Abstract
Blocking the migration of metastatic cancer cells is a major goal in the therapy of cancer. The receptor tyrosine kinase AXL is one of the main triggers for cancer cell migration in neoplasia of breast, colon, skin, thyroid and prostate. In our study we analyzed the effect of AXL inhibition on cell motility and viability in triple negative breast cancer cell lines overexpressing AXL. Thereby we reveal that the compound BMS777607, exhibiting the lowest IC50 values for inhibition of AXL kinase activity in the studied cell lines, attenuates cell motility to a lower extent than the kinase inhibitors MPCD84111 and SKI606. By analyzing the target kinases of MPCD84111 and SKI606 with kinase profiling assays we identified Lyn, a Src family kinase, as a target of both compounds. Knockdown of Lyn and the migration-related CRK-associated substrate (p130Cas), had a significant inhibitory effect on cell migration. Taken together, our findings highlight the importance of combinatorial or multikinase inhibition of non-receptor tyrosine kinases and AXL receptor tyrosine kinase in the therapy of triple negative breast cancer.
Abbreviations
| AKT | = | RAC-α serine/threonine-protein kinase |
| FAK | = | focal adhesion kinase |
| EGFR | = | epidermal growth factor receptor |
| ELISA | = | enzyme-linked immunosorbant assay |
| Gas6 | = | growth arrest specific 6 |
| MAPK | = | mitogen activated protein kinases |
| PI3K | = | phosphatidylinositol 3-kinase |
| Pyk2 | = | proline-rich tyrosine kinase 2 |
| RTK | = | receptor tyrosine kinase |
| siRNA | = | short interfering RNA |
| TKI | = | tyrosine kinase inhibitor |
| TNBC | = | triple negative breast cancer |
Introduction
The formation of metastasis resulting from migration of cells out of the primary tumor to invade distant sites in the organism is the number one reason for cancer patient mortality.Citation1-Citation3 Cell migration is complex and it is precisely regulated by multiple factors such cell-cell and cell-substrate contacts but also by soluble mediators, the binding of which to their receptors on the cell surface triggers a network of interconnected signaling pathways. There is a plethora of mediators and effectors that represent potential targets for inhibitors with the capacity to influence cell migration.Citation4 The receptor tyrosine kinase (RTK) AXL (also known as UFO) is one of these targets and known to play an important role in cancer progression, invasion, metastasis, drug resistance and is correlated to patient mortality.Citation5,6 AXL belongs to the TAM (Tyro3, AXL, Mer) RTK family,Citation7 with all 3 members of the family being highly homologous in their extracellular and catalytic domains.Citation6 The activation of AXL occurs when the growth arrest specific 6 (Gas6) ligand binds to its extracellular domain,Citation8 which results in the activation of downstream signaling pathways such as mitogen activated protein kinases (MAPK), phosphatidylinositol 3-kinase (PI3K) RAC-α serine/threonine-protein kinase (AKT) and NF-КB (Nuclear Factor Kappa B).Citation9,10 AXL is frequently overexpressed in human cancers including lymphocytic leukemia, breast, colon, skin, thyroid and prostate cancer.Citation11-Citation17 Down-regulation of AXL by siRNA attenuates the migration and invasion of breast,Citation18 ovarian,Citation19 hepatocellular carcinoma,Citation20 mesothelioma,Citation21 prostateCitation22 and pancreatic cancer.Citation23 Furthermore it has been shown that the inhibition of AXL results in the reduction of cancer progression and metastatic potential and induces apoptosis in cancer cells.Citation19,24
These observations suggest that AXL is an excellent target to counteract cancer and recently several AXL inhibitors have been described.Citation25
The aim of our study was to elucidate the impact of 3 tyrosine kinase inhibitors (TKI), namely SKI606, BMS777607 and MPCD84111 on Axl phosphorylation and the migration as well as viability of triple negative breast cancer (TNBC) cell lines.
SKI-606 (bosutinib) was described as a dual Src/Abl inhibitor exhibiting a significant effect on proliferation of colon cancer and chronic myelogenous leukemia.Citation26 It was also demonstrated that SKI606 inhibits AXL at 0.58 μmol/L concentrations and blocks migration and invasion of breast cancer cell lines.Citation12
BMS-777607 was described as a selective MET, RON (also known as MST1R) inhibitor but also shown to target AXL and Tyro3 kinase at low nanomolar concentrations.Citation27 BMS777607 inhibits AXL at 1.1 nM in a biochemical enzyme assay.Citation28
MPCD84111 is a quinolinyloxyphenylsulfonamides tyrosine kinase inhibitor (TKI) patented as an AXL inhibitor by the Max Planck Society under patent application example 12 from WO2011045084.
Our results prove that inhibition of migration by AXL inhibitors is independent form AXL kinase phosphorylation. Additionally, we highlight the importance of multikinase inhibition in the therapy of TNBC.
Results
BMS777607, SKI606 and MPCD84111 exhibit different potential on AXL RTK inhibition
Overexpression of the RTK AXL and its implication in the migration of TNBC cell lines has already been demonstrated in publications and also by our studies (Fig. S2 and S3).Citation12,18,29 To determine the impact of 2 potential (BMS777607 and SKI606) and one patented (MPCD84111) AXL TKI on AXL phosphorylation we used 3 AXL-expressing TNBC cell lines.Citation12,30,31
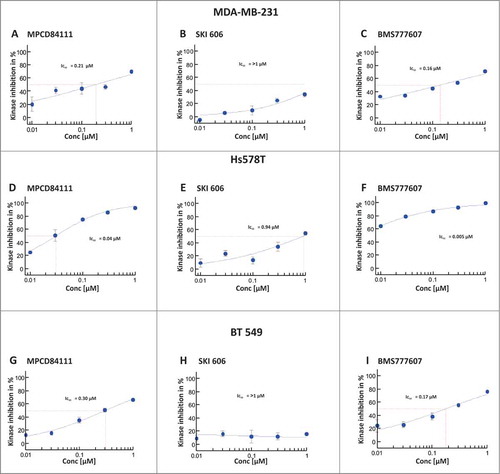
The IC50 values of AXL phosphorylation, were measured after 1 h of inhibitor treatment and subsequent Gas6 (250 ng/ml) stimulation. MPCD84111 displayed IC50 values of 0.21 μM in MDA-MB-231, 0.30 μM in BT549 and 0.04 μM in Hs578T cells (; Fig. S1A-C). SKI606 was less efficient in comparison to MPCD84111 and BMS777607 having an IC50 value of >1 μM in MDA-MB-231 and BT549 and 0.94 μM in Hs578T cells (; Fig. S1A-C). BMS777607 inhibited the phosphorylation of AXL with IC50 values of 0.16 μM in MDA-MB-231, of 0.17 μM in BT549 cells and of 0.005 μM in Hs578T cells (; Fig. S1A-C). In conclusion, the strongest inhibition of AXL phosphorylation was achieved by BMS777607 followed by MPCD84111 and SKI606.
