Abstract
CDK4 and CDK6 bound to D-type cyclins are master integrators of G1 phase cell cycle regulations by initiating the inactivating phosphorylation of the central oncosuppressor pRb. Because of their frequent deregulation in cancer, cyclin D-CDK4/6 complexes are emerging as especially promising therapeutic targets. The specific CDK4/6 inhibitor PD0332991 is currently tested in a growing number of phase II/III clinical trials against a variety of pRb-proficient chemotherapy-resistant cancers. We have previously shown that PD0332991 inhibits not only CDK4/6 activity but also the activation by phosphorylation of the bulk of cyclin D-CDK4 complexes stabilized by p21 binding. Here we show that PD0332991 has either a positive or a negative impact on the activation of cyclin D-CDK4/6 complexes, depending on their binding to p21. Indeed, whereas PD0332991 inhibits the phosphorylation and activity of p21-bound CDK4/6, it specifically stabilized activated cyclin D3-CDK4/6 complexes devoid of p21 and p27. After elimination of PD0332991, these activated cyclin D3-CDK4/6 complexes persisted for at least 24 h, resulting in paradoxical cell cycle entry in the absence of a mitogenic stimulation. This unsuspected positive effect of PD0332991 on cyclin D3-CDK4/6 activation should be carefully assessed in the clinical evaluation of PD0332991, which until now only involves discontinuous administration protocols.
Abbreviations
| 2D | = | 2-dimensional |
| BrdU | = | bromodeoxyuridine |
| CAK | = | CDK-activating kinase |
| CDK | = | cyclin-dependent kinase |
| FBS | = | fetal bovine serum |
| IP | = | immunoprecipitation |
| PAGE | = | polyacrylamide gel electrophoresis |
| PBS | = | phosphate buffer saline |
| PD033 | = | PD0332991 |
| pRb | = | retinoblastoma susceptibility protein |
| SDS | = | sodium dodecyl sulfate |
| SEM | = | standard error of the mean |
Introduction
Eukaryotic cell cycle progression is tightly regulated by cyclin-dependent kinase (CDK) complexes. CDK4 and CDK6, activated by D-type cyclins induced by mitogens, initiate in G1 phase the phosphorylation of the tumor suppressor pRb.Citation1–4 This leads to pRb inactivation and release of the E2F transcription factor activity necessary for DNA synthesis and cell cycle progression. pRb phosphorylation is then maintained independently of D-type cyclins, and hence of mitogens, by a positive feedback loop linking pRb to E2F-dependent transcription of cyclin E, which leads to CDK2 activation and further phosphorylation of pRb.Citation5 In addition, cyclin D-CDK4/6 complexes play a second, noncatalytic function in G1 phase progression through the sequestration of the Cip/Kip CDK inhibitors p21 and p27, thereby facilitating CDK2 activation.Citation6 Activation of CDK4/6 is a multistep process that absolutely requires first the binding to a D-type cyclin, which is opposed by INK4 CDK inhibitors such as p16, and then an activating phosphorylation in the T-loop.Citation3,Citation7–9 In contrast to the weak T177 phosphorylation of CDK6, our previous work has identified the activating T172 phosphorylation of CDK4 as the last highly regulated step determining CDK4 activity.Citation8,Citation10–13 Whereas CDK7, the catalytic component of CDK-activating kinase (CAK), is clearly involved in CDK4/6 activation,Citation14,15 other proline-directed kinases could phosphorylate CDK4 but not CDK6 which lacks the adjacent proline present in the phosphoacceptor domain of CDK4.Citation13,15 The impacts of p21 and p27 on CDK4/6 activation are complex and remain much debated. They play positive roles by stabilizing cyclin D-CDK4/6 complexes and targeting them to nuclei but they can also inhibit CDK4/6 activity.Citation16,6 Less stable cyclin D3-CDK4 complexes in p21/p27 null cells are hyperactive.Citation17–19 How can p21 and p27 shift from an inhibitory to an activation mode is still poorly understood. One debated possibility is related to different stoichiometries of the binding of these proteins to cyclin-CDK complexes.Citation8,16,20 On the other hand, as first exemplified by T187 phosphorylation of p27,Citation21 phosphorylations of Cip/Kip proteins, including by oncogenic tyrosine kinases, have also emerged as other potential mechanisms for CDK regulation.Citation22–24 Consistent with this idea, we have recently demonstrated that S130 phosphorylation of p21 inside the cyclin D-CDK4/6 complexes is catalyzed by other active CDK4/6 and CDK2 complexes and is required for the activating T172 phosphorylation of p21-bound CDK4 complexes.Citation15 Later at G1/S transition, S130 phosphorylation of p21 leads to its recognition by the SCF/Skp2 ubiquitin ligase complex and proteasomal degradation of cyclin/CDK-bound p21, hence contributing to CDK2 activation.Citation25,26
Aberrant regulation of cell cycle is a hallmark of cancer.Citation27 CDK4/6 activity is deregulated through various genetic alterations in many human tumors. These include amplification or mutation of the CDK4 and CDK6 genes, amplification of the genes encoding D-type cyclins and deletion or silencing of the CDKN2A/B gene encoding the INK4 inhibitors p16 and p15.Citation28,29 Such a deregulation is crucial for various oncogenic transformation processes suggesting that many cancer cells are addicted to high CDK4/6 activity.Citation30,31 By contrast, normal development of most tissues can take place in the absence of cyclin D-CDK4/6 complexes.Citation32–34 CDK4/6 activity thus appears as a promising therapeutic target for cancer treatment.Citation35 Several highly selective inhibitors of CDK4 and CDK6 are currently being tested in phase II/III clinical trials against a variety of pRb-proficient chemotherapy-resistant cancers (ClinicalTrials.gov).Citation36 Among them, PD0332991Citation37 (palbociclib, Pfizer) is the most advanced one. Preclinical studies have demonstrated that PD0332991 induces G1 arrest in pRb-positive cell lines and suppresses the growth of various tumors in xenografts.Citation38–43 In different cancer models, treatment with PD0332991 has not only a cytostatic effect but also triggers either senescence or apoptotic cell death of tumoral cells.Citation30,42,44,45 In the currently tested discontinuous oral treatments (e.g. given for 14 consecutive days in 21-day cycles) PD0332991 is generally well tolerated with cytopenia being the main side effect.Citation46–48 Preliminary reports indicate that PD0332991 induces an ‘unprecedented improvement of progression-free survival’ of women with advanced breast cancer.Citation49 This compound received in 2013 the FDA ‘Breakthrough Therapy’ status allowing an accelerated clinical evaluation.Citation50
In this study, we report the serendipitous observation that interruption of PD0332991 treatment paradoxically induces pRb phosphorylation and DNA synthesis in serum-deprived quiescent cells. This prompted us to further characterize the effects of PD0332991 on CDK4/6 complexes and to find out that this compound unexpectedly stabilizes activated cyclin D3-CDK4/6 complexes that are devoid of p21 and p27.
