizumab (n = 10 per treatment group). At daily intervals up to day 5 following treatment, animals were injected with 10 mg/kg (i.v.) of H33342 Hoechst fluorescent dye (Life Technologies), which acts as a marker of blood perfusion in tumors.Citation30 After 1 minute, animals were euthanized and tumors removed, snap frozen in Tissue-Tek® O.C.T™ (Sakura) and sectioned. Images were captured and assembled using ImageJ (NIH) and Motic Images Assembly 1.0 Pro (Motic China Group Co. Ltd.). H33342 staining was quantitated again using ImageJ software. Sections were then stained with Hematoxylin and Eosin (H&E) (Sigma-Aldrich) to enable the tumor area to be determined. The percent of tumor area occupied by vasculature was then determined.
Orthotopic RCC tumor model
Female Balb/c mice at 6 to 8 weeks old were injected orthotopically under the kidney capsule with 5×104 Renca cells in 10 μl PBS. Animal health and weight was monitored daily for the initial 5 d then every second day. After 11 days, 5 animals were euthanized to check for tumor establishment prior to commencing treatment. For the vascular disruption study mice were injected with a single (i.v.) dose of 32 mg/kg BNC105P or saline. Twenty 4 hours post-administration animals were injected with H33342 Hoechst Fluorescent dye. After 1 minute, animals were euthanized and tumors and lungs removed, snap frozen in Tissue-Tek® O.C.T™ (Sakura) and sectioned. Images were captured and assembled using ImageJ (NIH) and Motic Images Assembly 1.0 Pro (Motic China Group Co. Ltd.). Sections were then stained with H&E.
For the molecular characterization study, the mice were injected with a single dose (i.v.) of 32 mg/kg BNC105P or saline. Kidney bearing tumors were collected 24 hours post-administration, formalin fixed and paraffin embedded prior to immunohistochemistry (IHC).
For the overall survival studies, the animals were randomized into groups (n = 15) and the treatment schedule commenced on Day 12 post-inoculation (considered Day 1 of treatment). Animals were treated with saline (control) (i.v.), vehicle (control) (p.o.), 30 mg/kg pazopanib (p.o.) or 16 mg/kg BNC105P (i.v.) as monotherapies or in combination. Pazopanib or vehicle were dosed daily and BNC105P on Day 2 and 9 of a 21 day treatment cycle. The animals were monitored daily and euthanized at an ethical endpoint based on body condition and/or clinical signs.
Immunohistochemistry
Tumors from Caki-1 or MDA-MB-231 xenografts in Balb/c nude mice were collected in Tissue-Tek® O.C.T™ (Sakura) at specified time points after a single dose of BNC105P (i.v.). The tumors were sectioned and stained for VEGFA (Abcam #AB46154), phosphorylation of mTOR (Ser2448) (Cell Signaling #2976) or phosphorylation of 4E-BP-1 (Thr37/46) (Cell Signaling #2855). DAB detection was used followed by a counterstain with Hematoxylin.
Tumor bearing kidneys from Renca orthotopic inoculated animals were removed 24 hours post-BNC105P treatment, formalin fixed and paraffin embedded. Sections were dewaxed and stained for GLUT-1 (Acris #AP06144PU-N), HIF-1α (Santa Cruz #sc-10790), PERK (Cell Signaling #5683), eIF2α (Cell Signaling #5324), ph4E-BP-1 (Thr37/46) (Cell Signaling #2855) and VEGF (Abcam #AB46154). Fast-Red (Sigma-Aldrich) detection was used followed by a counterstain with Hematoxylin.
Microscopy
Using an Olympus BX51 microscope and cellSens Standard (Version 1.5) software, images were captured on an Olympus DP72 camera. Composite images were prepared using Microsoft Image Composite Editor (Version 1.4.4.0).
Statistical analysis
Results for tumor growth inhibition assays were analyzed for significance using a one-way ANOVA and post-hoc Tukey's Multiple Comparison Test (P < 0 .05). Outliers in the vascular disruption assay were removed using Grubbs’ Test. Survival assays were analyzed using a Kalpan Meyer plot and Log Rank test for significance. All analysis was performed using GraphPad Prism (Version 4). A synergistic relationship was calculated where FBNC105 and Feverolimus was the fractional response of tumor growth inhibition to BNC105 and everolimus, respectively. If the observed response (Robs) was greater than the additive response (Radd) = FBNC105 + Feverolimus – (FBNC105 * Feverolimus), the relationship was defined as synergistic.Citation31
Disclosure of Potential Conflicts of Interest
All authors are employees of and have ownership interests in Bionomics Ltd.
