Abstract
The presence of γH2AX foci on apparently intact mitotic chromosomes is controversial because they challenge the assumed relationship between γH2AX foci and DNA double-strand breaks (DSBs). In this work, we show that after irradiation during interphase, a variety of γH2AX foci are scored in mitotic cells. Surprisingly, approximately 80% of the γH2AX foci spread over apparently undamaged chromatin at Terminal or Interstitial positions and they can display variable sizes, thus being classified as Small, Medium and Big foci. Chromosome and chromatid breaks that reach mitosis are spotted with Big (60%) and Medium (30%) Terminal γH2AX foci, but very rarely are they signaled with Small γH2AX foci. To evaluate if Interstitial γH2AX foci might be signatures of misrejoining, an mFISH analysis was performed on the same slides. The results show that Interstitial γH2AX foci lying on apparently intact chromatin do not mark sites of misrejoining, and that misrejoined events were never signaled by a γH2AX foci during mitosis. Finally, when analyzing the presence of other DNA-damage response (DDR) factors we found that all γH2AX foci—regardless their coincidence with a visible break—always colocalized with MRE11, but not with 53BP1. This pattern suggests that these γH2AX foci may be hallmarks of both microscopically visible and invisible DNA damage, in which an active, although incomplete or halted DDR is taking place.
Abbreviations
| AU | = | arbitrary units |
| DSB | = | DNA double-strand break |
| FISH | = | fluorescence in situ hybridization |
| IRIF | = | ionizing radiation induced foci |
| FI | = | fluorescence intensity |
| mFISH | = | multicolor fluorescence in situ hybridization |
| MRN complex | = | MRE11-Rad50-Nbs1 complex |
| SD | = | standard deviation |
| TIF | = | telomere-dysfunction induced foci |
Introduction
DNA double-strand breaks (DSBs) are hazardous lesions for the genome, and effective repair relies on the rapid sensing and signaling of the damage. Sensing of the DNA damage involves the phosphorylation of histone H2AX molecules to generate a γH2AX domain that extends over megabase stretches of DNA on both sides of the DNA break.Citation1-3 A general response of the cell to the presence of DSBs is the appearance of phosphorylated H2AX foci because it occurs during all kinds of endogenous DNA damaging events such as apoptosis, class switch, V(D)J and meiotic recombination. Blocking of replication forks also leads to γH2AX foci formation, although the fraction of γH2AX foci that actually represent broken forks seems to be significantly lower than the fraction of γH2AX foci that represent DSBs of exogenous or endogenous origin.Citation4 Finally, DNA breakage generated by exogenous agents such as ionizing radiation or radiomimetic drugs also results in extensive formation of γH2AX foci.Citation3,Citation5-7 The spreading of H2AX phosphorylation functions both as a repair protein docking platform and as a chromatin remodelling factor that exposes the break sites and favors the recruitment and function of several repair proteins.Citation8,9 In this regard γH2AX knockout mice have increased ionizing radiation sensitivity accompanied by genomic instability due to DNA repair deficiencies.Citation10,11
Phosphorylated H2AX forms foci immediately after DNA damage induction by ionizing radiation. These γH2AX IRIF (Ionizing Radiation Induced Foci) are detectable with immunostaining or cytometry techniques as soon as 3 minutes afterwards, and the maximum number of foci is detected 30–60 minutes after irradiation.Citation6 It is generally not possible to independently determine the presence of DSBs because visualization of γH2AX foci is several orders of magnitude more sensitive than other methods of DSB detection.Citation12 Each γH2AX focus contains at least several hundreds of γH2AX molecules, and the number of foci has been found to closely