Results
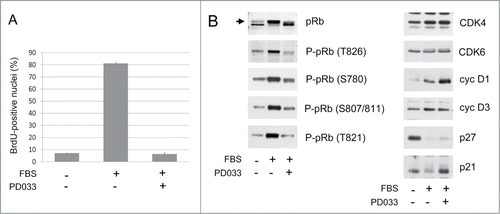
T98G glioblastoma cells are defective for CDKN2A,B,C and sensitive to CDK4/6 inhibition.Citation40 Continuous treatment of these cells with 250 nM PD0332991 for 16 h completely prevented their serum-induced entry into S-phase (). As expected, this was associated with a reduction of the CDK4/6-specific phosphorylations of pRb at T826, S780 and S807/811 and with an increase of the hypophosphorylated form of pRb. CDK4/6 inhibition did not affect the expression of CDK4 and cyclin D3 but PD0332991 increased the levels of cyclin D1 (), as observed by others.Citation41,42,51,52 Also interestingly, PD0332991 treatment prevented the disappearance of p21 but not of p27 (). p21 and p27 are marked for proteasomal degradation by the SCF/Skp2 ubiquitin ligase complex depending on their phosphorylation at S130 and T187, respectively.Citation21,25 The differential effect of PD0332991 on p21 was consistent with our observation that S130 phosphorylation of p21 is mainly effected by CDK4 or CDK6,Citation15 whereas T187 of p27 is phosphorylated by CDK2 but not by CDK4.Citation21 This p21 accumulation might also prevent the export and degradation of cyclin D1,Citation53 thus explaining in part the accumulation of cyclin D1 induced by PD0332991. Nevertheless, other mechanisms might concur to cyclin D1 accumulation in PD0332991-arrested cells. As an early marker of conversion to senescence (geroconversion), MEK-dependent hyperinduction of cyclin D1 in response to PD0332991Citation52 was also observed in the absence of a large increase of p21.Citation42
Figure 1. Inhibition of DNA synthesis and pRb phosphorylation by continuous PD0332991 treatment. (A, B) Quiescent T98G cells were stimulated (+) or not stimulated (−) with 10 % FBS for 16 h in the presence (+) or in the absence (−) of 250 nM PD0332991. (A) DNA synthesis was evaluated from duplicate dishes by counting the percentage of nuclei having incorporated BrdU during the last 30 min of stimulation (mean + range from duplicate dishes). (B) Western Blotting analyses from whole cell lysates with the indicated antibodies. Arrow indicates the hyperphosphorylated forms of pRb.
Arrest of PD0332991 treatment induces DNA synthesis and pRb phosphorylations in serum-deprived cells
In control conditions of experiments that were designed to investigate kinetics of cell cycle recovery after withdrawal of PD0332991 treatment, we unexpectedly discovered that a pre-treatment of T98G cells with PD0332991 sufficed to induce DNA synthesis, as analyzed 16 h or 24 h after elimination of PD0332991 in cells that were continuously maintained without serum (). In these experiments, cells were serum-deprived for 3 d with or without PD0332991 and then rapidly rinsed twice with PBS and subsequently incubated in culture medium without serum and PD0332991. This paradoxical induction of DNA synthesis in response to the arrest of a PD0332991 pre-treatment was confirmed in the MCF7 breast carcinoma cell line (), a well-described model of cell cycle arrest by PD0332991.Citation41
Figure 2. Withdrawal of PD0332991 induces DNA synthesis and pRb phosphorylation in serum-deprived cells. As a pre-treatment, T98G (A,C,D) and MCF7 cells (B) were serum starved during 3 d in the presence (+) or absence (−) of a 250 nM PD0332991. Cells were then washed twice in PBS to eliminate PD0332991 and cultured for the indicated times without serum. DNA synthesis (A,B) was evaluated from duplicate dishes by counting the percentage of nuclei having incorporated BrdU during the last 30 min of treatment. (A) Results from T98G cells are means + SEM from 2 independent experiments. (B) Results from MCF7 cells are means + range from duplicate dishes. (C) Western Blotting analyses with the indicated antibodies from whole cell lysates. Arrow indicates the hyperphosphorylated forms of pRb. (D) Densitometric quantitation of protein gel blotting detections of pRb phosphorylation on different residues at the 24 h time point. Results show for each phospho-specific pRb antibody the ratio between cells that were pre-treated or untreated with PD0332991 (fold change (+ PD/ −PD)).
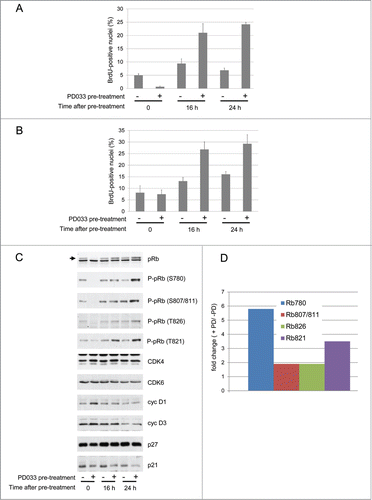
To understand how the discontinued treatment with PD0332991 could allow the cells to exit their quiescent state despite the absence of serum, we analyzed the phosphorylation of pRb. As shown in , cells that have been pre-treated with PD0332991 exhibited an elevated pRb phosphorylation compared to untreated counterparts. The effect was more pronounced 24 h after PD0332991 withdrawal and it could not be explained by modulations of the expression of cyclin D1 (which declined upon PD0332991 removal), cyclin D3, CDK4 or CDK6. p21 accumulation was nevertheless reduced in PD0332991-pretreated cells ().
Interestingly, not all CDK4/6-specific phosphorylation sites of pRb were equally affected. The effect of PD0332991 pre-treatment on the S780 phosphorylation was stronger compared to the other phosphorylation sites (S807/811 and T826)(). Quantification of the different phosphorylations of pRb detected 24 h after the withdrawal of PD0332991 revealed a fold change of about 2 for the phosphorylations at S807/811 and T826, whereas the phosphorylation of S780 increased about 6 times compared to untreated cells (). T821 phosphorylation was also more strongly increased (). We have previously demonstrated that S780 of pRb is more efficiently phosphorylated by cyclin D3-CDK4/6 complexes, whereas cyclin D1 more efficiently drives the phosphorylation at S807/811 and T826.Citation54 Moreover T821 is a specific target of CDK6 in addition to CDK2.Citation55 Our results suggested that pre-treatment of T98G cells with PD0332991 could somehow increase CDK4/6 activity and that this impact could more selectively target cyclin D3-associated activity.
