Abstract
Wnt/β-catenin and Hedgehog/Gli signalings play key roles in multiple biogenesis such as embryonic development and tissue homeostasis. Dysregulations of these 2 pathways are frequently found in most cancers, particularly in colon cancer. Their crosstalk has been increasingly appreciated as an important mechanism in regulating colon cancer progression. Our studies into the link between Wnt/β-catenin and Hedgehog/Gli signalings in colon cancer revealed several possible crosstalk points and suggested potential therapeutic strategies for colon cancer.
Introduction
Colon cancer is one of the major malignancies worldwide. Its pathogenesis is complex and requires the accumulated alteration of multiple genes and pathways. More than 80% of colon cancer cases harbor aberrant Wnt/β-catenin signaling that regulate colon cancer progression through intracellular mechanism or interaction with tumor microenvironment and cancer stem cells. In colon cancer, Wnt/β-catenin signaling is activated via genetic or epigenetic changes of its components, and further derived by oncogenes or tumor suppressors outside canonical signaling pathway. In addition to Wnt/β-catenin signaling, Hedgehog/Gli signaling has also attracted increasing attention due to its important roles in colon tumor maintenance. Hedgehog/Gli signaling is activated by canonical and non-canonical signaling routes in colon cancer. Accumulating evidences have suggested that Hedgehog/Gli signaling acts on colon cancer in an autocrine, paracrine or cancer stem cell fashions. Recent studies have revealed that Wnt/β-catenin and Hedgehog/Gli signalings are not independent cascades in colon cancer. In light of this, we focused our studies on the current advancements on their correlation which, more importantly, could lead to potential strategies for colon cancer therapy.
Wnt/β-catenin Signaling Pathway
The discovery of Wnt gene in Drosophila and its homologous gene in vertebrates laid the foundation for an evolutionary conserved signaling cascade which is commonly referred to as canonical Wnt signaling pathway. In the absence of wnt ligands, β-catenin usually locates in cell membrane where it interacts with E-cadherin and mediates intercellular adhesion.Citation1 Free cytoplasm β-catenin is destabilized by a destruction complex including adenomatous polyposis coli (APC), Axin, glycogen synthase kinase-3β (GSK3β) and casein kinase 1α (CK1α).Citation2,3 When wnts interact with Frizzled and LRP, Wnt/β-catenin signaling is activated. Both Dishevelled (Dvl) and Axin are recruited to cell membrane, followed by GSK3β inhibition and β-catenin releasing from a destruction complex. β-catenin is transported into cell nucleus and forms complex with T-cell transcription factor (TCF)/lymphoid enhancer-binding factor (LEF). In the participation of coactivatiors cAMP response element-binding protein (CREB)-binding protein (CBP), a series of target genes are expressed, which include proteins associated with cell proliferation, apoptosis, invasion and angiogenesis.
Wnt/β-catenin Signaling and Colon Cancer
Human hereditary and sporadic colon cancers usually develop from the earliest aberrant crypt foci to larger adenomas, which will progress to carcinoma and invasive adenocarcinomas. Genetic or epigenetic changes of Wnt pathway components are frequently found in most colon cancer cases which lead to aberrant Wnt/β-catenin signaling (). Found in about 80% of colon tumors, APC mutations are one of the earliest events in colon cancer progression.Citation4,5 APC mutations usually occur in a mutation cluster region that is important for binding to Axin and forming destruction complex, and lead to produce truncated form of APC as well as the stabilization of β-catenin. In addition, wildtype APC, containing both nuclear localization sequences (NLS) and nuclear export sequences (NES), acts to shuttle β-catenin between cell nucleus and cytoplasm. Lacking nuclear export sequences (NES), truncated APC is incompetent for β-catenin shuttling. Among the 10% of colon cancer cases that contain wildtype APC, β-catenin is mutated by point or in frame deletion of serine and threonine residues. These mutations render β-catenin difficult for ubiquitination and degradation by cellular proteosomes. Moreover, Axin mutation is found in some microsatellite instability (MSI) colon tumor cases. Axin is an important component of destruction complex and its inactive mutations could disturb the process of β-catenin degradation. Epigenetic alterations have occurred in colon cancer. Components of Wnt/β-catenin signaling, such as sFRP1, WIF-1 and DKK1, are silenced by hypermethylation, which contributes to aberrant Wnt/β-catenin signaling in colon cancer.Citation6 While colorectal adenomas progressing to invasive carcinoma, further genetic changes in oncogenes or tumor suppressors are found to aggravate the activation of Wnt/β-catenin signaling (). Oncogenic activation of KRAS is frequently observed in colon cancer which can increase β-catenin stability through inhibiting GSK-3β by KRAS/PI3K/AKT signaling.Citation7 Inactive mutation of tumor suppressor p53 is also found in colorectal cancer and related to the stabilization of β-catenin.Citation8,9 Loss expression of phosphatase and tensin homolog (PTEN) has been associated with colorectal cancer's aggressive capacity.Citation10-12 PTEN knockdown can increase the accumulation of β-catenin in cell nucleus.Citation10,11
Figure 1. The mechanism that result in aberrant Wnt/β-catenin signaling in colon cancer. There include genetic like inactivating and activating mutations or epigenetic changes like DNA hypermethylation of Wnt pathway components; Meanwhile, another cause involves genetic and/or expression changes of oncogenes or tumor suppressors outside Wnt pathway.