correlate with the number of radiation-induced DSBs at doses lower than 5Gy, supporting the notion that the number of γH2AX foci and the number of DSBs are equivalent, at least in the early stages of repair.Citation3,Citation12-16 Very soon after irradiation γH2AX foci are numerous and small, and they disappear along with the resolution of DNA damage. Indeed, the kinetics of formation and disappearance of γH2AX foci closely correlated with the induction and repair of DSBs.Citation12,14,17 Based on all these results, the scoring of γH2AX foci has become extremely useful to monitor DSB induction and resolution. Nonetheless, most of the data regarding the correlation between γH2AX foci disappearance and DSB repair has been obtained from interphase nuclei, and little is known about how mitotic cells deal with DSBs. Surprisingly, when analyzing γH2AX foci on mitotic chromosomes, some of these persistent foci have been detected in apparently intact chromosomes.Citation18-22
Mitotic mouse germ cells irradiated at G2/M presented most γH2AX foci on apparently normal chromosomes, leading the authors to suggest that phosphorylation of H2AX can remain after DNA end ligation.Citation18 Other studies have also reported discrepancies between the high number of γH2AX foci scored on seemingly intact chromosomes and the lower amount of unrepaired DSBs remaining after irradiation, measured by techniques such as Pulsed Field Gel Electrophoresis or comet assays.Citation20,23,24 The authors suggested that the condensed structure of mitotic chromosomes might interfere with proper γH2AX foci disappearance, as the highly condensed mitotic chromatin may disturb or delay H2AX dephosphorylation, or its replacement by new non-phosphorylated histones, after the repair process has taken place.Citation20,25 Another explanation for these γH2AX foci on apparently intact chromosomes is that they may persist in sites where illegitimate broken ends have been tethered, as suggested by Suzuki and colleagues.Citation22 Indeed, they showed that most of the anaphase bridges analyzed after irradiation were enriched with γH2AX. Because anaphase bridges are the result of illegitimate rejoining between DNA broken ends, the authors proposed that phosphorylated H2AX histone may persist in rejoined breaks, maybe indicating the presence of an aberrant chromatin structure due to misrejoining events.Citation22 Finally, it has also been suggested that some γH2AX foci could be signatures of replicative stress that reach M phase. It has been described that replication stress may induce DSB formation leading to γH2AX foci appearance.Citation26 In line with this observation, UV-sensitive cells have more residual γH2AX foci in mitosis after G1 γ-irradiation.Citation24 In short, these results would imply that the disappearance of γH2AX foci does not necessarily coincide with the kinetics of DSB repair, and that γH2AX may persist in sites where DSBs have already been rejoined, thus, questioning the currently assumed relationship between γH2AX foci and the presence of unrepaired DSBs.
To explore this possibility, we have irradiated cells and examined mitotic chromosomes after DNA damage infliction. Chromosome rearrangements resulting from illegitimate repair have been identified, and the presence of γH2AX at the rejoining point has been evaluated with negative results. This suggests that residual γH2AX foci on apparently intact mitotic chromosomes do not correspond to scars that signal chromatin sites where misrejoining events had taken place. We have also examined the presence of other repair proteins at γH2AX foci of metaphase chromosomes. The data reveal that, although γH2AX foci do not always lie on sites of cytologically visible DNA damage, they do always co-localize with MRE11, a DNA damage signaling and repair factor, suggesting that γH2AX signaling indicates sites of unresolved DNA damage.