PD0332991 has an unexpected positive impact on the activation of cyclin D3-CDK4/6 complexes
To evaluate whether PD0332991 could have an unsuspected positive effect on cyclin D3-CDK4/6 complexes, we next analyzed the composition and the pRb-kinase activity of these complexes, after their immunoprecipitation using cyclin D3 or CDK6 antibodies, from T98G cells that were treated or not treated with PD0332991 for 16 h in the presence or in the absence of serum. Importantly, the kinase activity of the immunoprecipitated complexes was assayed in vitro, i.e., in the presence of a physiological 2 mM ATP concentration but in the absence of added PD0332991. Continuous treatment of cells with PD0332991 in the presence of serum dramatically enhanced this in vitro activity of CDK6 complexes and of cyclin D3 complexes containing both CDK4 and CDK6 (). This effect was not explained by greater amounts of CDK4/6 or cyclin D1/D3 in the complexes. PD0332991 treatment also increased the weak basal pRb-kinase activity associated with cyclin D3 and CDK6 in serum-deprived cells ().
Figure 3. PD0332991 cell treatment durably increases the activity of cyclin D3-CDK4/6 complexes measured in vitro. (A and B) Quiescent T98G cells were stimulated (+) or not stimulated (−) with 10% FBS for the indicated periods in the presence (+) or in the absence (−) of 250 nM PD0332991. In (B), cells that were treated for 16 h with FBS and PD0332991 (FBS+PD033 pre-treat.) were then rinsed twice with PBS to eliminate the inhibitor and put back (+) or not (−) in the presence of 250 nM PD0332991 for 1, 4, 8 or 24 h. (A and B) Cell lysates were immunoprecipitated (IP) with anti-cyclin D3 (D3) and anti-CDK6 antibodies. These immunoprecipitates were incubated in vitro with ATP and a pRb fragment. The incubation mixture was then separated by SDS-PAGE and immunoblotted with the indicated antibodies to detect co-immunoprecipitated proteins and the T826 phosphorylation of the pRb fragment (pRb-kinase). High and low exposures of the pRb-kinase blot are shown in (A).
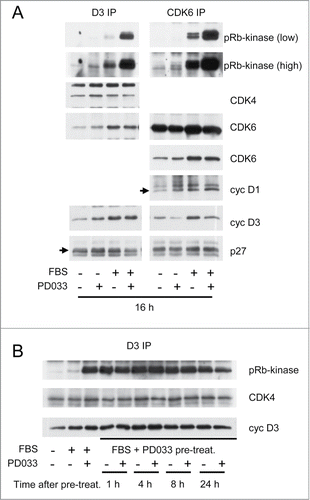
The positive effect of PD0332991 on the activation of cyclin D3-CDK4/6 complexes is fast and persists after elimination of the drug
To explain the impact of the pre-treatment with PD0332991 on the entry into S-phase observed in , the effect of the drug on the cyclin D3-associated in vitro activity should persist even after its elimination. Experiment described in shows that the dramatically increased activity of the cyclin D3 complexes was indeed maintained at least 24 h after PD0332991 withdrawal. Moreover, this stimulatory effect of PD0332991 on cyclin D3 complexes was fast as it was already detected 1 h after administration of the inhibitor (not shown).
The activating effect of PD0332991 on CDK4/6 is specific of cyclin D3 complexes that are not associated with p21 or p27
As illustrated in , the positive impact of PD0332991 on CDK4/6 activation was specific of the cyclin D3 complexes, as the compound rather decreased cyclin D1-bound activity despite the larger amount of cyclin D1 complexes in T98G cells. The selective impact on cyclin D3-bound complexes was consistent with the preferential impact of PD0332991 on the cyclin D3-dependent S780 phosphorylation of pRb versus the other CDK4/6-specific phosphorylations (as shown in ).
Figure 4. PD0332991 cell treatment specifically increases the activity of p21/p27-free cyclin D3-CDK4/6 complexes. Serum-deprived T98G (A) and HCT116 (B) cells were stimulated (+) or not stimulated (−) with 10 % FBS for the indicated periods in the presence (+) or in the absence (−) of 250 nM PD0332991. Cell lysates were immunoprecipitated (IP) with anti-cyclin D1 (D1), anti-cyclin D3 (D3), anti-p21 or anti-p27 antibodies. These immunoprecipitates were incubated in vitro with ATP and a pRb fragment. The incubation mixture was then separated by SDS-PAGE and immunoblotted with the indicated antibodies to detect co-immunoprecipitated proteins and the T826 phosphorylation of the pRb fragment (pRb-kinase). High and low exposures of the pRb-kinase and p21 blots are shown in (B).
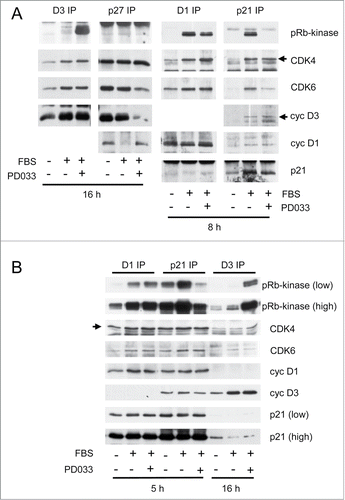
On the other hand, as previously shown, PD0332991 treatment suppressed the pRb-kinase activity associated with p21 (), which we explained by the inhibition of CDK4/6-dependent S130 phosphorylation of p21, which in turn prevents the activating T172 phosphorylation of p21-bound CDK4.Citation15 The opposite impact of PD0332991 on cyclin D3- and p21- containing complexes necessarily implied that the cyclin D3-associated complexes that were durably activated by PD0332991 could not be associated with p21. They could not be associated with p27 either, as in T98G cells p27 containing complexes are almost inactiveCitation8 and were not activated by PD0332991 (). Therefore, PD0332991 appeared to favor the formation of cyclin D3-CDK4/6 complexes that were devoid of p21 or p27. Interestingly, PD0332991 might more specifically increase the presence of p21/p27-free cyclin D3-CDK6 complexes in T98G cells, as a reduction of CDK6 association was observed in p27 and p21 immunoprecipitates in response to PD0332991, whereas the association of CDK6 with cyclin D1 and cyclin D3 was unaffected (). The association of cyclin D3 with p27 was also reduced ().
Specific activation of cyclin D3 complexes by PD0332991 was also observed in HCT116 human colon carcinoma cells (). In these cells too, PD0332991 oppositely affected the activation of complexes associated with cyclin D3 or p21.