Funding
All work was supported by Bionomics Ltd.
References
- Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res 2004; 10:415-27; PMID:14760060; http://dx.doi.org/10.1158/1078-0432.CCR-0642-03
- Cai SX. Small molecule vascular disrupting agents: Potential new drugs for cancer treatment. Recent Pat Anticancer Drug Discov 2007; 2:79-101; PMID:18221055; http://dx.doi.org/10.2174/157489207779561462
- Kremmidiotis G, Leske AF, Lavranos TC, Beaumont D, Gasic J, Hall A, O'Callaghan M, Matthews CA, Flynn B. BNC105: a novel tubulin polymerization inhibitor that selectively disrupts tumor vasculature and displays single-agent antitumor efficacy. Mol Cancer Ther 2010; 9:1562-73; PMID:20515948; http://dx.doi.org/10.1158/1535-7163.MCT-09-0815
- Stevenson JP, Rosen M, Sun W, Gallagher M, Haller DG, Vaughn D, Giantonio B, Zimmer R, Petros WP, Stratford M, et al. Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol 2003; 21:4428-38; PMID:14645433; http://dx.doi.org/10.1200/JCO.2003.12.986
- Horsman MR, Siemann DW. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies Cancer Res 2006; 66:11520-39.; PMID:17178843; http://dx.doi.org/10.1158/0008-5472.CAN-06-2848
- Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer 2005; 5:423-35; PMID:15928673; http://dx.doi.org/10.1038/nrc1628
- Iversen AB1, Busk M, Horsman MR Induction of hypoxia by vascular disrupting agents and the significance for their combination with radiation therapy. Acta Oncol. 2013; 52:1320-6; PMID:23988183; http://dx.doi.org/10.3109/0284186X.2013.825050
- Yeung SC, She M, Yang H, Pan J, Sun L, Chaplin D. Combination chemotherapy including combretastatin A4 phosphate and paclitaxel is effective against anaplastic thyroid cancer in a nude mouse xenograft model.J Clin Endocrinol Metab 2007; 92:2902-9.; PMID:17550961; http://dx.doi.org/10.1210/jc.2007-0027
- Zweifel M, Jayson GC, Reed NS, Osborne R, Hassan B, Ledermann J, Shreeves G, Poupard L, Lu SP, Balkissoon J, et al. Phase II trial of combretastatin A4 phosphate, carboplatin, and paclitaxel in patients with platinum-resistant ovarian cancer. Ann Oncol 2011; 22:2036-41; PMID:21273348; http://dx.doi.org/10.1093/annonc/mdq708
- Lara PN Jr, Douillard JY, Nakagawa K, von Pawel J, McKeage MJ, Albert I, Losonczy G, Reck M, Heo DS, Fan X, et al. Phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent Vadimezan (ASA404) in advanced non–small-cell lung cancer. J Clin Oncol 2011; 29:2965-71; PMID:21709202; http://dx.doi.org/10.1200/JCO.2011.35.0660
- Siemann DW, Shi W. Dual targeting of tumor vasculature: Combining Avastin and vascular disrupting agents (CA4P or OXi4503). Anticancer res 2008; 28:2027-31; PMID:18751370
- Rischin D, Bibby DC, Chong G, Kremmidiotis G, Leske AF, Matthews CA, Wong SS, Rosen MA, Desai J. Clinical, pharmacodynamic, and pharmacokinetic evaluation of BNC105P: a phase I trial of a novel vascular disrupting agent and inhibitor of cancer cell proliferation. Clin Cancer res 2011; 17:5152-60; PMID:21690571; http://dx.doi.org/10.1158/1078-0432.CCR-11-0937
- Nowak AK, Brown C, Millward MJ, Creaney J, Byrne MJ, Hughes B, Kremmidiotis G, Bibby DC, Leske AF, Mitchell PL, et al. A phase II clinical trial of the vascular disrupting agent BNC105P as second line chemotherapy for advanced Malignant Pleural Mesothelioma. Lung Cancer 2013; 81:422-7; PMID:23787063; http://dx.doi.org/10.1016/j.lungcan.2013.05.006
- Drevs J, Hofmann I, Hugenschmidt H, Wittig C, Madjar H, Müller M, Wood J, Martiny-Baron G, Unger C, Marmé D. Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Res 2000;60:4819-24.; PMID:10987292
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356:115-24; PMID:17215529; http://dx.doi.org/10.1056/NEJMoa065044