Figure 1. Effect of inhibitors on AXL phosphorylation in TNBC cell lines. Serum starved MDA-MB-231 (top), Hs578T (middle) and BT549 (bottom) cells were treated with the correspondent inhibitor concentration. After 1 h incubation with the inhibitor the cells were stimulated with 250 ng/ml Gas6 for 30 min. Phosphorylation of AXL RTK was measured by pY-AXL-ELISA. The IC50 values were determined as percentage of the untreated DMSO control. The results shown are means and standard deviation calculated from 3 independent experiments. The strongest inhibitory effect on AXL phosphorylation was achieved by BMS777607 followed by MPCD84111 and SKI606.
The most potent AXL inhibitor BMS777607 does not inhibits significantly the migration and viability of TNBC cells
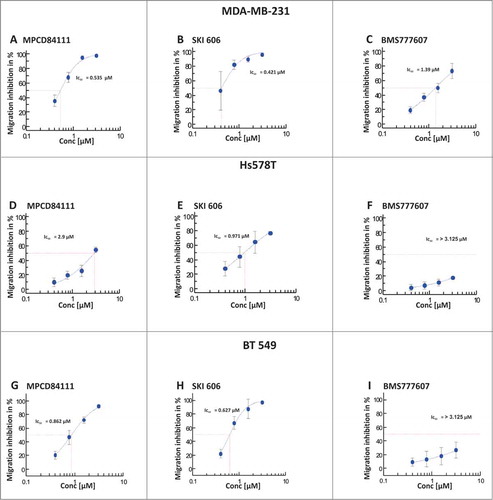
We determined the inhibitory effect of BMS777607, MPCD84111 and SKI606 on cell migration using Boyden chamber assays. The IC50 value for MPCD84111 was 0.535 μM in MDA-MB-231 (), 2.9 μM in Hs578T () and 0.862 μM in BT549 () cells. SKI606 inhibited the migration with an IC50 value of 0.421 μM in MDA-MB-231 () of 0.971 μM in Hs578T () and 0.627 μM in BT549 () cells. In contrast to SKI606 and MPCD84111, BMS777607 had an IC50 value of 1.39 μM in MDA-MB-231 (), >3.125 μM in Hs578T () and >3.125 μM in BT549 () cells. Comparing the IC50 values we found that Hs578T cell were less sensitive to TKI treatment than MDA-MB-231 cells. Among the 3 analyzed AXL TKIs SKI606 had the lowest IC50 value for inhibition of migration followed by MPCD84111 and BMS777607.
Figure 2. Effect of AXL TKIs on the migration of TNBC cell lines. The impact of MPCD84111, SKI606 and BMS777607 on cell motility was analyzed with Boyden chamber assay. The cells were allowed to migrate toward to the 1% FCS containing lower chamber for 3.5 h. MDA-MB-231 (top) and BT549 (bottom) were more sensitive to TKI treatment than Hs578T cells (middle). Among the 3 analyzed AXL TKIs SKI606 had the lowest IC50 value for migration inhibition. The IC50 values were determined as percentage of the untreated DMSO control. The results shown are means and standard deviation calculated from 3 independent experiments.
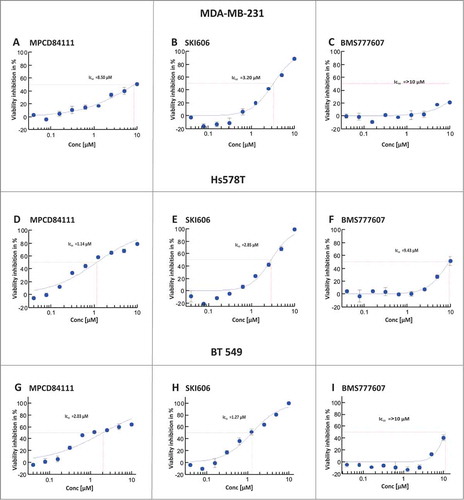
Additionally we analyzed the effect of AXL TKIs on cell viability. As illustrated in , SKI606 and MPCD84111 had a significant inhibitory effect on Hs578T and BT549 cell viability. MPCD84111 exhibited an IC50 value of 1.14 μM in Hs578T and 2.03 μM in BT549 (), SKI606 showed an IC50 value of 2.85 μM in Hs578T and 1.27 μM in BT549 cells () and BMS777607 had an IC50 value of 9.43 μM in Hs578T cells and >10 μM in BT549 cells (). The MDA-MB-231 cell line was less sensitive to TKI treatment than Hs578T, with IC50 values of 8.50 μM for MPCD84111 and 3.20 μM for SKI606, more than 10 μM for BMS777607 ().
Figure 3. Effect of AXL TKIs on the viability of TNBC cells. The cell viability of the inhibitor treated MDA-MB-231, Hs578T and BT549 was determined after 72 h. The IC50 values were calculated as a percentage of the untreated DMSO control. SKI606 and MPCD84111 show an inhibitory effect on Hs578T (middle) and BT549 (bottom) viability. The MDA-MB-231 cell line (top) was less sensitive to TKI treatment showing efficient reduction of viability only after treatment with SKI606. The results shown are means and standard deviation taken from 3 independent experiments.
The inhibitory concentrations for migration and viability were higher than the IC50 value for inhibition of the AXL kinase activity, suggesting that the effect on migration and viability is not mediated through AXL RTK.