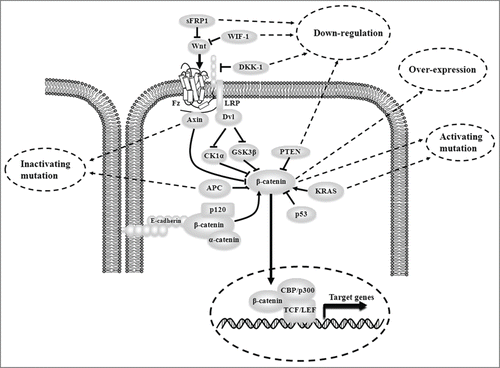
Active Wnt/β-catenin signaling stimulates colon cancer development through its downstream cancer-related targets such as c-myc, Cyclin D1, Cox-2, MMPs, uPAR, VEGF, etc. In addition to intracellular mechanism, Wnt/β-catenin signaling also plays an important role in colon cancer progression by interacting with tumor microenvironment and cancer stem cells. In tumor microenvironment, growth factors secreted by stromal cells could enhance the activity of Wnt/β-catenin. Hepatocyte growth factor receptor (HGFR) forms a complex with β-catenin.Citation13 When HGF binds to HGFR, β-catenin is dissociated from HGFR/β-catenin complex, followed by tyrosine phosphorylation. Finally, Wnt/β-catenin activity is enhanced in the same manner as KRAS activation mutation. Other factors such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF) and transforming growth factor β (TGF β) can enhance Wnt/β-catenin signaling by p68.Citation14 PDGF, EGF and TGF β promote tyrosine phosphorylation of p68, which inhibits β-catenin phosphorylation. Cancer stem cells (CSCs) are small parts of cancer cells, which can self–renew and differentiate to generate many types of tumor cells. In 2007, O’Brien and Ricci-Vitiani discovered colon cancer stem cells.Citation15,16 High Wnt activity can designate colon cancer stem cell population.Citation17
Hedgehog/Gli Signaling Pathway
Firstly identified in Drospphila melanogaster, Hedgehog/Gli signaling derives its name from an intercellular signaling ligand called Hedgehog. In vertebrates, there are at least 3 Hh ligands: Sonic hedgehog (Shh), Indian Hh (Ihh) and Desert Hh (Dhh). Lipid modified Hh ligands are secreted in the participation of Dispatched protein (Disp) and then interact with reception system consisting of Patched (PTCH) and Smoothened (SMO). SMO is a crucial signal transducer of Hedgehog pathway, signaling to a cytoplasmic transduction cascade that ultimately regulates transcriptional activators Gli proteins. There are 3 different Gli proteins: Gli1, Gli2 and Gli3. Gli1 acts as a transcriptional activator. Gli2 and Gli3 are bifunctional transcription factors which exist in 2 forms: their full-length ones as transcriptional activators and truncated N-terminal fragment as transcriptional repressors.Citation18 In the absence of Hh ligands, PTCH localizes to primary cilium and blocks SMO activity by preventing SMO from entering the cilium.Citation19,20 Consequently, Gli transcription factors in cilia are phosphorylated by PKA, GSK3β and CK1α, followed by cleavage to form Gli repressor and inhibiting target genes transcription.Citation21 When hedgehog ligands bind to PTCH, SMO is transported to cilia. SMO interacts with suppressor of fused (Sufu) to relieve its inhibitory effect on Gli.Citation22,23 When activated, Gli triggers the transcription of target genes.
Abnormal Hedgehog/Gli Signaling in Colon Cancer
It has been suggested that aberrant Hedgehog/Gli signaling plays a crucial role in colon cancer progression. The persistent activation of Hedgehog/Gli signaling in colon cancer is driven in a ligand-dependent manner, which is mediated through either canonical or non-canonical signaling routes (). Hedgehog/Gli signaling related components, Shh, PTCH1, SMO and Gli, are observed to be over-expressed in colon tumor.Citation24-27 A positive correlation between tumor progression and expression levels of Shh, PTCH1 or SMO has been suggested.Citation27 In non-canonical signaling routes, increased Gli function is regulated by multiple oncogenes and tumor suppressors, which consequently leads to aberrant Hedgehog signaling during colon carcinogenesis. Oncogenes BRAF are frequently observed with activating mutations in colon cancer.Citation28 Oncogenic KRAS mutant could promote Gli1 by downstream RAF/MEK/ERK and PI3K/AKT signaling cascades.Citation29,30 In addition, inactive p53 and lost PTEN expression have been proven to result in up-regulated Gli1 activity in colon cancer cells.Citation30
Figure 2. The mechanism that result in aberrant Hedgehog/Gli signaling in colon cancer. There include canonical and non-canonical signaling routes. Canonical routes involve in the over-expression of Hh ligand, PTCH1, SMO and Gli. Non-canonical signaling routes include genetic or expression changes of multiple oncogenes and tumor suppressors such as p53, KRAS, BRAF and PTEN.