The activating effect of PD0332991 on cyclin D3-CDK4/6 does not involve an increase of the activating phosphorylations of CDK4/6
PD0332991 could enhance cyclin D3-associated pRb-kinase activity by increasing the activating phosphorylation of CDK4 or CDK6. The relative presence of phosphorylated and non-phosphorylated forms of CDK4 and CDK6 in the different complexes was assessed as previouslyCitation8 using 2D-gel electrophoresis. We have previously identified the activated phosphorylated forms of CDK4 and CDK6 as the most negatively charged ones using different approaches: [Citation32P] phosphate incorporation, a phosphospecific antibody, in vitro phosphorylation by recombinant CAK, and analysis of T172A-mutated CDK4 or T177A-mutated CDK6.Citation8,13 Treatment of HCT116 cells () and T98G cells (not shown) with PD0332991 did not increase the phosphorylation of cyclin D3-bound CDK4. Cyclin D3-bound CDK6 was not detectably phosphorylated as previously shown,Citation13 even in PD0332991-treated cells (). Instead, PD0332991 treatment reduced the faint phosphorylation of CDK6 detected in cyclin D1 and p21 immunoprecipitates and, as previously shown,Citation15 the phosphorylation of p21-bound CDK4 (). Finally, as evaluated by 2D-gel electrophoresis separation, PD0332991 did not grossly affect the posttranslational profile of cyclin D3 () and it similarly reduced the S130 phosphorylation of p21 either bound to cyclin D3 or cyclin D1 (), as previously shown.Citation15
Figure 5. PD0332991 cell treatment neither increases the activating phosphorylation of CDK4/6 nor modifies the 2D-gel electrophoresis profile of cyclin D3. Serum-deprived HCT116 cells were stimulated (+) or not stimulated (−) with 10 % FBS for the indicated periods in the presence (+) or in the absence (−) of 250 nM PD0332991. Cell lysates were immunoprecipitated (IP) with anti-cyclin D1 (D1), anti-cyclin D3 (D3) or anti-p21 antibodies and these immunoprecipitates were separated by 2D gel electrophoresis followed by immunodetection of CDK4 and CDK6 (A), cyclin D3 (cyc D3) (B) or p21 (C). Black arrows in (A) indicate the position of T172-phosphorylated CDK4 and T177-phosphorylated CDK6. Noteworthy, detection of the very minor phosphorylated form of CDK6 in (A) required the overexposure of the blots. Colored arrows in (C) indicate the main phosphorylated forms of p21. 1P 130, 1P 98 and 2P 98,130 indicate p21 phosphorylated at S130, S98 or both sites, respectively, as previously identified and characterized.Citation15
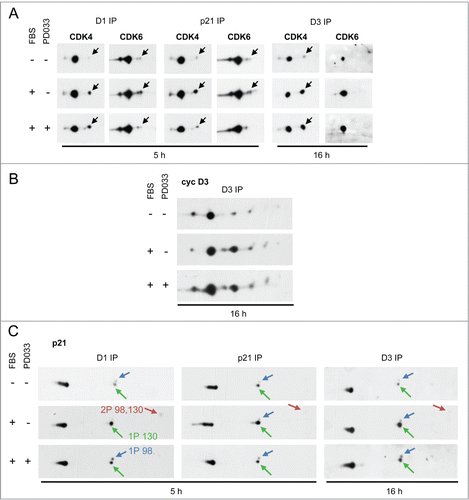
PD0332991 stabilizes and activates p21-free cyclin D3-CDK4 complexes
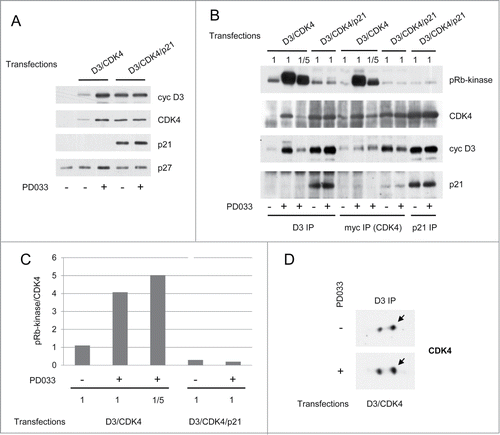
In the experiments described above, the activity of CDK4 complexes could not be investigated directly due to the lack of a CDK4 antibody that preserves activity. In order to directly compare the effect of PD0332991 cell treatment on cyclin D3-CDK4 complexes bound or not to p21, we transfected vectors of myc-tagged CDK4 and cyclin D3 with or without a p21 vector in CHO cells which do not express endogenous p21. As previously observed with p27,Citation8 co-expression of p21 with cyclin D3 and CDK4 increased their expression levels () and stability (not shown) possibly due to the stabilization of these proteins in complexes assembled by p21. In the absence of p21 expression, PD0332991 cell treatment also increased the total amount and stability of cyclin D3 and CDK4 ( and data not shown), which was associated with a much increased formation of cyclin D3-CDK4 complexes (). This effect of PD0332991 was not observed in the presence of a p21 co-expression, suggesting that PD0332991 and p21 competed for the stabilization of cyclin D3-CDK4 complexes (). PD0332991 not only increased the amount of cyclin D3-CDK4 complexes, but it even more potently stimulated their in vitro pRb-kinase activity as seen in co-immunoprecipitations using anti-cyclin D3 or anti-myc (CDK4) antibodies (). When comparing similar amounts of cyclin D3-CDK4 complexes purified from cells that were treated or not treated with PD0332991 (), or when normalizing the pRb-kinase activity to the amount of CDK4 in complexes, 4–5-fold increases of activity were observed in response to PD0332991 (). Again, activation of cyclin D3-CDK4 complexes by PD0332991 was not associated with an increased phosphorylation of CDK4 (). Finally, the effects of PD0332991 on the formation and activity of cyclin D3-CDK4 complexes did not result from a cell cycle inhibition, as CHO cells were completely insensitive to PD0332991-induced cell cycle arrest (data not shown).
Figure 6. PD0332991 stabilizes and activates p21-free cyclin D3-CDK4 complexes. CHO cells were transfected for 48 h with plasmids encoding cyclin D3 (D3) and myc-CDK4 alone or together with p21. Transfections were done in the absence (−) or presence (+) of 1 μM PD0332991. (A) Western Blotting analyses with the indicated antibodies from whole cell lysates. (B) Cell lysates were immunoprecipitated (IP) with anti-cyclin D3 (D3), anti-myc or anti-p21 antibodies. These immunoprecipitates were incubated in vitro with ATP and a pRb fragment. The incubation mixture was then separated by SDS-PAGE and immunoblotted with the indicated antibodies to detect co-immunoprecipitated proteins and the T826 phosphorylation of the pRb fragment (pRb-kinase). As CHO cells transfected with cyclin D3 and CDK4 alone and treated with PD0332991 expressed approximately 5 times more cyclin D3 and CDK4 than their untreated counterparts (as quantified from whole cell lysate immunodetections in (A)), immunoprecipitations from PD0332991-treated cells were also performed with a 1/5 volume of cell lysate (1/5), in order to compare the pRb-kinase activity of similar amounts of CDK4 complexes from cells cultured with or without PD0332991. (C) Western blotting detections obtained from the myc (CDK4)-immunoprecipitations in (B) were subjected to densitometric analysis and the ratio of pRb-kinase activity versus CDK4 was calculated. (D) Cell lysates were immunoprecipitated (IP) with anti-cyclin D3 (D3) antibody and separated by 2D-gel electrophoresis followed by CDK4 immunodetection.