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010; 28:1061-8; PMID:20100962; http://dx.doi.org/10.1200/JCO.2009.23.9764
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27:3584-90; PMID:19487381; http://dx.doi.org/10.1200/JCO.2008.20.1293
- Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369:722-31; PMID:23964934; http://dx.doi.org/10.1056/NEJMoa1303989
- Spear MA, LoRusso P, Mita A, Mita M. Vascular disrupting agents (VDA) in oncology: Advancing towards new therapeutic paradigms in the clinic. Curr Drug Targets 2011; 12:2009-15; PMID:21777190; http://dx.doi.org/10.2174/138945011798829366
- Siemann DW, Bibby MC, Dark GG, Dicker AP, Eskens FA, Horsman MR, Marmé D, Lorusso PM. Differentiation and definition of vascular-targeted therapies. Clin Cancer Res 2005; 11:416-20.; PMID:15701823
- Nathan P, Zweifel M, Padhani AR, Koh DM, Ng M, Collins DJ, Harris A, Carden C, Smythe J, Fisher N, et al. Phase I trial of Combretastatin A4 Phosphate (CA4P) in combination with Bevacizumab in patients with advanced cancer. Clin Cancer Res 2012; 18:3428-39; PMID:22645052; http://dx.doi.org/10.1158/1078-0432.CCR-11-3376
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011; 11:393-410.; PMID:21606941; http://dx.doi.org/10.1038/nrc3064
- Ke Q, Costa. Hypoxia-Inducible Factor-1 (HIF-1). Mol Pharmacol 2006; 70:1469-80; PMID:16887934; http://dx.doi.org/10.1124/mol.106.027029
- Shinojima T, Oya M, Takayanagi A, Mizuno R, Shimizu N, Murai M. Renal cancer cells lacking hypoxia inducible factor (HIF)-1alpha expression maintain vascular endothelial growth factor expression through HIF-2alpha. Carcinogenesis 2007; 28:529-36; PMID:16920734; http://dx.doi.org/10.1093/carcin/bgl143
- Lin Z, Weinberg JM, Malhotra R, Merritt SE, Holzman LB, Brosius FC 3rd. GLUT-1 reduces hypoxia-induced apoptosis and JNK pathway activation. J Physiol Endocrinol Metab 2000; 278:E958-66
- Kazemi S, Mounir Z, Baltzis D, Raven JF, Wang S, Krishnamoorthy JL, Pluquet O, Pelletier J, Koromilas AE. A novel function of eIF2alpha kinases as inducers of the phosphoinositide-3 kinase signaling pathway. Mol Biol Cell 2007; 18:3635-44; PMID:17596516; http://dx.doi.org/10.1091/mbc.E07-01-0053
- Ellis L, Shah, Hammers H, Lehet K, Sotomayer P, Azabdaftari G, Seshadri M, Pili R. Vascular disruption in combination with mTOR inhibition in renal cell carcinoma. Mol Cancer Ther 2012; 11:383-92; PMID:22084164; http://dx.doi.org/10.1158/1535-7163.MCT-11-0748
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, et al. RECORD-1 study group. Phase 3 trial of Everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer 2010; 116:4256-65; PMID:20549832; http://dx.doi.org/10.1002/cncr.25219
- Flynn BL, Gill GS, Grobelny DW, Chaplin JH, Paul D, Leske AF, Lavranos TC, Chalmers DK, Charman SA, Kostewicz E et al. Discovery of 7-hydroxy-6-methoxy-2-methyl-3-(3,4,5-trimethoxybenzoyl)benzo[b]furan (BNC105), a tubulin polymerization inhibitor with potent antiproliferative and tumor vascular disrupting properties. J Med Chem 2011; 54:6014-27; PMID:21774499; http://dx.doi.org/10.1021/jm200454y
- Trotter MJ, Olive PL, Chaplin DJ. Effect of vascular marker Hoechst 33342 on tumour perfusion and cardiovascular function in the mouse. Br J Cancer 1990; 62:903-8; PMID:2257217; http://dx.doi.org/10.1038/bjc.1990.406
- Selvendiran K, Ahmed S, Dayton A, Kuppusamy ML, Rivera BK, Kálai T, Hideg K, Kuppusamy P. HO-3867, a curcumin analog, sensitizes cisplatin-resistant ovarian carcinoma, leading to therapeutic synergy through STAT3 inhibition. Cancer Biol Ther 2011; 12:837-45; PMID:21885917; http://dx.doi.org/10.4161/cbt.12.9.17713