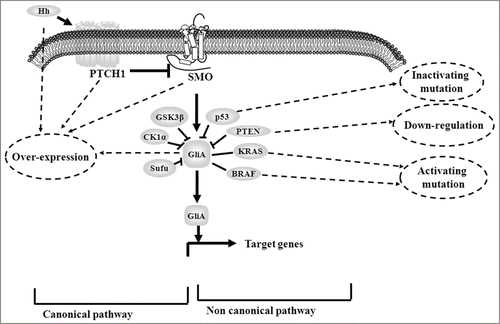
The mechanisms of aberrant Hedgehog/Gli signaling associated with cancer are diverse.Citation31 In colon cancer, the precise mechanism is still unclear. The current understanding of the role of Hedgehog signaling in colon cancer can be summarized below. First, Hedgehog signaling acts on colon cancer in an autocrine manner. Tumor cell secretes Hh ligands and then binds itself to trigger the transcription of target genes, which stimulates proliferation and survival of tumor cells. This view is supported by the observation that the hyperplastic polyps, adenomas and adenocarcinomas of colon express Shh, PTCH1 and SMO.Citation32,33 Furthermore, cyclopamine, an inhibitor of Hedgehog signaling, inhibits the growth of colon cancer cell lines.Citation34 Active Hedgehog signaling is expressed in tumor epithelial cells rather than in the stroma.Citation35 Secondly, Hedgehog signaling acts in a paracrine fashion. Tumor cell secretes Hh ligands, which stimulates the surrounding stroma to produce a mitogen. The mitogen then promotes tumor cell growth and survival. This has been supported by data that suggest both epithelium and mesenchymal cells in gut express Hedgehog signaling.Citation32,33 The xenografts of tumor cell are inhibited by Hedgehog signaling antagonist in the stroma.Citation36 Finally, Hedgehog signaling is important for the maintenance of cancer stem cells. Studies showed that Hedgehog signaling activation is essential to stem cell survival and expansion.Citation35
Crosstalk between Wnt/β-catenin and Hedgehog/Gli Signalings in Colon Cancer
While Wnt/β-catenin and Hedgehog/Gli signalings are crucially involved in colon cancer processes, crosstalk between them has been identified to be important for colon cancer recurrence, invasion and metastasis.Citation30,Citation37-42 To summarize, possible molecular crosstalk between 2 pathways include the following ():
Figure 3. Crosstalk between Wnt/β-catenin and Hedgehog/Gli signalings in colon cancer. Firstly, both pathways are regulated by common modulators such as GSK3β, CK1α, Sufu, p53, PTEN, SMO and KRAS; Secondly, β-catenin interacts with Gli1 by Gli3R and their downstream targets (such as CBD-BP and c-myc for β-catenin; Hh, Snail, sFRP1 and Wnt for Gli1). Finally, β-catenin and Gli1 have antagonistic role in regulating TCF and its downstream target genes.
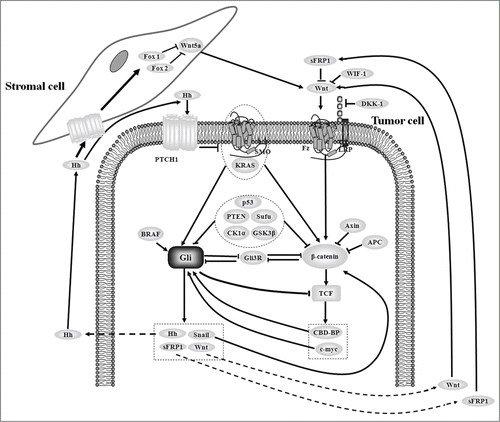
First, both Wnt/β-catenin and Hedgehog/Gli signalings are regulated by molecules such as GSK3β, CK1α, Sufu, p53, PTEN, SMO and KRAS. Two protein kinases GSK3β and CK1α negatively regulate both β-catenin and Gli1. In Wnt/β-catenin signaling, GSK3β, CK1α and β-catenin bind to Axin. Axin mediates β-catenin phosphorylation in Ser 45 by CK1α, which allows GSK3β to phosphorylate Thr 41, Ser 37 and Ser 33 of β-catenin.Citation2,3 Phosphorylated β-catenin is recognized, ubiquitinylated by β-TrCP and ultimately degraded.Citation43 Furthermore, in Hedgehog/Gli signaling both GSK3β and CK1α phosphorylate full-length Gli3.Citation44 Phosphorylated Gli3 is recognized by β-TrCP and leads to the degradation of C-terminal peptides to generate Gli3R, which in turn inhibits Gli1 activity.Citation45 Sufu, as a suppressor of the Fused kinase, is a negative regulator of both Wnt/β-catenin and Hedgehog/Gli signaling pathways. Sufu not only interacts with Gli1 but also binds to β-catenin to control their nuclear–cytoplasmic distributions.Citation42,46,47 In addition, inactive mutation of p53 or loss of PTEN in colon cancer could stimulate both pathways by activating β-catenin and Gli1.Citation10,30 Studies have shown that high levels of functional p53 could lead to a reduction of the amount and transcriptional activity of β-catenin.Citation8,9 PTEN knockdown can increase the stabilization of β-catenin through activating PI3K/AKT pathway.Citation10,11 For Hedgehog/Gli signaling, knockdown of p53 increases Gli1 activity whereas over-expression of p53 inhibits Gli1 activity in colon cancer cells.Citation30 Either enhanced levels of PTEN or inhibition of downstream AKT could disturb Gli1 activity.Citation30 SMO is an upstream active factor of Gli1 in Hedgehog signaling.Citation19 Studies have shown an inhibition of SMO could reduce protein levels of active β-catenin and induce its nuclear exclusion, which is independent on the effect of Gli.Citation39 Activated KRAS could stimulate the action of 2 pathways in colon cancer.Citation7,29,30,48,49 KRAS mutation up-regulates β-catenin through the inhibition of GSK-3β mediated by PI3K/AKT signaling.Citation7 Studies have shown that the inhibitor U0126 for RAS/RAF/MEK/ERK signaling could repress Gli1 activity in human colon cancer cells.Citation29,30 Overexpression of both KRAS mutants and the downstream component AKT enhance Gli1 activity in colon cancer cells.Citation30