To conclude, this study highlights an unexpected positive impact of PD0332991 on CDK4/6 involving the stabilization and the activation of cyclin D3-CDK4/6 complexes devoid of p21 and p27. This persistent effect is likely to generate the paradoxical mitogenic response that could be observed upon cessation of PD0332991 cell treatment.
Discussion
Many cancers harbor genetic alterations leading to aberrant activation of cyclin D-CDK4/6 complexes while keeping a normal pRb expression. In several cancer models, deregulation of CDK4/6 leads to addiction to the activity of these kinases and the CDK4/6 inhibitor PD0332991 induces either apoptosis or senescence. Direct inhibition of CDK4/6 activity thus appears as a promising strategy to treat cancers by reactivating a normal pRb function, a hope which is encouraged by recent preliminary reports from phase II clinical trials.Citation36 We have recently shown that the activation of CDK4 by phosphorylation is a central node in the cell cycle decision. Indeed, CDK4 phosphorylation is not only a converging target of various signaling cascades,Citation10–12,Citation56 but it is also influenced by positive feedbacks mediated by CDK4 and CDK2 to sustain CDK4 activation.Citation15 Most cyclin D-CDK4/6 complexes exist in cells in stoichiometric association with their stabilizing partners p21 or p27, and we have shown that PD0332991 also interferes with the activation of p21-bound CDK4 by preventing the CDK4/6-dependent phosphorylation of p21 at S130 and hence T172 phosphorylation of CDK4.Citation15 Therefore, by different mechanisms, PD0332991 interferes both with activity and activation of CDK4/6.
Here, we report a completely opposite impact of cell treatment with PD0332991 on cyclin D3-CDK4/6 complexes that are unbound to p21 or p27. This paradoxical positive effect was associated neither with an increased phosphorylation of CDK4 or CDK6 nor with a modification of the 2D-gel electrophoresis profile of cyclin D3. Collectively, our data suggest that PD0332991 durably stabilizes the assembly of cyclin D3-CDK6 and cyclin D3-CDK4 complexes that are devoid of p21 and p27 and, as a result, become hyperactive upon arrest of PD0332991 treatment. Even in cells such as T98G and MCF-7 that are bona fide models of inhibition of pRb phosphorylation and G1 cell cycle arrest in response to CDK4/6 inhibition by PD0332991,Citation40,41 this accumulation of activated p21/p27-free cyclin D3-CDK4/6 complexes was sufficient to generate a mitogenic response in serum-deprived quiescent cells upon PD0332991 withdrawal.
How could PD0332991 favor the formation of cyclin D3-CDK4/6 complexes that are not bound to p21 or p27 ? p21 and p27 bind the CDK moiety of cyclin-CDK complexes by inserting their 310 helix into the catalytic ATP-binding cleft, thus inhibiting the activity.Citation57–59 It is therefore likely that PD0332991, as a high affinity ATP-competitive drug, would also compete for p21/p27 binding to CDK4 and CDK6. Moreover, PD0332991 appeared to somehow mimic the effect of p21 binding as a stabilizing factor for cyclin D3-CDK4/6 complexes. Curiously, unlike p21 binding, the stabilizing effect of PD0332991 on cyclin D3-CDK4/6 did not require a stable interaction but it was durable, even persisting 24 h after withdrawal of the drug. It also remains unclear why this effect specifically affects complexes associated with cyclin D3 and not those associated with cyclin D1. Cyclin D3-CDK6 complexes were also shown to be specifically more resistant to p21 and p27, perhaps due to their higher intrinsic stability.Citation60 Further crystallographic and biophysical investigations should evaluate conformational changes induced by PD0332991 interaction with CDK4 and CDK6 and their impact on the association with D-type cyclins. Noteworthy, direct conformational changes were also invoked to explain the dimerization/transactivation effects of ATP-competitive RAF inhibitors on wild-type RAF, leading to paradoxical activation of the MAPK pathway and enhanced proliferation.Citation61,62
PD0332991 is undeniably the most promising cell cycle based therapy to date for most pRb-positive cancers. Acquired resistance to this drug may arise through activation of CDK2.Citation41,63 Another side effect of PD0332991 treatment could be the acquisition of a more invasive phenotype as observed in pancreatic cancer cell lines.Citation51 The clinical efficacy of PD0332991 and other CDK4/6 inhibitors, either as a monotherapy or in combination with various other drugs, was until now only tested in discontinuous treatments with one week off treatment every 2 or 3 weeks (clinicaltrials.gov).Citation36 Especially in tumors that express high levels of cyclin D3, the undesired aspect of PD0332991 pharmacology demonstrated here could thus allow hyper-activation of cyclin D3-CDK4/6 complexes and proliferation of cancer cells during the treatment interruption. This might explain tumor response failures and favor acquired resistances, suggesting that discontinuous PD0332991 protocols might need to be carefully considered. On the other hand, a burst of tumoral cell proliferation upon withdrawal of PD0332991 treatment might also open a window of increased responsiveness to genotoxic chemo/radiotherapeutics. This might be exploited in sequential treatments which would alternate PD0332991 and genotoxic therapeutics. Finally, it remains to be verified whether such a stabilization of activated CDK4/6 complexes could also occur in response to the other clinically assessed CDK4/6 inhibitors including LEE-011 and LY2835219. Unfortunately these molecules were not made widely available for academic research.