Results
γH2AX foci on mitotic chromosomes show variable sizes and fluorescence intensities
Metaphase spreads from exponentially growing cells were obtained from non-irradiated and irradiated lymphoblastoid cells derived from a healthy donor. The karyotype stability, repair efficiency and normality of the DNA-repair kinetics of this cell line have been evaluated in previous studies.Citation27,28 After immunostaining of phosphorylated H2AX, mitotic chromosomes displayed some γH2AX foci of big size, and these were accompanied by a population of much smaller foci, but still clearly different from the background. Previous studies have reported the presence of γH2AX foci of variable sizes in mitotic chromosomes.Citation18,21,24 Thus, 665 γH2AX foci were scored in 68 metaphase spreads using the Foci Picker 3D plugin from ImageJ, and settings were used that allowed identification and counting of all types of γH2AX foci. Using this approach, the mean number of γH2AX foci per metaphase was 7.42 before irradiation and increased to 11.1 forty-eight hours after irradiation. Although the increase in the total number of γH2AX foci after irradiation was statistically significant (Fisher's Exact Test; p = 0.01), the number of γH2AX foci in unirradiated cells was unexpectedly high. Importantly, most of the mitotic γH2AX foci scored in unirradiated cells were visually small and of low fluorescence intensity and they usually appeared as a single focus in one of the chromatids. In contrast, the fraction of bigger and brighter γH2AX foci clearly increased after irradiation, and these foci spanned through both chromatids as isolocus paired foci. Because the Foci Picker 3D plugin provides measures the fluorescence intensity (FI) of all scored foci measured in arbitrary units, γH2AX foci scored before and after irradiation were classified into 3 categories according to their FI (Supplemental ): Small, corresponding to the foci with the lower FI (58.37 ± 3.77 AU; Arbitrary Units); Medium, corresponding to the foci with median FI (124.97 ± 3.34 AU); and Big, corresponding to the foci with the highest FI scored (179.78 ± 4.40 AU) (). This γH2AX foci classification was statistically validated as the Small, Medium and Big foci showed significant differences in their FI both before and after irradiation (Dunn's Multiple Comparisons Test; P < 0.05 to P < 0.001; ). Next, foci were also classified regarding their position in the chromosome (). Terminal γH2AX foci were (i) those located at telomeric or sub-telomeric positions in complete chromosomes and (ii) those located at the end of a broken chromosome, co-localizing with the break. Interstitial γH2AX foci were (i) those located within an apparently intact chromosome or chromatid arm and (ii) those that co-localized with a chromatid break. Most of the scored γH2AX foci were Terminal foci both before (∼80%) and after irradiation (76.6%) (). In the unirradiated metaphases, these Terminal foci were mostly Small (52.2%), and very few Big foci (6.2%) were found. However, after irradiation, the relative frequency of Small Terminal foci dropped to almost half (27.3%; Fisher's Exact Test; P < 0.0001) while Big Terminal foci increased 5 times and reached 31.4% (Fisher's Exact Test; P < 0.0001; ). Therefore, the absolute frequency of Big Terminal foci increases after irradiation (0.458 vs 3.477 γH2AX foci/cell) while that of Small Terminal γH2AX foci remains stable (3.875 vs 3.027 γH2AX foci/cell; ). The proportion of Medium Terminal foci did not significantly change after irradiation.
Table 1. Classification of γH2AX foci according to their fluorescence intensity. Foci are classified in Small, Medium or Big according to their Fluorescence Intensity (FI), which is given in arbitrary units (AU). Mtp: number of metaphases analyzed in each slide; N: number of γH2AX foci scored in each category and slide; Mean and SD: mean FI and standard deviation (SD) of all foci scored for a given category and slide; Mean and SD of all measures: mean and SD of all means obtained for each category. Each slide corresponds to a different experiment
Table 2. Classification of γH2AX foci according to their fluorescence intensity and position. Number, type and position within the chromosome of the γH2AX foci scored in 5 different slides (see ). N: number of metaphases analyzed. The frequency per cell is shown in brackets. Superscript letters indicate statistically significant differences in the number of γH2AX foci scored within the same categories before and after irradiation (Fisher's Exact Test; P < 0.0001)
Figure 1. γH2AX foci in mitotic chromosomes present different fluorescence intensities. (A) 665 γH2AX foci from 68 metaphases were scored using FociPicker 3D. The Fluorescence Intensity (FI) of each focus was plotted and these measures were used to classify them. The FI values of all scored foci ranged from 25 to 225 (arbitrary units; AU) and were classified into 3 categories: Small, corresponding to the foci with the lower FI (from 25 to 92.9 AU); Medium, corresponding to the foci with median FI (from 93 to 158.9 AU); and Big, corresponding to the foci with the highest FI (from 159 to 225 AU). The mean FI of Small foci (S) was statistically different from that of Medium (M) and Big foci (B) both before and after irradiation (Si, Mi, Bi). The same differences were found for Medium and Big foci (Dunn's Multiple Comparisons Test; P < 0.05 to P < 0.001). The means and standard deviation (SD) shown in the graph are calculated from 2 different slides in non irradiated cells and from 3 different slides in irradiated cells (). (B) Examples of γH2AX foci of different FI located either at a Terminal or at an Interstitial position within the chromosome are shown. (C) γH2AX foci were classified regarding their FI (Big, Medium or Small) and their position within the chromosome (Terminal or Interstitial). The percentages of each type of foci are shown. Asterisks show statistically significant differences in the number of foci (Fisher's Exact Test; P < 0.0001).