Secondly, β-catenin interacts with Gli1 by Gli3R and each downstream target. A few studies have demonstrated the interaction between β-catenin and Gli1 in colon cancer in multiple ways. In colon cancer, Gli3R displays mutual antagonism with β-catenin and Gli1 respectively.Citation30,35,50 Gli3R inhibits β-catenin activity through interacting with the C-terminal transactivation domain of β-catenin and thereby antagonizes the active forms of β-catenin.Citation50 The effects that Gli1 enhancing and Gli3R repressing cell proliferation are mutually rescued in co-transfected cells.Citation35 An over-expression of Gli1 decreases the mRNA levels of Gli3R, and the transcription of Gli1 is also repressed by the enhanced Gli3R expression.Citation30 Thus, β-catenin and Gli1 could regulate each other by Gli3R. In addition, β-catenin could regulate Gli1 by downstream targets of CBD-BP and c-myc. β-catenin induces the expression of an RNA-binding protein CBD-BP, which in turn binds to the coding region of Gli1 mRNA and stabilizes it.Citation38 Enhanced expression of c-myc, a key target of β-catenin, by transfection of plasmids containing c-myc cDNA in colon cancer cells induces elevate Gli1 mRNA levels.Citation30 Moreover, c-myc overexpression could repress Gli3, which forms Gli3R and then exerts mutual antagonism with Gli1.Citation30 Gli1 also regulates β-catenin through its targets: Snail, Wnt, Shh and sFRP1. Gli1 could induce Snail expression, which then interacts with β-catenin and stimulates its transcriptional activity.Citation51,52 Wnt2b, Wnt4 and Wnt7b are shown to be target proteins of Gli1Citation53; while they are the upstream ligands of Wnt/β-catenin signaling, they could trigger downstream cascades to promote the stability of β-catenin. Another target protein, sFRP1, is an antagonist of Wnt ligands which indirectly inhibits the activity of downstream β-catenin.Citation54,55 In addition, Gli1 could up-regulate Shh expression, which is secreted and acts on stromal cells. Stromal cells responding to Shh enhance Foxf1 and Foxf2 expression, which inhibits mesenchymal expression of Wnt5a and leads to suppression of β-cateninCitation56.
Finally, β-catenin and Gli1 have antagonistic roles in regulating TCF and downstream target genes in metastatic colon cancer. Even if β-catenin is still highly expressed in metastatic colon cancer, TCF's targets are decreased in contrast to that in non-metastatic stage.Citation30 In addition, studies have suggested enhanced Gli1 could attenuate TCF activity and its downstream targets in colon cancer cells.Citation30
Implications for Colon Cancer Therapy
The crosstalk between Wnt/β-catenin and Hedgehog/Gli signalings plays an important role in colon cancer and hence is an interesting therapeutic target. The therapeutic strategies may include the following 2 aspects:
(1) Targeting common regulators of both Wnt/β-catenin and Hedgehog/Gli signalings. Targeting common activators or suppressors that lead to a blockage of both pathways is theoretically effective to colon cancer suppression. The potential targets are described as the following: i. SMO. Cyclopamine is a natural Hedgehog pathway antagonist and acts by binding to and inhibiting SMO. Cyclopamine treatment could induce colon cancer cells apoptosis in vitro and rescue the lethality and intestinal phenotypes owing to APC loss in mice.Citation34,35,37 Vismodegib (GDC-0449), a cyclopamine derivative, could delay colon tumor growth in mice.Citation36 ii. KRAS. KRAS suppression by antisense oligonucleotides decreases colon cancer cell viability.Citation57 Inhibitor of KRAS membrane localization, farnesyltransferase (FTS) could inhibit colon cancer cell growth by reducing KRAS and up-regulating p53 and p21.Citation58 In addition, some inhibitors of downstream RAF-MEK-ERK/MAPK cascade of KRAS such as PD 098059, U0126, and CI-1040 have shown antagonistic effects on colon cancer cells.Citation29,30,59 iii. CK1α and GSK3β. Small molecular pyrvinium could activate CK1α, which in turn suppresses Wnt signaling and colon cancer cell proliferation.Citation60 PX-316 that indirectly activates GSK3β by repressing upstream AKT could present antitumor activity against colon cancer in mice.Citation61 iv. p53. Restoring p53 function have been developed as a novel therapeutic approaches.Citation62 In colon cancer, adenovirus mediated p53 gene transfer could induce apoptosis and inhibit growth and angiogenesis.Citation63 In addition, overexpression of Sufu, PTEN or Gli3R has shown anti-tumor effects on colon cancer cells.Citation35,42,64 So they may be developed to be effective therapeutic molecules for colon cancer.
(2) Combined inhibition of Wnt/β-catenin and Hedgehog/Gli signalings
In addition to common regulators, there are independent mechanism that mediates Wnt/β-catenin and Hedgehog/Gli signalings respectively (). The combination of inhibitors that separately disturb Wnt/β-catenin or Hedgehog/Gli signaling may be a feasible approach for colon cancer treatment.