SKI606 and MPCD84111 display similarities in their target profile
Next, we wanted to understand why SKI606 and MPCD-84111 stronger block the migration of the TNBC cell lines than BMS777607. Therefore we performed a Cellular Target Profiling assay in Hs578T cell lysates to determine the targets of the compounds. The target spectrum of BMS777607 was reported by Schroeder et al.,Citation28 therefore we focused on the characterization of SKI606 and MPCD84111.
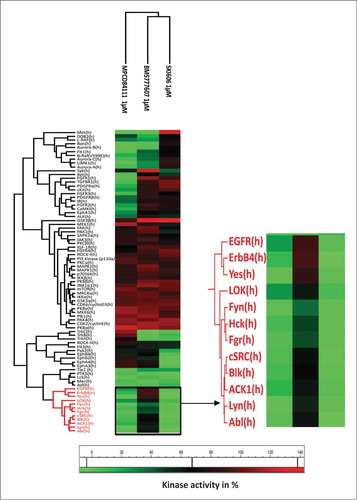
The Cellular-Target-Profiling assay revealed that SKI606 mainly binds to the SRC-family kinases (Lyn, Src, Fyn and Yes), MAPK-family members, Ephrins and some of the PKC-family members. MPCD84111 displays a strong affinity for AXL, MET and Aurora B kinase and similarly to SKI606 binds to Lyn, Yes and Src (). Between the 2 analyzed inhibitor SKI606 has with 40% more target than MPCD84111. This result is not surprising in the context that SKI606 potentially inhibits 148 kinases with a Kd-value below 1 μM.Citation32 After comparing these data to the target profile of BMS777607,Citation28 we concluded that BMS777607 and MPCD84111 possess common targets such as Met and Aurora B. Binding to AXL kinase was confirmed for all 3 TKIs.
Table 1. Target profiles of SKI606 and MPCD84111. Tables show the Kd values of the proteins targeted by SKI606 (A) and MPCD84111 (B) in Hs578T cell lysates
On the basis of Cellular Target Profiling assay and Ambit phage based competition binding assay data, 84 kinases were selected to further examine the compounds inhibitory potential in Kinase Profiler assay by Millipore, at 1 μM concentration. As expected from the results of the Cellular target profiling assay results, the Src family kinases and few other kinases (Abl, PTK5, EGFR, ErbB4, Lok, Tie2) had less than 50% activity after treatment with SKI606 and MPCD84111, whereas BMS777607 and MPCD84111 generally affected the Met-related kinases (Met, Ron) and the Aurora kinases (Aurora A/B/C) (). In conclusion, the profiling assays revealed that SKI606 and MPCD84111 commonly affect migration related kinases such as Src-family kinases, which might explain their significant effect on cell migration.
Lyn and p130Cas affect the migration of triple negative breast cancer cells
Among the Src-family kinases targeted by SKI606 and MPCD84111, Lyn is the only one being significantly overexpressed in TNBC cell lines.Citation33 Based on these result we selected Lyn to analyze its function on the migration and viability of MDA-MB-231, Hs578T and BT549 cells. It is known that Lyn interactsCitation34 with one of the members of the focal adhesion complex, namely p130Cas.Citation35 Therefore we analyzed p130Cas function in the migration and viability of MDA-MB-231, Hs578T and BT549 cells. To evaluate a functional connection between the selected proteins and cell migration we used a siRNA knockdown. The siRNA knockdown efficiency was validated by Western blot analysis ().
Figure 5. Effects of siRNA-mediated knockdown of Lyn and p130Cas on migration and viability of human TNBC cell lines. The knockdown of Lyn and p130Cas significantly reduced migration of MDA-MB-231, Hs578T and BT549 cells as determined by Boyden chamber assay (A). In MDA-MB-231 and Hs578T cell lines, knockdown of Lyn had no significant effect on the level of viability, while in BT549 cells all siRNA treatments had significant effects on the viability levels as determined by CellTiter-Glo assay (B). Knockdown of Lyn and p130Cas was confirmed by Western blot analysis (C). Tubulin represents the loading control. The results shown are means and standard deviation taken from 3 independent experiments.*, P ˂ 0.0001, Dunnett's Multiple Comparison Test.
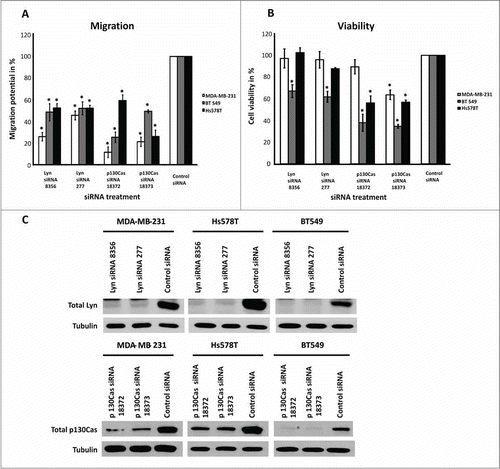
The knockdown of Lyn and p130Cas led to a significant decrease in the migration of all cell lines (). In MDA-MB-231 and Hs578T cells, knockdown of Lyn had no significant impact on the cell viability while the knockdown of p130Cas showed a clear effect (). In BT549 cells all siRNA treatments had a significant effect on cell viability ().
Effect of AXL receptor tyrosine kinase inhibitors on intracellular migration related pathways in triple negative breast cancer cells
In the next step we characterized the effect of AXL TKIs on migration related signaling pathways. Serum starved cells were treated with 1 μM of SKI606, BMS777607 or MPCD84111 for 1.5 or 48 h in serum free medium.
As illustrated in , MPCD84111 caused an inhibition of phosphorylation of Lyn, Pyk2, FAK, p130Cas in all cell lines. There was no significant impact on phosphorylation of c-Src, showing that the inhibition of the FAK-p130Cas-complex is not dependent on phospho-c-Src in these cell lines. SKI606 had an inhibitory effect on the phosphorylation of Pyk2, FAK, p130Cas and Lyn comparable to that of MPCD84111. In contrast to MPCD84111, SKI606 inhibited c-Src Tyr 416. There were no significant changes in the phosphorylation of Lyn after 1.5 h of treatment with MPCD84111 and SKI606 in Hs578T and BT549 cells. BMS777607 led to a less significant inhibition of Lyn, Pyk2, FAK and p130Cas phosphorylation in all cell lines. At the same time BMS777607 was proven to inhibit c-Src in Hs578T cells after 1.5 h of treatment but finally the phosphorylation of c-Src recovered after 48 h ().