Materials and Methods
Cell culture, BrdU incorporation and transfection
T98G, HCT116 and CHO cells were cultured as described. Citation8,11,15 After starvation without FBS for 3 d, cells were growth stimulated by 10 % FBS. MCF7 cells were cultured as describedCitation64 in the presence of 5 % FBS and 6 ng/ml insulin. They were then starved for 3 d in DMEM without phenol red, insulin and FBS. PD0332991 (Selleck Chemicals) was dissolved in DMSO and used at a final concentration of 250 nM or 1 μM. In all control conditions, cells were given the same concentration of DMSO. DNA replicating cells were identified by a 30 min incubation with BrdU.Citation65 CHO cells were transfected for 48 h using JetPEI (Polyplus Transfection) with 6 μg of pcDNA3 vector encoding myc-tagged CDK4 and Xpress-cyclin D3, and 6 μg of PE vector encoding p21.Citation15
Immunoblot analyses
Equal amounts of whole cell extract proteins were separated according to molecular mass and immunodetected using the following antibodies: monoclonal antibodies against cyclin D1 (DCS-6), cyclin D3 (DCS-22) (from Neomarkers), anti-total pRb monoclonal antibody (#554136, BD PharMingen), monoclonal anti-phospho-pRb (T826) antibody (Abcam-Epitomics), monoclonal anti-phospho-pRb (S780 and S807/811) antibodies (Cell Signaling), polyclonal anti-phospho-pRb (T821) antibody (Biosource), monoclonal (DCS-31) or polyclonal (H-22) anti-CDK4 antibodies, polyclonal antibodies against p21 (C-19), p27 (C-15) and CDK6 (C-21) (all from Santa Cruz Biotechnology). Secondary antibodies were either coupled to horseradish peroxidase (Amersham Biosciences) for detection by enhanced chemiluminescence (Western Lightning, Perkin-Elmer) or to DyLight 680 and 800 (Pierce Biotechnology) for infrared fluorescence detection using the Odyssey scanner (LI-COR).
Immunoprecipitation
Co-immunoprecipitations were performed as describedCitation8,10 using monoclonal antibodies against cyclin D1 (DCS-11), cyclin D3 (DCS-28) (Neomarkers), a mixture of the K25020 anti-p27 monoclonal antibody from BD-PharMingen and the C-15 p27 polyclonal antibody (Santa Cruz Biotechnology), polyclonal antibodies against CDK6 (C-21) or p21 (C-19) (Santa Cruz Biotechnology) and monoclonal anti-myc tag (9E10) (Santa Cruz Biotechnology).
pRb-kinase assay
As described,Citation8,10 immunoprecipitated protein complexes were incubated with 2 mM ATP and a recombinant pRb fragment (Sigma), before SDS-PAGE separation of the incubation mixture and western blotting detection of the T826-phosphorylation of the pRb fragment, cyclin D1, cyclin D3, CDK4, CDK6, p21 and p27.
Two-dimensional (2D)-gel electrophoresis
As described,Citation8 immunoprecipitated protein complexes were denatured in a buffer containing 7 M urea and 2 M thiourea. Proteins were separated by isoelectric focusing on immobilized linear pH gradient (pH 3 to 10) strips, separated by SDS-PAGE and immunoblotted.
Disclosure of Potential Conflict of Interest
No conflicts of interest were disclosed.
Acknowledgments
HCT116 and MCF7 cells were provided by Robert Fisher (Mount Sinai School of Medicine, New York) and Geert Berx (VIB, University of Ghent), respectively. p21 expression plasmid was provided by Ludger Hengst (Innsbruck Medical University). SP is a FRS-FNRS Scientific Research Worker, BC is a fellow of the Fonds pour la Formation à la Recherche dans l’Industrie et l’Agriculture (FRIA), and PPR is a Senior Research Associate of the FRS-FNRS. We thank Dr Eric Raspé for helpful discussions and Prof. Jacques Dumont for continued interest and support.
Additional information
Funding
References
- Sherr CJ. D-type cyclins. Trends Biochem Sci 1995; 20:187-90; PMID:7610482; http://dx.doi.org/10.1016/S0968-0004(00)89005-2
- Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway and the restriction point. Curr Opin Cell Biol 1996; 8:805-14; PMID:8939678; http://dx.doi.org/10.1016/S0955-0674(96)80081-0
- Bockstaele L, Coulonval K, Kooken H, Paternot S, Roger PP. Regulation of CDK4. Cell Division 2006; 1:25; PMID:17092340; http://dx.doi.org/10.1186/1747-1028-1-25
- Choi YJ, Anders L. Signaling through cyclin D-dependent kinases. Oncogene 2014; 33:1890-903; PMID:23644662; http://dx.doi.org/10.1038/onc.2013.137
- Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol 1998; 18:753-61; PMID:9447971.
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999; 13:1501-12; PMID:10385618; http://dx.doi.org/10.1101/gad.13.12.1501
- Kato JY, Matsuoka M, Strom DK, Sherr CJ. Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase. Mol Cell Biol 1994; 14:2713-21; PMID:8139570; http://dx.doi.org/10.1128/MCB.14.4.2713
- Bockstaele L, Kooken H, Libert F, Paternot S, Dumont JE, de Launoit Y, Roger PP, Coulonval K. Regulated activating Thr172 phosphorylation of cyclin-dependent kinase 4(CDK4): its relationship with cyclins and CDK "inhibitors." Mol Cell Biol 2006; 26:5070-85; PMID:16782892; http://dx.doi.org/10.1128/MCB.02006-05
- Paternot S, Bockstaele L, Bisteau X, Kooken H, Coulonval K, Roger PP. Rb inactivation in cell cycle and cancer: The puzzle of highly regulated activating phosphorylation of CDK4 vs. constitutively active CDK-activating kinase. Cell Cycle 2010; 9:689-99; PMID:20107323; http://dx.doi.org/10.4161/cc.9.4.10611
- Paternot S, Coulonval K, Dumont JE, Roger PP. Cyclic AMP-dependent phosphorylation of cyclin D3-bound CDK4 determines the passage through the cell cycle restriction point in thyroid epithelial cells. J Biol Chem 2003; 278:26533-40; PMID:12730225; http://dx.doi.org/10.1074/jbc.M302492200
- Paternot S, Roger PP. Combined inhibition of MEK and mammalian target of rapamycin abolishes phosphorylation of cyclin-dependent kinase 4 in glioblastoma cell lines and prevents their proliferation. Cancer Res 2009; 69:4577-81; PMID:19458076; http://dx.doi.org/10.1158/0008-5472.CAN-08-3260