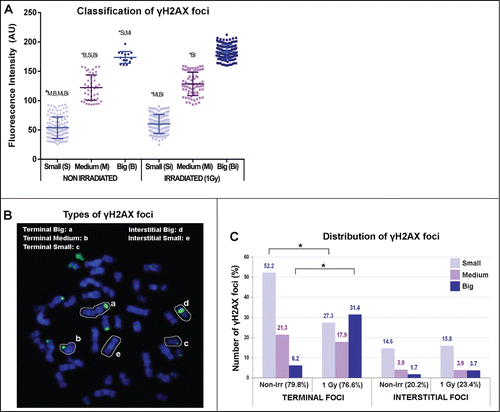
Thus, mitotic chromosomes showed Terminal and Interstitial γH2AX foci of variable FI. While the number of Interstitial foci did not increase 48 hours after irradiation and they were mostly Small γH2AX, irradiation of cells favored the appearance of Big Terminal γH2AX foci (). In this regard, the mean FI of all foci arising after irradiation was higher than that of γH2AX foci scored before irradiation (; Man-Whitney test; P < 0.0001). Among these irradiation-induced foci, the FI of Terminal foci was specially increased (Dunn's Multiple Comparision Test; P < 0.001).
Chromosome and chromatid breaks show Big γH2AX foci
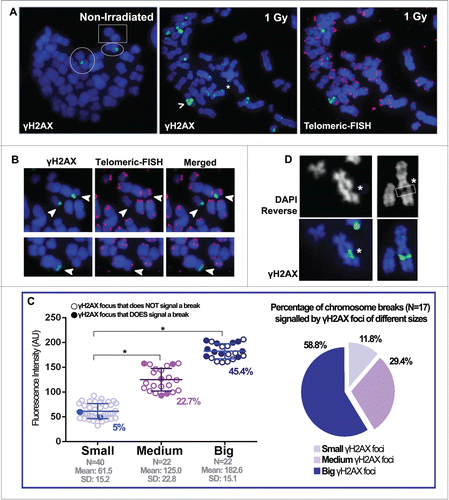
After γH2AX foci scoring and classification, we aimed to determine the chromosome and chromatid breaks and gaps, if present, that lay beneath all of these foci. Although chromosome morphology was highly preserved, identification of terminal chromosome breaks and acentric fragments was not unequivocal using DAPI staining alone. Thus, a telomeric FISH was performed on the same slides, and this approach enabled us to clearly identify terminal double-strand breaks and unrejoined acentric fragments, as demonstrated by the absence of telomeric signals at one end (). Before irradiation, the frequency of both chromosome and chromatid breaks per cell was 0.298 (analyzed in 47 metaphases), and it increased to 0.51 after irradiation (analyzed in 55 metaphases). Clearly, the frequency of breaks was significantly lower than the number of γH2AX foci scored per cell. After irradiation, 14 of the metaphases with more breaks and with high quality chromosome spreads and hybridization were selected. All the γH2AX foci in these metaphases were scored and their FI was measured (). Out of 84 Terminal foci scored, only 17 of them corresponded to microscopically visible breaks (20.2%). It is worth noting that all γH2AX foci that signaled chromosome breaks appeared as double foci in the same locus of both chromatids. These γH2AX foci-signaling breaks were mostly Big (45.5%), followed by Medium γH2AX foci (22.7%). Only rarely, chromosome breaks were signaled by Small γH2AX foci (5%) (). Therefore, most of the scored breaks in the metaphase spreads (∼60%) were signaled with Big Terminal foci (). Similarly, all chromatid breaks scored presented Big Interstitial γH2AX foci, clearly spanning through both sides of the chromatid break (). Chromosome gaps, in which chromatin is not broken but it is clearly damaged, were also decorated with Big Interstitial foci (). To sum up, chromatin that reaches mitosis in a broken or damaged state is most often decorated with Big Terminal or Interstitial γH2AX foci respectively, even at long times after irradiation. These results are in accordance with the observed increase in the number of Big Terminal γH2AX foci after irradiation ( and ), reflecting the presence of radiation-induced breaks that remain unrepaired.