Figure 6. Effect of AXL RTK inhibitors on intracellular signaling pathways. Immunoblots of lysates of MDA-MB-231 (A), Hs578T (B) and BT549 (C) starved cells treated with 1 μM of MPCD84111, SKI606 or BMS777607 for 1.5 or 48 h using antibodies against phospho-Pyk2 (pY580), phospho-FAK (pY576/577), phospho-p130Cas (pY410), phospho-Lyn (pY507), phospho-Src (pY416) and Tubulin (represents the loading control).
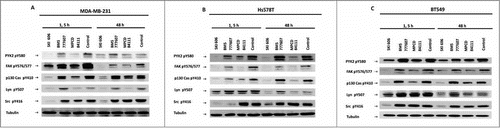
These results are consistent with our findings that SKI606 and MPCD84111 inhibit cell migration due to inhibition of essential migration related kinases such as p130Cas or Lyn.
Discussion
Triple negative breast cancer, which accounts up to 10% to 27% of breast cancer cases, is characterized by the absence of HER2, estrogen and progesterone receptor and by elevated phosphorylation of AXL, FAK, Met, Lyn, p130Cas and EGFR.Citation29,36,37
In this study we analyzed the efficacy of 3 AXL TKIs BMS777607, MPCD84111 and SKI606 exhibiting different selectivity profilesCitation28,32 on the migration and viability of AXL overexpressing TNBC cell lines,Citation30,31,38 namely MDA-MB-231, Hs578T and BT549.
Several studies demonstrated that the inhibition of AXL expression by AXL specific siRNAs attenuates the migration of TNBC cell lines.Citation12,18,39 Although these studies established the role of AXL protein in promoting TNBC cell migration, the phosphorylation status of AXL in breast cancer cell migration has not been clarified. In this study we elucidate the function of the AXL phosphorylation on the migration of TNBC cells. Therefore we used a selective AXL inhibitor BMS777607 and 2 AXL targeting multikinase inhibitor, SKI606 and MPCD84111 in our experiments. We show that in contrast to SKI606 and MPCD84111, the most specific and efficient AXL inhibitor BMS777607 was not capable to block the migration of TNBC cells. The inhibition values of migration were significantly higher than those determined for inhibition of AXL phosphorylation, suggesting that migration of the 3 studied TNBC cell lines does not depend on AXL phosporylation. Similar to our study Tan et al. analyzed the inhibitory potential of SKI606 on the migration of non small lung cancer cell lines. They showed that the inhibitory effect of SKI606 on migration was independent from AXL RTK.Citation40 This study is in line with our conclusion that efficiency of SKI606 and MPCD84111 is not correlated with the AXL phosphorylation inhibition. In contrast to our conclusion Zhang et al. correlated SKI606 migration inhibitory potential on TNBC cell with the compound effect on AXL phosphorlyation, which is contradictory because the IC50 values for the inhibition of migration were much higher than the values for the inhibition of AXL phosphorylation.Citation12 Similar discrepancy between AXL and invasion inhibition by the AXL inhibitor R428 can be observed in the study of Holland et al., were the IC50 value for invasion was 3000 nM whereas the IC50 value for AXL phosphorylation inhibition was more than 200 times lower exhibiting a value of 14 nM.Citation41
Based on our results and on the publication of Vouriluoto et al. where it was shown that AXL kinase protein with gate keeper mutation has no effect on the migration of MDA-MB-231 cells,Citation39 we conclude that AXL might have a kinase independent function.
We can not exclude that AXL protein itself might play an important role as adhesion or scaffolding protein in cell migration as implicated by other publications by specific AXL siRNA knockdown experiments.Citation12,18 As a scaffolding protein AXL could have influence on multiple non-RTKs, serving as docking sites for as Lyn, Src, PI3K (p85), ZAP70, Syk, c-SRC, Lck, GRB2 and PLCγ.Citation11,42 Antibodies against AXL and sAXL might interfere with the protein function of AXL shown by several publications.Citation19,43 However association and transactivation of AXL with EGFR and c-Met would limit the effects of targeted therapies.Citation44,45
To comprehend the efficiency of SKI606 and MPCD84111 on the migration of TNBC cells we examined the targets of SKI606 and MPCD84111. Based on our experiments we concluded that SKI606 has 40% more cellular targets than MPCD84111 but still both compounds commonly affect the migration related Src family kinases ( and ). This could explain their significant inhibitory effect on the migration of TNBC cell lines. Among the Src family kinases targeted by SKI606 and MPCD84111 (Yes, Fyn, Hck, Fgr, c-Src, Blk, Lyn), we analyzed the role of Lyn. Lyn has been described as an important target in haematopoietic and solid tumors including TNBC, and was proven to be one of the most overexpressed kinase in TNBC cell lines.Citation29,33 Lyn plays an important role in the migration of cancer cells and was shown that can interact with p130Cas, one of the member of the focal adhesion complex.Citation34 Association of p130Cas with motility, invasion and survival of TNBC cells has been shown before by several publications.Citation29,46,47 Therefore additionally we analyzed the function of p130Cas in the migration and viability of TNBC cells. We proved that the siRNA-mediated knockdown of Lyn and p130Cas attenuated the migration of all 3 TNBC cell lines. Consequently our results underline the importance of Lyn and p130Cas in the migration of TNBC cell lines. At the same time the AXL TKIs SKI606 and MPCD84111 inhibit the phosphorylation of Pyk2, FAK, p130Cas and Lyn all 3 TNBC cell lines, while BMS777607 had no effect on these signaling molecules. This might explain why BMS777607, being the most efficient AXL TKI published until now, does not inhibit migration of TNBC cells. Despite other reports we show that Src inhibition attenuates breast cancer cell migration,Citation48,49 our study proves that the strong inhibitory effect on migration exerted by MPCD84111 does not depend on the inhibition of c-Src phosphorylation. Thus, the migration of TNBC cell lines described here does not depend on the activity of c-Src kinase but on other tyrosine kinases such as p130Cas and Lyn.