- Rocha AS, Paternot S, Coulonval K, Dumont JE, Soares P, Roger PP. Cyclic AMP inhibits the proliferation of thyroid carcinoma cell lines through regulation of CDK4 phosphorylation. Mol Biol Cell 2008; 19:4814-25; PMID:18799615; http://dx.doi.org/10.1091/mbc.E08-06-0617
- Bockstaele L, Bisteau X, Paternot S, Roger PP. Differential regulation of cyclin-dependent kinase 4 (CDK4) and CDK6, evidence that CDK4 might not be activated by CDK7, and design of a CDK6 activating mutation. Mol Cell Biol 2009; 29:4188-200; PMID:19487459; http://dx.doi.org/10.1128/MCB.01823-08
- Merzel Schachter M., Merrick KA, Larochelle S, Hirschi A, Zhang C, Shokat K, Rubin SM, Fisher RP. A Cdk7-Cdk4 T-Loop phosphorylation cascade promotes G1 progression. Mol Cell 2013; 50:250-60; PMID:23622515; http://dx.doi.org/10.1016/j.molcel.2013.04.003
- Bisteau X, Paternot S, Colleoni B, Ecker K, Coulonval K, De Groote P, Declercq W, Hengst L, Roger PP. CDK4 T172 phosphorylation is central in a CDK7-dependent bidirectional CDK4/CDK2 interplay mediated by p21 phosphorylation at the restriction point. PLoS Genet 2013; 9:e1003546; PMID:23737759; http://dx.doi.org/10.1371/journal.pgen.1003546
- LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev 1997; 11:847-62; PMID:9106657; http://dx.doi.org/10.1101/gad.11.7.847
- Bagui TK, Jackson RJ, Agrawal D, Pledger WJ. Analysis of cyclin D3-cdk4 complexes in fibroblasts expressing and lacking p27(kip1) and p21(cip1). Mol Cell Biol 2000; 20:8748-57; PMID:11073976; http: //dx.doi.org /10.1128/MCB.20.23.8748-8757.2000
- Bagui TK, Mohapatra S, Haura E, Pledger WJ. P27Kip1 and p21Cip1 are not required for the formation of active D cyclin-cdk4 complexes. Mol Cell Biol 2003; 23:7285-90; PMID:14517297; http://dx.doi.org/10.1128/MCB.23.20.7285-7290.2003
- Sugimoto M, Martin N, Wilks DP, Tamai K, Huot TJ, Pantoja C, Okumura K, Serrano M, Hara E. Activation of cyclin D1-kinase in murine fibroblasts lacking both p21(Cip1) and p27(Kip1). Oncogene 2002; 21:8067-74; PMID:12444543; http://dx.doi.org/10.1038/sj.onc.1206019
- Blain SW, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J Biol Chem 1997; 272:25863-72; PMID:9325318; http://dx.doi.org/10.1074/jbc.272.41.25863
- Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev 1997; 11:1464-78; PMID:9192873; http://dx.doi.org/10.1101/gad.11.11.1464
- Grimmler M, Wang Y, Mund T, Cilensek Z, Keidel EM, Waddell MB, Jakel H, Kullmann M, Kriwacki RW, Hengst L. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 2007; 128:269-80; PMID:17254966; http://dx.doi.org/10.1016/j.cell.2006.11.047
- Jakel H, Peschel I, Kunze C, Weinl C, Hengst L. Regulation of p27 (Kip1) by mitogen-induced tyrosine phosphorylation. Cell Cycle 2012; 11:1910-7; PMID:22580455; http://dx.doi.org/10.4161/cc.19957
- Ray A, James MK, Larochelle S, Fisher RP, Blain SW. p27Kip1 inhibits cyclin D-cyclin-dependent kinase 4 by two independent modes. Mol Cell Biol 2009; 29:986-99; PMID:19075005; http://dx.doi.org/10.1128/MCB.00898-08
- Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem 2003; 278:25752-7; PMID:12730199; http://dx.doi.org/10.1074/jbc.M301774200
- Zhu H, Nie L, Maki CG. Cdk2-dependent Inhibition of p21 stability via a C-terminal cyclin-binding motif. J Biol Chem 2005; 280:29282-8; PMID:15964852; http://dx.doi.org/10.1074/jbc.M407352200
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell 2002; 2:103-12; PMID:12204530; http://dx.doi.org/10.1016/S1535-6108(02)00102-2
- Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta 2002; 1602:73-87; PMID:11960696; http://dx.doi.org/10.1016/S0304-419X(02)00037-9
- Choi YJ, Li X, Hydbring P, Sanda T, Stefano J, Christie AL, Signoretti S, Look AT, Kung AL, von Boehmer H, et al. The requirement for cyclin d function in tumor maintenance. Cancer Cell 2012; 22:438-51; PMID:23079655; http://dx.doi.org/10.1016/j.ccr.2012.09.015
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009; 9:153-66; PMID:19238148; http://dx.doi.org/10.1038/nrc2602
- Kozar K, Sicinski P. Cell cycle progression without cyclin D-CDK4 and cyclin D-CDK6 complexes. Cell Cycle 2005; 4:388-91; PMID:15738651; http://dx.doi.org/10.4161/cc.4.3.1551
- Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 2004; 118:493-504; PMID:15315761; http://dx.doi.org/10.1016/j.cell.2004.08.002
- Berthet C, Klarmann KD, Hilton MB, Suh HC, Keller JR, Kiyokawa H, Kaldis P. Combined loss of Cdk2 and Cdk4 results in embryonic lethality and Rb hypophosphorylation. Dev Cell 2006; 10:563-73; PMID:16678773; http://dx.doi.org/10.1016/j.devcel.2006.03.004
- Knudsen ES, Wang JY. Targeting the RB-pathway in cancer therapy. Clin Cancer Res 2010; 16:1094-9; PMID:20145169; http://dx.doi.org/10.1158/1078-0432.CCR-09-0787
- Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin Cancer Res 2014; 20(13):3379-83; PMID:24795392; http://dx.doi.org/10.1158/1078-0432.CCR-13-1551
- Toogood PL, Harvey PJ, Repine JT, Sheehan DJ, VanderWel SN, Zhou H, Keller PR, McNamara DJ, Sherry D, Zhu T et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem 2005; 48:2388-406; PMID:15801831; http://dx.doi.org/10.1021/jm049354h
- Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004; 3:1427-38; PMID:15542782
- Baughn LB, Di Liberto M, Wu K, Toogood PL, Louie T, Gottschalk R, Niesvizky R, Cho H, Ely S, Moore MA, et al. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res 2006; 66:7661-7; PMID:16885367; http://dx.doi.org/10.1158/0008-5472.CAN-06-1098
- Michaud K, Solomon DA, Oermann E, Kim JS, Zhong WZ, Prados MD, Ozawa T, James CD, Waldman T. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res 2010; 70:3228-38; PMID:20354191; http://dx.doi.org/10.1158/0008-5472.CAN-09-4559
- Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene 2010; 29:4018-32; PMID:20473330; http://dx.doi.org/10.1038/onc.2010.154