Figure 2. Big and small γH2AX foci decorate mitotic chromosomes before and after irradiation. (A) Left: metaphase from a non-irradiated cell where very few γH2AX foci can be scored. The circles highlight a chromosome with a Big Terminal γH2AX foci and a chromosome with a Medium Interstitial γH2AX foci. The square highlights a chromosome with a Small Interstital γH2AX foci. Middle: metaphase spread obtained 48 hours after 1 Gy irradiation, in which many γH2AX foci can be seen located both interstitially and at the chromosome ends. A Big Interstitial γH2AX focus spotting a chromatid break (>) and a Small Terminal γH2AX focus (*) are highlighted. Right: after γH2AX foci scoring, telomeric FISH was applied to the same slides. (B) Telomeric FISH allows identification of terminal chromosome breaks. Terminal γH2AX foci are highlighted with white arrows. In the top row, both γH2AX foci correspond to chromosome breaks, evidenced by the absence of telomeric signals in the affected chromosomes. In the bottom row, the Terminal γH2AX focus does not correspond with a break, as telomeric signals are present. (C) Left: the Terminal γH2AX foci from 14 metaphases were scored after irradiation and classified regarding their FI. Small, Medium and Big foci were statistically different (*) (ANOVA test; P < 0.001). The number of foci scored (N), as well as the mean FI and SD values for each category are shown. In each group, those γH2AX foci that signal chromosome breaks are highlighted using filled circles and their percentage is given below. Right: the chromosome breaks scored are classified according to the size of the γH2AX foci that signals them. (D) Left column: a chromatid break (*) scored after DAPI reverse analysis marked with a Big Interstitial γH2AX foci that spans over both sides of the broken chromatid. Right column: the white box (*) signals a chromosome gap, also marked with a Big Interstitial γH2AX foci.
Small γH2AX foci spot apparently intact chromatin sites
The previous scoring demonstrated that, while most of the breaks are signaled with Big or Medium γH2AX foci, many of the γH2AX foci did not coincide with chromosome or chromatid breaks or gaps. Surprisingly, those Terminal γH2AX foci not signaling a break co-localized with apparently normal telomere signals (). It is worth noting that after applying telomeric FISH the γH2AX foci were still visible, allowing for a clear co-localization between γH2AX foci and telomeres when a dual-band fluorescence filter was used (). Only in rare occasions (less than 5%) these foci co-localized with split or abnormally small telomere signals ().
Figure 3. Many γH2AX foci locate on apparently normal telomeres. (A) Many of the γH2AX foci scored after irradiation are located at the terminal ends of chromosomes and seem to lie on apparently intact chromatin (>). Most of the telomeres lying beneath these foci show a normal size and structure (>) except for a few shorter telomeric signals (*). (B) Examples of eroded (* top row, right image) or split (* low row, right image) telomeres decorated with γH2AX foci that co-exist with apparently normal telomeres. In turn, some of these apparently normal telomeres can also be signaled with a terminal γH2AX focus (white circle). (C) Western blot showing the presence of wild-type p53 in the cells used. The first lane is a protein molecular weight marker. As expected, the phospho-p53 signal clearly increased after irradiation (IR: irradiation).