We also demonstrate that viability inhibition is not correlated with the migration attenuation, as the growth inhibitory concentrations of the investigated inhibitors (MPCD84111, BMS777607 and SKI606) were significantly higher than the IC50 of migration inhibition. Thus we exclude viability inhibition being responsible for the reduction of cell migration in TNBC cells by the analyzed AXL inhibitors.
The present study reveals that efficacy of SKI606 and MPCD84111 to block TNBC cell migration is independent from AXL kinase phosphorylation. Although we cannot exclude AXL important role in modulation of tumor associated vasculature and immune cell functionCitation43 and modulation of natural killer cells by the TAM receptors in vivo.Citation50 Our data show that SKI606 and MPCD84111 inhibit breast cancer cell migration through migration related kinases, namely Lyn and p130Cas. However tumor cell migration can not be blocked completely by inhibition of Lyn or p130Cas (). Our results underline previous findings that TNBC cell lines are not addicted to a single signaling pathway.Citation29 Based on these findings we conclude that the use of multikinase inhibitors such as SKI606 and MPCD84111 which target migration related kinases might improve the treatment of TNBC.
Material and Methods
Cell lines
Experiments were performed on 3 different triple negative basal breast cancer cell lines. MDA-MB-231 cells were obtained from Stefano Iacobelli (Department of Oncology and Experimental Medicine, University Foundation ‘G. D’Annunzio’ Chieti-Pescara, Chieti, Italy) Hs578T cells were a kind gift from U3 Pharma (Martinsried, Germany) and BT549 cells were obtained from the American Type Culture Collection. MDA-MB-231 cells were grown at 37°C in Dulbecco's modified Eagle's medium (GIBCO, Invitrogen, Cat no: 41965-039) supplemented with 1% sodium pyruvate (GE Healthcare, Cat no: 11360-039.), Hs578T and BT549 cells in RPMI 1640 medium (GIBCO, Invitrogen, Cat no: 31870-025), supplemented with 1% glutamine (GIBCO, Invitrogen Cat. no. 25030-081) and the medium of the BT549 cells with 0.001% human insulin (GE Heathcare). All media were supplemented with 10% fetal calf serum (FCS, GIBCO, Cat. no. 16000-044), 100 U/ml penicillin, and 100 μg/ml streptomycin (PAA, Cat. no: P11-010). All cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Short interfering RNA transfections
AXL, Lyn and BCAR1 short interfering RNAs were purchased from Ambion (Invitrogen). The sequence for AXL 1845 siRNA was sense: 5′GGAACUGCAUGCUGAAUGAtt3′; antisense: 5′UCAUUCAGCAUGCAGUUCCtg3′, for AXL 1847 siRNA was sense: 5′CAGCGAGAUUUAUGACUAUtt3′; antisense: 5′AUAGUCAUAAAUCUCGCUGtt3′ for BCAR1 18372 siRNA was sense: 5′GGAUGGAGGACUAUGACUAtt3′; antisense: 5′UAGUCAUAGUCCUCCAUCCag3′, for BCAR1 18373 siRNA was sense: 5′GAGUUUGAGAAGACCCAGAtt3′; antisense: 5′UCUGGGUCUUCUCAAACUCct3′, for Lyn 8356 siRNA was sense: 5′CUAGAGUAAUUGAAGAUAAtt3′; antisense: 5′UUAUCUUCAAUUACUCUAGca 3′, for Lyn 277 siRNA was sense: 5′GGAACAAGGAGACAUUGUGtt3′; antisense: 5′CACAAUGUCUCCUUGUUCCtc3′. Ambion control # 1 siRNA was used as a control. Briefly 120,000 cells were plated in 6 well plates (Corning) and after 24 h transfected with a final concentration of 40 nmol siRNA for 48 h. The Lipofectamine RNAiMAX was obtained from Invitrogen (Cat. no. 13778-075) and used according to the manufacturer's instruction.
Tyrosine kinase inhibitors
SKI606 and MPCD84111 were obtained from Vichem Chemie (Budapest, Hungary). MPCD84111 is patented under application example 12 from WO2011045084. BMS777607 was a kind gift from LDC Discovery Center GmbH (Dortmund, Germany). All inhibitors were dissolved in DMSO and stored at room temperature in 10 mM stock solution.
Cellular profiling assay
The assay was performed by Kinaxo on s578T cell lysates. The assay combines different technology platforms, such as the stable isotope labeling by amino acids (SILAC) in cell culture or isobaric tags for relative and absolute quatification (ITRAQ). These technologies are used together with affinity-based separation methods with data showing the binding affinities of the compound to the corresponding targets. The assay is described in detail in the publication of Contradt et al., published in 2011.Citation51
Kinase profiler assay
The assay was performed according to the manufacturer instruction by Millipore on 84 kinases with 1 μM compound concentration.
Cell viability and migration assay
We measured cell viability using a luciferase-coupled ATP quantitation assay (CellTiter-Glo; Promega, Cat no: G7571). Cells were dispensed at 1000 cells/100 μl/well in tissue-culture treated 96-well white bottom assay plates (PerkinElmer). After 24 h incubation the cells were treated with the indicated inhibitor concentration from the inhibitor dilution, except the siRNA treated cells. After inhibitor addition, plates were incubated for 72 h at 7°C in humidified atmosphere containing 5% CO2.
At the end of the incubation period, CellTiter-Glo reagent was added, to the contents of the plates, mixed by an orbital shaker for 2 min and incubated for 15 min. The luminescent signal was recorded using a Microplate Luminometer LB96V (Berthold Technologies). The experiments were performed at least 3 times.
The motility of the cells was determined by Boyden chamber assay. 50,000 cells were plated on top of a membrane with 8 μm pores (BD Transduction Laboratories, Heidelberg, Germany) in 300 μl of medium with 0.1% of FCS. As chemoattractant 1% FCS in 700 μl of medium was used. After incubation for 3.5 h at 37°C the cells remaining in the insert were removed with a cotton swab and the cells on the bottom of the filter were fixed with 0.05% Crystal violet/20% methanol (Sigma, Cat no: 32213-2). The cells which had traversed the membrane were counted and images were taken with 10 x objectives under brightfield illumination using a charge-coupled device camera- mounted on a Axiovert 300 Microscope (Zeiss) and analyzed with MetaVue software. At least 5 random fields were counted per well.