- Leontieva OV, Blagosklonny MV. CDK4/6-inhibiting drug substitutes for p21 and p16 in senescence: duration of cell cycle arrest and MTOR activity determine geroconversion. Cell Cycle 2013; 12:3063-9; PMID:23974099; http://dx.doi.org/10.4161/cc.26130
- Dean JL, McClendon AK, Hickey TE, Butler LM, Tilley WD, Witkiewicz AK, Knudsen ES. Therapeutic response to CDK4/6 inhibition in breast cancer defined by ex vivo analyses of human tumors. Cell Cycle 2012; 11:2756-61; PMID:22767154; http://dx.doi.org/10.4161/cc.21195
- Puyol M, Martin A, Dubus P, Mulero F, Pizcueta P, Khan G, Guerra C, Santamaria D, Barbacid M. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 2010; 18:63-73; PMID:20609353; http://dx.doi.org/10.1016/j.ccr.2010.05.025
- Sawai CM, Freund J, Oh P, Ndiaye-Lobry D, Bretz JC, Strikoudis A, Genesca L, Trimarchi T, Kelliher MA, Clark M, et al. Therapeutic targeting of the cyclin D3:CDK4/6 complex in T cell leukemia. Cancer Cell 2012; 22:452-65; PMID:23079656; http://dx.doi.org/10.1016/j.ccr.2012.09.016
- Schwartz GK, Lorusso PM, Dickson MA, Randolph SS, Shaik MN, Wilner KD, Courtney R, O'Dwyer PJ. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1). Br J Cancer 2011; 104:1862-8; PMID:21610706; http://dx.doi.org/10.1038/bjc.2011.177
- Leonard JP, LaCasce AS, Smith MR, Noy A, Chirieac LR, Rodig SJ, Yu JQ, Vallabhajosula S, Schoder H, English P, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood 2012; 119:4597-607; PMID:22383795; http://dx.doi.org/10.1182/blood-2011-10-388298
- Dickson MA, Tap WD, Keohan ML, D'Angelo SP, Gounder MM, Antonescu CR, Landa J, Qin LX, Rathbone DD, Condy MM, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol 2013; 31:2024-8; PMID:23569312; http://dx.doi.org/10.1200/JCO.2012.46.5476
- Finn RS, Crown JP, Lang I, Boer K, Bondarenko I, Kulyk S, Ettl J, Patel R, Pinter T, Schmidt M, et al. Results of a randomized phase 2 study of PD 0332991, a cyclin-dependent kinase (CDK) 4/6 inhibitor, in combination with letrozole vs letrozole alone for first-line treatment of ER+/HER2– advanced breast cancer (BC). Cancer Research 2012; 72: Abstract nr S1-6; http://dx.doi.org/10.1158/1538-7445.AM2012-3858.
- Guha M. Blockbuster dreams for Pfizer's CDK inhibitor. Nat Biotechnol 2013; 31:187; PMID:23471056; http://dx.doi.org/10.1038/nbt0313-187a
- Liu F, Korc M. Cdk4/6 inhibition induces epithelial-mesenchymal transition and enhances invasiveness in pancreatic cancer cells. Mol Cancer Ther 2012; 11:2138-48; PMID:22869556; http://dx.doi.org/10.1158/1535-7163.MCT-12-0562
- Leontieva OV, Demidenko ZN, Blagosklonny MV. MEK drives cyclin D1 hyperelevation during geroconversion. Cell Death Differ 2013; 20:1241-9; PMID:23852369; http://dx.doi.org/10.1038/cdd.2013.86
- Alt JR, Gladden AB, Diehl JA. p21(Cip1) Promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J Biol Chem 2002; 277:8517-23; PMID:11751903; http://dx.doi.org/10.1074/jbc.M108867200
- Paternot S, Arsenijevic T, Coulonval K, Bockstaele L, Dumont JE, Roger PP. Distinct specificities of pRb phosphorylation by CDK4 activated by cyclin D1 or cyclin D3: differential involvement in the distinct mitogenic modes of thyroid epithelial cells. Cell Cycle 2006; 5:61-70; PMID:16294008; http://dx.doi.org/10.4161/cc.5.1.2265
- Takaki T, Fukasawa K, Suzuki-Takahashi I, Semba K, Kitagawa M, Taya Y, Hirai H. Preferences for phosphorylation sites in the retinoblastoma protein of D-type cyclin-dependent kinases, Cdk4 and Cdk6, in vitro. J Biochem (Tokyo) 2005; 137:381-6; PMID:15809340; http://dx.doi.org/10.1093/jb/mvi050
- Blancquaert S, Wang L, Paternot S, Coulonval K, Dumont JE, Harris TE, Roger PP. Cyclic AMP-dependent activation of mammalian target of rapamycin (mTOR) in thyroid cells. implication in mitogenesis and activation of CDK4. Mol Endocrinol 2010; 24:1453-68; PMID:20484410; http://dx.doi.org/10.1210/me.2010-0087
- Pavletich NP. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol 1999; 287:821-8; PMID:10222191; http://dx.doi.org/10.1006/jmbi.1999.2640
- Wang Y, Fisher JC, Mathew R, Ou L, Otieno S, Sublet J, Xiao L, Chen J, Roussel MF, Kriwacki RW. Intrinsic disorder mediates the diverse regulatory functions of the Cdk inhibitor p21. Nat Chem Biol 2011; 7:214-21; PMID:21358637; http://dx.doi.org/10.1038/nchembio.536
- Chen J, Saha P, Kornbluth S, Dynlacht BD, Dutta A. Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol 1996; 16:4673-82; PMID:8756624
- Lin J, Jinno S, Okayama H. Cdk6-cyclin D3 complex evades inhibition by inhibitor proteins and uniquely controls cell's proliferation competence. Oncogene 2001; 20:2000-9; PMID:11360184; http://dx.doi.org/10.1038/sj.onc.1204375
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010; 464:427-30; PMID:20179705; http://dx.doi.org/10.1038/nature08902
- Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G. et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010; 464:431-5; PMID:20130576; http://dx.doi.org/10.1038/nature08833
- Wang L, Wang J, Blaser BW, Duchemin AM, Kusewitt DF, Liu T, Caligiuri MA, Briesewitz R. Pharmacologic inhibition of CDK4/6: mechanistic evidence for selective activity or acquired resistance in acute myeloid leukemia. Blood 2007; 110:2075-83; PMID:17537993; http://dx.doi.org/10.1182/blood-2007-02-071266
- Coopman PJ, Bracke ME, Lissitzky JC, De Bruyne GK, Van Roy FM, Foidart JM, Mareel MM. Influence of basement membrane molecules on directional migration of human breast cell lines in vitro. J Cell Sci 1991; 98 ( Pt 3):395-401; PMID:2055965
- Roger PP, Baptist M, Dumont JE. A mechanism generating heterogeneity in thyroid epithelial cells: suppression of the thyrotropin/cAMP-dependent mitogenic pathway after cell division induced by cAMP-independent factors. J Cell Biol 1992; 117:383-93; PMID:1313816; http://dx.doi.org/10.1083/jcb.117.2.383