The potential targets of Wnt/β-catenin signaling include (): i. Upstream mediators of β-catenin: SFRP, APC and Axin. Re-expression of SFRP in colon cancer cells leads to attenuated Wnt signaling as well as cell death.Citation65 Recombinant adenovirus (Ad-CBR) that constitutively expresses the central third of APC could block nuclear translocation of β-catenin and inhibit β-catenin/TCF-4-mediated transactivation, which consequently induces colon cancer cells apoptosis.Citation66 Adenovirus mediated gene transfer of Axin could induce colorectal cancer cells apoptosis.Citation67 These data suggest SFRP, APC and Axin may be effective therapeutic molecules for colon cancer. ii. β-catenin. Because of the elevated β-catenin in most of colon cancers, approaches such as decreasing β-catenin expression by antisense, RNA interference, protein knockdown and disturbing β-catenin/LEF/TCF nuclear complex have been suggested.Citation68 The use of β-catenin antisense oligonucleotides in colon cancer decreases β-catenin level, TCF transcrption and Cyclin D1 expression. Meanwhile, there is a reduction in cell proliferation and invasiveness in vitro and inhibition of tumor growth in vivo.Citation69,70 Expressing siRNA targeting β-catenin decreases TCF transcription and leads to an increase in G1 cell cycle arrest and promotion of cell differentiationCitation71. Cong et al. constructed a chimeric protein with β-catenin binding domain of E-cadherin fused to β-TrCP ubiquitin protein ligase (β-TrCP-E-cadherin), which can knock down cytosolic β-catenin.Citation72 Expression of β-TrCP-E-cadherin can affect growth and clonogenic ability in colon cancer cells and lead to lose their tumorigenic ability in nude mice.Citation72 Emami and his colleagues screened an inhibitor called ICG-001, which disturbs β-catenin/LEF/TCF nuclear complex by specifically targeting the coactivator CBP.Citation73 This agent could reduce colon cancer growth both in vitro and in vivo.Citation73 iii. Downstream effectors of β-catenin. Because downstream targets of β-catenin play an important role in colon cancer development, targeting these genes can be effective for tumor therapy. COX-2 inhibitors such as NSAIDS have been proven to suppress colon cancer.Citation74
The important role of Hedgehog/Gli signaling in various cancers has caused attention on targeting it. According to the crosstalk between 2 pathways, the potential targets apart from above common regulators include (): i. BRAF. Inhibition of BRAF by RNA interference increases apoptosis in colon cancer cells.Citation75 ii. Gli1. A few small molecular inhibitors for Gli1, GANT61, have been identified. Treatment with GANT61 induces DNA damage and cell death in colon cancer cells.Citation29,76
Concluding Remarks
Both Wnt/β-catenin and Hedgehog/Gli signalings are frequently activated in colon cancer. These 2 pathways have been shown to coordinate or cross-regulate at multiple aspects during colon cancer development. Despite understanding of the interaction between Wnt/β-catenin and Hedgehog/Gli signalings, detailed molecular mechanisms need to be clarified. The development and identification of selective inhibitors based on the crosstalk between Wnt/β-catenin and Hedgehog/Gli signalings provide approaches for preventing and treating colon cancer progression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Natural Sciences Foundation of China (No. 31271516, No. 21207084); Research Fund for the Doctoral Program of Higher Education of China (20111401110011),China Postdoctoral Science Foundation (2012M521178) and Natural Sciences Foundation of Shanxi (2014011027–5).
References
- Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee SR, Zhao Y, Harris DC, Zheng G. E-cadherin/beta-catenin complex and the epithelial barrier. J Biomed Biotechnol 2011; 2011:567305; PMID:22007144
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev 2002; 16:1066-76; PMID:12000790; http://dx.doi.org/10.1101/gad.230302
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 2002; 108:837-47; PMID:11955436; http://dx.doi.org/10.1016/S0092-8674(02)00685-2
- Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 2006; 25:7531-7; PMID:17143297; http://dx.doi.org/10.1038/sj.onc.1210059
- The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487:330-7; PMID:22810696; http://dx.doi.org/10.1038/nature11252
- Qi J, Zhu YQ, Luo J, Tao WH. Hypermethylation and expression regulation of secreted frizzled-related protein genes in colorectal tumor. World J Gastroenterol 2006; 12:7113-7; PMID:17131472
- Li J, Mizukami Y, Zhang X, Jo WS, Chung DC. Oncogenic K-ras stimulates Wnt signaling in colon cancer through inhibition of GSK-3beta. Gastroenterology 2005; 128:1907-18; PMID:15940626; http://dx.doi.org/10.1053/j.gastro.2005.02.067