Western blot analysis
Starved cells were incubated with 1 μM of indicated inhibitor concentration for 1.5 or 48 h 100 micrograms of total protein lysate were denatured with sample buffer containing SDS and 2-mercaptoethanol at 95°C for 5 min and electrophoretically separated on a 4% to 12% polyacrylamide gel, transferred to a nitrocellulose membrane, and blocked with NET-gelatine. Antibodies raised against p-Lyn (Tyr507) # 2731, Lyn # 2732, p-Src (Tyr 416) #2101, p-Pyk (Tyr 580) #3291, p FAK (Tyr576/577) # 3281, p p130Cas (Tyr 410) #4011, p130 Cas # 13846 were obtained from Cell Signaling Technologies. Anti AXL antibody # sc-1096 was purchased from Santa Cruz. Anti- tubulin #T1926 was used from Sigma- Aldrich. The antibodies were used at 1:2,000 dilutions with overnight incubation at 4°C in NET-gelatine. The secondary antibodies HRP-coupled goat anti-rabbit (Bio-Rad) and goat anti-mouse (Sigma) were used at 1:10,000 dilutions in NET-gelatine. Detection of bound antibodies was performed by using an ECL substrate reaction (Perkin Elmer, Cat no: NEL103001EA) and exposed to Kodak X-Omat (Amersham Biosciences) film.
Immunoprecipitation
The homemade anti AXL capture antibody was pre-coupled to 40 μl A-Sepharose beads (GE Healthcare) in lysis buffer for 1 hour. After incubation were washed 3 times with lysis buffer. Cell lysates and pre-coupled antibody-beads were incubated at 4°C for 16 hours. The precipitates were washed 3 times with 1 ml lysis buffer, suspended in Laemmli buffer and boiled for 10 minutes. The AXL protein phosphorylation was analyzed by western blot using the homemade anti-p-Tyr clone 4G10 antibody. The total level of AXL was detected by using the anti-AXL # 4566 (Cell Signaling) and the anti-AXL sc-1096 (Santa Cruz) antibodies.
ELISA (enzyme-linked immunosorbant assay)
AXL phosphorylation was determined using phosphotyrosine AXL enzyme-linked immunosorbant assay (pY-AXL-ELISA). 75,000 cells were seeded onto 6-well plates after 24 h incubation were starved for 24 h and treated with serial diluted inhibitor concentrations for 1 h. After 1 h of the incubation with the inhibitors the cells were stimulated with 250 ng/ml Gas6 (R&D Systems) for 30 min. Cells were lysed on ice in 400 μl lysis buffer (50 mM HEPES (pH 7.5), 150 mM NaCl, 1 mM EGTA, 10% Glycerol (Roth, Cat. no: 4043.2), 1% Triton X-100 (Roth, Cat. no. 668), 100 mM NaF, 10 mM Na4P2O7·10 H2O, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and 10 mg/ml aprotinin) for 15 min.
96-well Nunc MicroWell™ plates (Fischer Scientific GmbH) which had been coated overnight with homemade anti-AXL capture antibody 2 μg/ml (clone 259/2, IgG1 isotype) in PBS (100 μl/well) were blocked with PBS-0.05% Tween®20 (Sigma, P1379-1) + 10% FCS for 4 h at 37°C. Plates were washed 5 times with PBS/0.05% Tween®20 and 95 μl of lysate was transferred per well for overnight incubation at 4°C. Plates were washed 5 times with PBS/0.05% Tween®20 (Sigma) For detection of phosphorylated tyrosine we used homemade biotynilated 4G10-antibody (0.5 μg/ml) in PBS/0.05%Tween®20/10% FCS (100 μl/well) and incubated the 96-well plate for 2 h at room temperature. The anti-phosphotyrosine mouse monoclonal antibody 4G10 was biotynilated with Sulfo-NHS®-Biotin according to the suppliers protocol (Pierce) and purified by Mirco Bio-Spin 6 Chromatography Columns (BIO RAD Laboratories) using PBS as dilutent. Plates were washed 5 times with PBS/0.05% Tween®20. For binding to biotin alkaline phosphatase-conjugated strepavidin SA110 (Millipore) (1:4,000) was used in PBS/0.05% Tween 20 + 10% FCS (100 μl/well) and incubated for 30 min at room temperature. Plates were washed 5 times with PBS-0.05% Tween®20. For fluorimetric detection of alkaline phosphatase AttoPhos Substrate Set (Roche Diagnostics GmbH) was used (100 μl/well). The fluorimetric signal was quantified after 90 min at 430/560 nm wavelength using a TECAN Ultra Evolution plate reader (Tecan Deutschland GmbH).
Statistical and data analysis
Assays were performed as biological triplicates, comparing inhibitor treated cell to DMSO control. IC50 values were determined from dose response curve generated by XLfit5.1.0 software (IDBS). For statistical analysis Dunnett's Multiple Comparison Test was performed using GraphPad Prism 5 (GraphPad Software, Inc..). At values p ˂ 0.0001 differences were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
956634_Supplementary_Materials.zip
Download Zip (3.3 MB)Acknowledgments
We thank Stefano Iacobelli (Department of Oncology and Experimental Medicine, Chieti, Italy) for providing the MDA-MB-231 cell line, U3 Pharma for the Hs578T cell line and LDC Discovery Center GmbH (Dortmund, Germany) for BMS777607. We also thank Vijay Kumar Ulaganathan and Pál Gyulavári for technical support and Gregor Kirfel (University of Bonn, Germany) for critical reading of the manuscript.