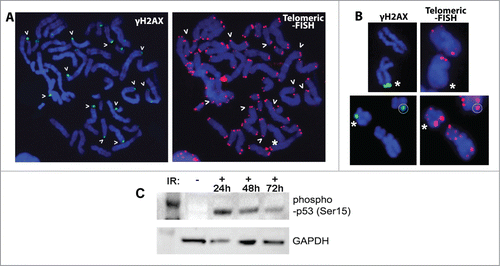
It has been described that in a p53- or p21-deficient background, telomere signaling by γH2AX is significantly increased due to progressive telomere shortening.Citation29 Although telomeres were clearly visible and the chromosomes did not display any feature related to telomere shortening such as chromosome fusions, a Western blot was performed to disregard p53 deficiency (). The results demonstrated that the cell line used is proficient in p53 radiation-induced phosphorylation, ruling out p53 deficiency as the triggering event in the appearance of γH2AX foci at metaphase telomeres. Thus, Small and Medium Terminal γH2AX foci do mostly co-localize with apparently intact chromatin at telomeric or sub-telomeric positions, and they do not spot visible discontinuities in chromatin integrity.
Interstitial γH2AX foci do not flag misrepair events
As stated before, all the chromatid breaks and gaps scored in the samples were decorated with Big γH2AX foci. Nonetheless, most of the Interstitial γH2AX foci (95%) spanned over apparently intact chromatin sites and they did not correspond to any visible chromatin discontinuity or alteration, regardless of their size. It has been proposed that residual foci in apparently intact metaphase chromosomes could indicate aberrant chromatin structure as a result of illegitimate rejoining.Citation22 To test this possibility, we performed mFISH on the same chromosome slides in which γH2AX immunostaining and telomeric FISH had been already performed. Among these irradiated cells, 14 metaphases with high quality mFISH hybridization were analyzed.
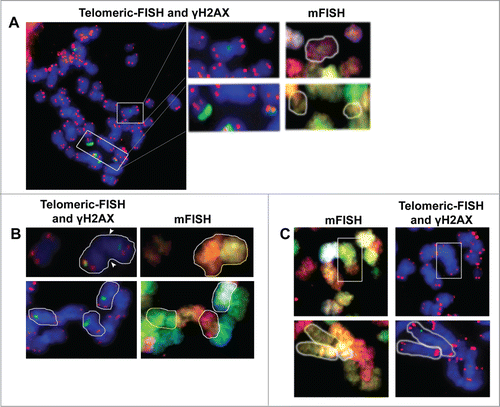
Firstly, Interstitial γH2AX foci of these metaphases were relocated, and mFISH analysis was used to identify misrejoining events on the affected chromosomes. Out of 76 Interstitial γH2AX foci, 30.6% were Small, 9.4% were Medium and 7.5% were Big, and only 2 of the Big Interstital foci corresponded to chromatid breaks (10.5%). Next, we evaluated the co-localization of these 74 Interstitial foci with the joining site of dicentric or translocated chromosomes. None of these foci signaled any misrejoined event (). To explore all possibilities, the reverse approach was tried; 11 misrejoined events were scored in these metaphases: 2 rings, a compound acentric fragment and 8 translocations. Again, none of these illegitimate rejoining events were found to display γH2AX foci at the misrejoining point (). The results show that radiation-induced illegitimate rejoining events identified in metaphase do not present residual γH2AX foci, and cannot thus be considered as scars that spot the rejoining site.