- Levina E, Oren M, Ben-Ze’ev A. Downregulation of beta-catenin by p53 involves changes in the rate of beta-catenin phosphorylation and Axin dynamics. Oncogene 2004; 23:4444-53; PMID:15064706; http://dx.doi.org/10.1038/sj.onc.1207587
- Sadot E, Geiger B, Oren M, Ben-Ze’ev A. Down-regulation of beta-catenin by activated p53. Mol Cell Biol 2001; 21:6768-81; PMID:11564862; http://dx.doi.org/10.1128/MCB.21.20.6768-6781.2001
- Rychahou PG, Kang J, Gulhati P, Doan HQ, Chen LA, Xiao SY, Chung DH, Evers BM. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc Natl Acad Sci 2008; 105:20315-20; http://dx.doi.org/10.1073/pnas.0810715105
- Bowen KA, Doan HQ, Zhou BP, Wang Q, Zhou Y, Rychahou PG, Evers BM. PTEN loss induces epithelial–mesenchymal transition in human colon cancer cells. Anticancer Res 2009; 29:4439-49; PMID:20032390
- Sawai H, Yasuda A, Ochi N, Ma J, Matsuo Y, Wakasugi T, Takahashi H, Funahashi H, Sato M, Takeyama H. Loss of PTEN expression is associated with colorectal cancer liver metastasis and poor patient survival. BMC Gastroenterol 2008; 8:56; PMID:19036165; http://dx.doi.org/10.1186/1471-230X-8-56
- Rasola A, Fassetta M, De Bacco F, D’Alessandro L, Gramaglia D, Di Renzo MF, Comoglio PM. A positive feedback loop between hepatocyte growth factor receptor and beta-catenin sustains colorectal cancer cell invasive growth. Oncogene 2007; 26:1078-87; PMID:16953230; http://dx.doi.org/10.1038/sj.onc.1209859
- Yang L, Lin C, Liu ZR. P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from beta-catenin. Cell 2006; 127:139-55; PMID:17018282; http://dx.doi.org/10.1016/j.cell.2006.08.036
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007; 445:106-10; http://dx.doi.org/10.1038/nature05372
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007; 445:111-5; PMID:17122771; http://dx.doi.org/10.1038/nature05384
- Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 2010; 12:468-76; PMID:20418870; http://dx.doi.org/10.1038/ncb2048
- Jacob J, Briscoe J. Gli proteins and the control of spinal-cord patterning. EMBO Rep 2003; 4:761-5; PMID:12897799; http://dx.doi.org/10.1038/sj.embor.embor896
- Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci U S A 2009; 106:3196-201; PMID:19218434; http://dx.doi.org/10.1073/pnas.0813373106
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science 2007; 317:372-6; PMID:17641202; http://dx.doi.org/10.1126/science.1139740
- Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH Jr, Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med 2009; 15:1055-61; PMID:19701205; http://dx.doi.org/10.1038/nm.2011
- Kogerman PGT, Kogerman L, Krause D, Undén AB, Sandstedt B, Toftgård R, Zaphiropoulos PG. Mammalian suppressor of fused modulates nuclearcytoplastic shuttling of Gli1. Nat Cell Biol 1999; 1:312-9; PMID:10559945; http://dx.doi.org/10.1038/13031
- Ding Q, Fukami S, Meng X, Nishizaki Y, Zhang X, Sasaki H, Dlugosz A, Nakafuku M, Hui C. Mouse suppressor of fused is a negative regulator of sonic hedgehog signaling and alters the subcellular distribution of Gli1. Curr Biol 1999; 9:1119-22; PMID:10531011; http://dx.doi.org/10.1016/S0960-9822(99)80482-5
- Yoshikawa K, Shimada M, Miyamoto H, Higashijima J, Miyatani T, Nishioka M, Kurita N, Iwata T, Uehara H. Sonic hedgehog relates to colorectal carcinogenesis. J Gastroenterol 2009; 44:1113-7; PMID:19662327; http://dx.doi.org/10.1007/s00535-009-0110-2
- Wang H, Li YY, Wu YY, Nie YQ. Expression and clinical significance of hedgehog signaling pathway related components in colorectal cancer. Asian Pac J Cancer Prev 2012; 13:2319-24; PMID:22901214; http://dx.doi.org/10.7314/APJCP.2012.13.5.2319
- Oniscu A, James RM, Morris RG, Bader S, Malcomson RDG, Harrison DJ. Expression of Sonic hedgehog pathway genes is altered in colonic neoplasia. J Pathol 2004; 203:909-17; PMID:15258993; http://dx.doi.org/10.1002/path.1591
- Bian YH, Huang SH, Yang L, Ma XL, Xie JW, Zhang HW. Sonic hedgehog-Gli1 pathway in colorectal adenocarcinomas. World J Gastroenterol 2007; 13:1659-65; PMID:17461467; http://dx.doi.org/10.3748/wjg.v13.i11.1659
- Rako I, Jakic-Razumovic J, Katalinic D, Sertic J, Plestina S. Mutation pattern of KRAS and BRAF oncogenes in colorectal cancer patients. Neoplasma 2012; 59:376-83; PMID:22489692; http://dx.doi.org/10.4149/neo_2012_049
- Mazumdar T, DeVecchio J, Agyeman A, Shi T, Houghton JA. The GLI genes as the molecular switch in disrupting Hedgehog signaling in colon cancer. Oncotarget 2011; 2:638-45; PMID:21860067
- Varnat F, Siegl-Cachedenier I, Malerba M, Gervaz P, Ruiz i Altaba A. Loss of WNT-TCF addiction and enhancement of HH-GLI1 signalling define the metastatic transition of human colon carcinomas. EMBO Mol Med 2010; 2:440-57; PMID:20941789; http://dx.doi.org/10.1002/emmm.201000098