Funding
This work was supported by Max Planck Society and partly by KMR 12-1-2012-0074 of NFÜ/Hungary.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Suyama E, Kawasaki H, Wadhwa R, Taira K. Cell migration and metastasis as targets of small RNA-based molecular genetic analyses. J Muscle Res Cell Motil 2004; 25:303-8; PMID:15548858; http://dx.doi.org/10.1007/s10974-004-4343-7
- Weigelt B, Peterse JL, van ‘t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer 2005; 5:591-602; PMID:16056258; http://dx.doi.org/10.1038/nrc1670
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer 2006; 6:449-58; PMID:16723991; http://dx.doi.org/10.1038/nrc1886
- Stafford LJ, Vaidya KS, Welch DR. Metastasis suppressors genes in cancer. Int J Biochem Cell Biol 2008; 40:874-91; PMID:18280770; http://dx.doi.org/10.1016/j.biocel.2007.12.016
- Holland SJ, Powell MJ, Franci C, Chan EW, Friera AM, Atchison RE, McLaughlin J, Swift SE, Pali ES, Yam G, et al. Multiple roles for the receptor tyrosine kinase axl in tumor formation. Cancer Res 2005; 65:9294-303; PMID:16230391; http://dx.doi.org/10.1158/0008-5472.CAN-05-0993
- Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res 2008; 100:35-83; PMID:18620092; http://dx.doi.org/10.1016/S0065-230X(08)00002-X
- Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron 1991; 6:691-704; PMID:2025425; http://dx.doi.org/10.1016/0896-6273(91)90167-X
- Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev 2006:295-304; http://dx.doi.org/10.1016/j.cytogfr.2006.04.004
- Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol 1997; 17:4442-53; PMID:9234702
- Shankar SL, O’Guin K, Kim M, Varnum B, Lemke G, Brosnan CF, Shafit-Zagardo B. Gas6Axl signaling activates the phosphatidylinositol 3-kinaseAkt1 survival pathway to protect oligodendrocytes from tumor necrosis factor alpha-induced apoptosis. J Neurosci 2006; 26:5638-48; PMID:16723520; http://dx.doi.org/10.1523/JNEUROSCI.5063-05.2006
- Ghosh AK, Secreto C, Boysen J, Sassoon T, Shanafelt TD, Mukhopadhyay D, Kay NE. The novel receptor tyrosine kinase Axl is constitutively active in B-cell chronic lymphocytic leukemia and acts as a docking site of nonreceptor kinases: implications for therapy. Blood 2011; 117:1928-37; PMID:21135257; http://dx.doi.org/10.1182/blood-2010-09-305649
- Zhang YX, Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L, Szabadkai I, Daub H, Keri G, Ullrich A. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res 2008; 68:1905-15; PMID:18339872; http://dx.doi.org/10.1158/0008-5472.CAN-07-2661
- Gjerdrum C, Tiron C, Hoiby T, Stefansson I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A 2010; 107:1124-9; PMID:20080645; http://dx.doi.org/10.1073/pnas.0909333107
- Craven RJ, Xu LH, Weiner TM, Fridell YW, Dent GA, Srivastava S, Varnum B, Liu ET, Cance WG. Receptor tyrosine kinases expressed in metastatic colon cancer. Int J Cancer 1995; 60:791-7; PMID:7896447; http://dx.doi.org/10.1002/ijc.2910600611
- Tworkoski K, Singhal G, Szpakowski S, Zito CI, Bacchiocchi A, Muthusamy V, Bosenberg M, Krauthammer M, Halaban R, Stern DF. Phosphoproteomic screen identifies potential therapeutic targets in melanoma. Mol Cancer Res 2011; 9:801-12; PMID:21521745; http://dx.doi.org/10.1158/1541-7786.MCR-10-0512
- Avilla E, Guarino V, Visciano C, Liotti F, Svelto M, Krishnamoorthy G, Franco R, Melillo RM. Activation of TYRO3AXL tyrosine kinase receptors in thyroid cancer. Cancer Res 2011; 71:1792-804; PMID:21343401; http://dx.doi.org/10.1158/0008-5472.CAN-10-2186
- Paccez JD, Vasques GJ, Correa RG, Vasconcellos JF, Duncan K, Gu X, Bhasin M, Libermann TA, Zerbini LF. The receptor tyrosine kinase Axl is an essential regulator of prostate cancer proliferation and tumor growth and represents a new therapeutic target. Oncogene 2012; PMID:22410775
- Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J, Kallop D, Ludlam MJ, Pei L. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene 2009; 28:3442-55; PMID:19633687; http://dx.doi.org/10.1038/onc.2009.212
- Rankin EB, Fuh KC, Taylor TE, Krieg AJ, Musser M, Yuan J, Wei K, Kuo CJ, Longacre TA, Giaccia AJ. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res 2010; 70:7570-9; PMID:20858715; http://dx.doi.org/10.1158/0008-5472.CAN-10-1267
- Lee HJ, Jeng YM, Chen YL, Chung L, Yuan RH. Gas6Axl pathway promotes tumor invasion through the transcriptional activation of Slug in hepatocellular carcinoma. Carcinogenesis 2013; 19:19
- Ou WB, Corson JM, Flynn DL, Lu WP, Wise SC, Bueno R, Sugarbaker DJ, Fletcher JA. AXL regulates mesothelioma proliferation and invasiveness. Oncogene 2011; 30:1643-52; PMID:21132014; http://dx.doi.org/10.1038/onc.2010.555
- Paccez JD, Vasques GJ, Correa RG, Vasconcellos JF, Duncan K, Gu X, Bhasin M, Libermann TA, Zerbini LF. The receptor tyrosine kinase Axl is an essential regulator of prostate cancer proliferation and tumor growth and represents a new therapeutic target. Oncogene 2013; 32:689-98; PMID:22410775; http://dx.doi.org/10.1038/onc.2012.89
- Leconet W, Larbouret C, Chardes T, Thomas G, Neiveyans M, Busson M, Jarlier M, Radosevic-Robin N, Pugniere M, Bernex F, et al. Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy. Oncogene 2013; 18:487
- Keating AK, Kim GK, Jones AE, Donson AM, Ware K, Mulcahy JM, Salzberg DB, Foreman NK, Liang X, Thorburn A, et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther 2010; 9:1298-307; PMID:20423999; http://dx.doi.org/10.1158/1535-7163.MCT-09-0707
- Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther 2011; 10:1763-73; PMID:21933973; http://dx.doi.org/10.1158/1535-7163.MCT-11-0116
- Boschelli F, Arndt K, Gambacorti-Passerini C. Bosutinib: a review of preclinical studies in chronic myelogenous leukaemia. Eur J Cancer 2010; 46:1781-9; PMID:20399641; http://dx.doi.org/10.1016/j.ejca.2010.02.032
- Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012; 12:89-103; PMID:22270953; http://dx.doi.org/10.1038/nrc3205
- Schroeder GM, An Y, Cai ZW, Chen XT, Clark C, Cornelius LA, Dai J, Gullo-Brown J, Gupta A, Henley B, et al. Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. J Med Chem 2009; 52:1251-4; PMID:19260711; http://dx.doi.org/10.1021/jm801586s
- Hochgrafe F, Zhang L, O’Toole SA, Browne BC, Pinese M, Porta Cubas A, Lehrbach GM, Croucher DR, Rickwood D, Boulghourjian A, et al. Tyrosine phosphorylation profiling reveals the signaling network characteristics of Basal breast cancer cells. Cancer Res 2010; 70:9391-401; PMID:20861192; http://dx.doi.org/10.1158/0008-5472.CAN-10-0911
- Meric F, Lee WP, Sahin A, Zhang H, Kung HJ, Hung MC. Expression profile of tyrosine kinases in breast cancer. Clin Cancer Res 2002; 8:361-7; PMID:11839650
- Kohn KW, Zeeberg BR, Reinhold WC, Sunshine M, Luna A, Pommier Y. Gene expression profiles of the NCI-60 human tumor cell lines define molecular interaction networks governing cell migration processes. PLoS One 2012; 7:3 PMID:22570691; http://dx.doi.org/10.1371/journal.pone.0035716
- Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 2011; 29:1046-51; PMID:22037378; http://dx.doi.org/10.1038/nbt.1990
- Choi YL, Bocanegra M, Kwon MJ, Shin YK, Nam SJ, Yang JH, Kao J, Godwin AK, Pollack JR. LYN is a mediator of epithelial-mesenchymal transition and a target of dasatinib in breast cancer. Cancer Res 2010; 70:2296-306; PMID:20215510; http://dx.doi.org/10.1158/0008-5472.CAN-09-3141
- Geiger B. A role for p130Cas in mechanotransduction. Cell 2006; 127:879-81; PMID:17129774; http://dx.doi.org/10.1016/j.cell.2006.11.020
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta 2004; 5:2-3; PMID:15246682
- Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004; 10:5367-74; PMID:15328174; http://dx.doi.org/10.1158/1078-0432.CCR-04-0220
- Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol 2008; 26:2568-81; PMID:18487574; http://dx.doi.org/10.1200/JCO.2007.13.1748
- Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012; 44:852-60; PMID:22751098; http://dx.doi.org/10.1038/ng.2330
- Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB, Ivaska J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene 2011; 30:1436-48; PMID:21057535; http://dx.doi.org/10.1038/onc.2010.509
- Tan DS, Haaland B, Gan JM, Tham SC, Sinha I, Tan EH, Lim KH, Takano A, Krisna SS, Thu MM, et al. Bosutinib inhibits migration and invasion via ack1 in kras mutant non-small cell lung cancer. Mol Cancer 2014; 13:1476-4598; PMID:24461128; http://dx.doi.org/10.1186/1476-4598-13-13
- Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, Duan M, Torneros A, Yu J, Heckrodt TJ, et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res 2010; 70:1544-54; PMID:20145120; http://dx.doi.org/10.1158/0008-5472.CAN-09-2997
- Braunger J, Schleithoff L, Schulz AS, Kessler H, Lammers R, Ullrich A, Bartram CR, Janssen JW. Intracellular signaling of the UfoAxl receptor tyrosine kinase is mediated mainly by a multi-substrate docking-site. Oncogene 1997; 14:2619-31; PMID:9178760; http://dx.doi.org/10.1038/sj.onc.1201123
- Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, Weimer R, Wu Y, Pei L. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene 2010; 29:5254-64; PMID:20603615; http://dx.doi.org/10.1038/onc.2010.268
- Yeh CY, Shin SM, Yeh HH, Wu TJ, Shin JW, Chang TY, Raghavaraju G, Lee CT, Chiang JH, Tseng VS, et al. Transcriptional activation of the Axl and PDGFR-alpha by c-Met through a ras- and Src-independent mechanism in human bladder cancer. BMC Cancer 2011; 11:139; PMID:21496277; http://dx.doi.org/10.1186/1471-2407-11-139
- Meyer AS, Miller MA, Gertler FB, Lauffenburger DA. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Science Signaling 2013; 6:ra66; PMID:23921085; http://dx.doi.org/10.1126/scisignal.2004155
- Cunningham-Edmondson AC, Hanks SK. p130Cas substrate domain signaling promotes migration, invasion, and survival of estrogen receptor-negative breast cancer cells. Breast Cancer (London) 2009; 2009:39-52; PMID:21253442
- Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol 2006; 16:257-63; PMID:16581250; http://dx.doi.org/10.1016/j.tcb.2006.03.003
- Sanchez-Bailon MP, Calcabrini A, Gomez-Dominguez D, Morte B, Martin-Forero E, Gomez-Lopez G, Molinari A, Wagner KU, Martin-Perez J. Src kinases catalytic activity regulates proliferation, migration and invasiveness of MDA-MB-231 breast cancer cells. Cell Signal 2012; 24:1276-86; PMID:22570868; http://dx.doi.org/10.1016/j.cellsig.2012.02.011
- Vultur A, Buettner R, Kowolik C, Liang W, Smith D, Boschelli F, Jove R. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol Cancer Ther 2008; 7:1185-94; PMID:18483306; http://dx.doi.org/10.1158/1535-7163.MCT-08-0126
- Paolino M, Choidas A, Wallner S, Pranjic B, Uribesalgo I, Loeser S, Jamieson AM, Langdon WY, Ikeda F, Fededa JP, et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature 2014; 19; PMID:24553136; http://dx.doi.org/10.1038/nature12998
- Conradt L, Godl K, Schaab C, Tebbe A, Eser S, Diersch S, Michalski CW, Kleeff J, Schnieke A, Schmid RM, et al. Disclosure of erlotinib as a multikinase inhibitor in pancreatic ductal adenocarcinoma. Neoplasia 2011; 13:1026-34; PMID:22131878