- Barakat MT, Humke EW, Scott MP. Learning from Jekyll to control Hyde: Hedgehog signaling in development and cancer. Trends Mol Med 2010; 16:337-48; PMID:20696410; http://dx.doi.org/10.1016/j.molmed.2010.05.003
- Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 2003; 425:846-51; PMID:14520411; http://dx.doi.org/10.1038/nature01972
- Van Den Brink GR, Peppelenbosch MP. Expression of hedgehog pathway components in the adult colon. Gastroenterology 2006; 130:619; PMID:16472622; http://dx.doi.org/10.1053/j.gastro.2006.01.020
- Qualtrough D, Buda A, Gaffield W, Williams AC, Paraskeva C. Hedgehog signalling in colorectal tumour cells: induction of apoptosis with cyclopamine treatment. Int J Cancer 2004; 110:831-7; PMID:15170664; http://dx.doi.org/10.1002/ijc.20227
- Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruiz i Altaba A. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med 2009; 1:338-51; PMID:20049737; http://dx.doi.org/10.1002/emmm.200900039
- Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, et al. A paracrine requirement for hedgehog signalling in cancer. Nature 2008; 455:406-10; PMID:18754008; http://dx.doi.org/10.1038/nature07275
- Varnat F, Zacchetti G, Ruiz i Altaba A. Hedgehog pathway activity is required for the lethality and intestinal phenotypes of mice with hyperactive Wnt signaling. Mech Dev 2010; 127:73-81; PMID:19861162; http://dx.doi.org/10.1016/j.mod.2009.10.005
- Noubissi FK, Goswami S, Sanek NA, Kawakami K, Minamoto T, Moser A, Grinblat Y, Spiegelman VS. Wnt signaling stimulates transcriptional outcome of the Hedgehog pathway by stabilizing GLI1 mRNA. Cancer Res 2009; 69:8572-8; PMID:19887615; http://dx.doi.org/10.1158/0008-5472.CAN-09-1500
- Arimura S, Matsunaga A, Kitamura T, Aoki K, Aoki M, Taketo MM. Reduced level of smoothened suppresses intestinal tumorigenesis by down-regulation of Wnt signaling. Gastroenterology 2009; 137:629-38; PMID:19427313; http://dx.doi.org/10.1053/j.gastro.2009.04.059
- Maeda O, Kondo M, Fujita T, Usami N, Fukui T, Shimokata K, Ando T, Goto H, Sekido Y. Enhancement of GLI1-transcriptional activity by beta-catenin in human cancer cells. Oncol Rep 2006; 16:91-6; PMID:16786128
- van den Brink GR. Hedgehog Wnteraction in colorectal cancer. Gut 2006; 55:912-4; PMID:16766747; http://dx.doi.org/10.1136/gut.2005.085902
- Meng X, Poon R, Zhang X, Cheah A, Ding Q, Hui CC, Alman B. Suppressor of fused negatively regulates beta-catenin signaling. J Biol Chem 2001; 276:40113-9; PMID:11477086; http://dx.doi.org/10.1074/jbc.M105317200
- Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr Biol 1999; 9:207-10; PMID:10074433; http://dx.doi.org/10.1016/S0960-9822(99)80091-8
- Tempe D, Casas M, Karaz S, Blanchet-Tournier MF, Concordet JP. Multisite protein kinase A and glycogen synthase kinase 3beta phosphorylation leads to Gli3 ubiquitination by SCFbetaTrCP. Mol Cell Biol 2006; 26:4316-26; PMID:16705181; http://dx.doi.org/10.1128/MCB.02183-05
- Wang B, Li Y. Evidence for the direct involvement of {beta}TrCP in Gli3 protein processing. Proc Natl Acad Sci U S A 2006; 103:33-8; PMID:16371461; http://dx.doi.org/10.1073/pnas.0509927103
- Dunaeva M, Michelson P, Kogerman P, Toftgard R. Characterization of the physical interaction of Gli proteins with SUFU proteins. J Biol Chem 2003; 278:5116-22; PMID:12426310; http://dx.doi.org/10.1074/jbc.M209492200
- Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes. J Cell Biol 2010; 191:415-28; PMID:20956384; http://dx.doi.org/10.1083/jcb.201004108
- Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J Cell Biol 1995; 130:461-71; PMID:7542250; http://dx.doi.org/10.1083/jcb.130.2.461
- Janssen KP, Alberici P, Fsihi H, Gaspar C, Breukel C, Franken P, Rosty C, Abal M, El Marjou F, Smits R, et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 2006; 131:1096-109; PMID:17030180; http://dx.doi.org/10.1053/j.gastro.2006.08.011
- Ulloa F, Itasaki N, Briscoe J. Inhibitory Gli3 activity negatively regulates Wnt/beta-catenin signaling. Curr Biol 2007; 17:545-50; PMID:17331723; http://dx.doi.org/10.1016/j.cub.2007.01.062
- Louro ID, Bailey EC, Li X, South LS, McKie-Bell PR, Yoder BK, Huang CC, Johnson MR, Hill AE, Johnson RL, et al. Comparative gene expression profile analysis of GLI and c-MYC in an epithelial model of malignant transformation. Cancer Res 2002; 62:5867-73; PMID:12384550
- Stemmer V, de Craene B, Berx G, Behrens J. Snail promotes Wnt target gene expression and interacts with beta-catenin. Oncogene 2008; 27:5075-80; PMID:18469861; http://dx.doi.org/10.1038/onc.2008.140
- Li X, Deng W, Lobo-Ruppert SM, Ruppert JM. Gli1 acts through Snail and E-cadherin to promote nuclear signaling by beta-catenin. Oncogene 2007; 26:4489-98; PMID:17297467; http://dx.doi.org/10.1038/sj.onc.1210241
- Katoh Y, Katoh M. WNT antagonist, SFRP1, is Hedgehog signaling target. Int J Mol Med 2006; 17:171-5; PMID:16328026
- He J, Sheng T, Stelter AA, Li C, Zhang X, Sinha M, Luxon BA, Xie J. Suppressing Wnt signaling by the hedgehog pathway through sFRP-1. J Biol Chem 2006; 281:35598-602; PMID:17035233; http://dx.doi.org/10.1074/jbc.C600200200
- Ormestad M, Astorga J, Landgren H, Wang T, Johansson BR, Miura N, Carlsson P. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development 2006; 133:833-43; PMID:16439479; http://dx.doi.org/10.1242/dev.02252
- Lledo S, Alfonso R, Alino SF. Antisense gene therapy using anti-k-ras and antitelomerase oligonucleotides in colorectal cancer. Rev Esp Enferm Dig 2005; 97:472-80; PMID:16262526; http://dx.doi.org/10.4321/S1130-01082005000700002
- Halaschek-Wiener J, Wacheck V, Schlagbauer-Wadl H, Wolff K, Kloog Y, Jansen B. A novel Ras antagonist regulates both oncogenic Ras and the tumor suppressor p53 in colon cancer cells. Mol Med 2000; 6:693-704; PMID:11055588
- Jeon HK, Choi SU, Jung NP. Association of the ERK1/2 and p38 kinase pathways with nitric oxide-induced apoptosis and cell cycle arrest in colon cancer cells. Cell Biol Toxicol 2005; 21:115-25; PMID:16142585; http://dx.doi.org/10.1007/s10565-005-0148-8
- Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol 2010; 6:829-36; PMID:20890287; http://dx.doi.org/10.1038/nchembio.453
- Meuillet EJ, Ihle N, Baker AF, Gard JM, Stamper C, Williams R, Coon A, Mahadevan D, George BL, Kirkpatrick L, et al. In vivo molecular pharmacology and antitumor activity of the targeted Akt inhibitor PX-316. Oncol Res 2004; 14:513-27; PMID:15559765
- Stegh AH. Targeting the p53 signaling pathway in cancer therapy–the promises, challenges and perils. Expert Opin Ther Targets 2012; 16:67-83; PMID:22239435; http://dx.doi.org/10.1517/14728222.2011.643299
- Baek JH, Agarwal ML, Tubbs RR, Vladisavljevic A, Tomita H, Bukowski RM, Milsom JW, Kim JM, Kwak JY. In vivo recombinant adenovirus-mediated p53 gene therapy in a syngeneic rat model for colorectal cancer. J Korean Med Sci 2004; 19:834-41; PMID:15608394; http://dx.doi.org/10.3346/jkms.2004.19.6.834
- Xu SS, Shen WL, Ouyang SY. Inhibition of transfected PTEN on human colon cancer. World J Gastroenterol 2004; 10:3670-3; PMID:15534929
- Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet 2004; 36:417-22; PMID:15034581; http://dx.doi.org/10.1038/ng1330
- Shih IM, Yu J, He TC, Vogelstein B, Kinzler KW. The beta-catenin binding domain of adenomatous polyposis coli is sufficient for tumor suppression. Cancer Res 2000; 60:1671-6; PMID:10749138
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet 2000; 24:245-50; PMID:10700176; http://dx.doi.org/10.1038/73448
- Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W, et al. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr Cancer Drug Targets 2004; 4:653-71; PMID:15578921; http://dx.doi.org/10.2174/1568009043332709
- Roh H, Green DW, Boswell CB, Pippin JA, Drebin JA. Suppression of beta-catenin inhibits the neoplastic growth of APC-mutant colon cancer cells. Cancer Res 2001; 61:6563-8; PMID:11522655
- Green DW, Roh H, Pippin JA, Drebin JA. Beta-catenin antisense treatment decreases beta-catenin expression and tumor growth rate in colon carcinoma xenografts. J Surg Res 2001; 101:16-20; PMID:11676549; http://dx.doi.org/10.1006/jsre.2001.6241
- van de Wetering M, Oving I, Muncan V, Pon Fong MT, Brantjes H, van Leenen D, Holstege FC, Brummelkamp TR, Agami R, Clevers H. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep 2003; 4:609-15; PMID:12776180; http://dx.doi.org/10.1038/sj.embor.embor865
- Cong F, Zhang J, Pao W, Zhou P, Varmus H. A protein knockdown strategy to study the function of beta-catenin in tumorigenesis. BMC Mol Biol 2003; 4:10; PMID:14516475; http://dx.doi.org/10.1186/1471-2199-4-10
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription. Proc Natl Acad Sci U S A 2004; 101:12682-7; PMID:15314234; http://dx.doi.org/10.1073/pnas.0404875101
- Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin Oncol 2004; 31:12-21; PMID:15252926; http://dx.doi.org/10.1053/j.seminoncol.2004.03.041
- Subramanian F, Yamakawa A. Combination therapy targeting Raf-1 and MEK causes apoptosis of HCT116 colon cancer cells. Int J Oncol 2012; 41:1855-62; PMID:22922669
- Agyeman A, Mazumdar T, Houghton JA. Regulation of DNA damage following termination of hedgehog (HH) survival signaling at the level of the GLI genes in human colon cancer. Oncotarget 2012; 3:854-868; PMID:23097684