Figure 4. γH2AX foci do not flag misrejoining events. (A) From left to right. After γH2AX foci immunofluorescence, a telomeric-FISH analysis is performed on the same slides. In this metaphase, an apparently intact chromosome with a Medium Interstitial γH2AX focus (upper white box) and a broken chromosome and an acentric fragment with a Big and a Small Terminal γH2AX foci (both inside the lower white box) are highlighted. Top row: the mFISH analysis shows that the chromosome with the internal γH2AX focus is an intact chromosome 8 that has not suffered any misrejoining event. Low row: the 2 broken chromosomes with Terminal γH2AX foci are the 2 pieces resulting from the breakage of chromosome 16. (B) On the top row, a translocation between chromosomes 9 and 10 can be identified. This translocated chromosome has an Interstitial γH2AX focus that, clearly, does not lie on the joining point (denoted by white arrows). In the lower row, 3 chromosomes bearing Interstitial γH2AX foci have been highlighted. Telomeric FISH and mFISH images show that all of them are complete chromosomes that have not suffered any illegitimate rejoining event. (C) In a reverse approach, mFISH was used to identify misrejoining events such as translocations. Once identified, the telomeric and γH2AX foci labeling was analyzed. Top row: a (4;8) translocation is highlighted (white box) in which no γH2AX focus can be observed at the joining point. Low row: a reciprocal translocation between chromosomes 1 and 10 can be identified (white contour) and, again, no γH2AX foci can be observed at either joining site.
All γH2AX foci in intact chromosomes co-localizewith other DNA repair proteins
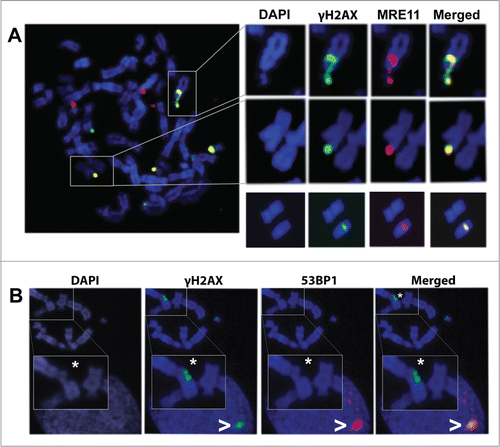
The results obtained this far demonstrate that once the chromosome break is repaired, no γH2AX foci can be detected at the rejoining point. Also, the results show that, while chromosome and chromatid breaks mostly coincide with residual Big γH2AX foci, not all of the γH2AX foci identified in the chromosome spreads actually co-localized with visible breaks or with microscopically visible chromatin alterations. It has been proposed that highly condensed chromatin during mitosis might interfere with H2AX dephosphorylation or replacement with new non-phosphorylated histones once repair is achieved.Citation20,25 This idea would imply that the repair process is already concluded at these sites, so that the presence of other repair proteins would be unexpected. To test this possibility we decided to perform a double immunofluorescence of γH2AX and MRE11 on mitotic chromosomes. MRE11 is one of the first proteins located at the DNA damage site to develop its nuclease function.Citation30 After double immunofluorescence of MRE11 and γH2AX, metaphase spreads were scored and analyzed. As expected, all visible chromosome and chromatid breaks already signaled by γH2AX foci, also presented with MRE11 foci. Surprisingly, all Terminal and Interstitial γH2AX foci spreading over apparently intact chromatin, including those Small Terminal foci located at telomeric regions, also co-localized with MRE11 foci in metaphase cells (). To further explore this subject, we performed double immunofluorescence of γH2AX and 53BP1 on mitotic chromosomes, as 53BP1 is another mediator protein known to form IRIF at sites of DSBs after radiation exposure.Citation31 Although 53BP1 and γH2AX foci did frequently coincide in the interphase nuclei of the same spreads, 53BP1 foci were absent from mitotic chromosomes, even in those presenting clearly visible chromosome or chromatid breaks (). These results are consistent with previous studies describing that 53BP1 dissociates from endogenously arising DSBs at the G2/M boundary.Citation32,33 To sum up, co-localization of the DDR proteins γH2AX and MRE11 suggests that they signal sites in which loading of the DDR answers to an unrepaired DNA lesion. Absence of 53BP1 from these mitotic foci may indicate that maintenance of a complete DDR is inefficient during mitosis.
Figure 5. Co-localization pattern of γH2AX foci with MRE11 and 53BP1. (A) A metaphase spread showing γH2AX and MRE11 co-localization. These 2 proteins co-localize at sites of chromatin damage such as a chromosome break (upper row) and at sites of apparently intact chromatin (middle row). In the lower row, an apparently intact chromosome from a different metaphase bears an internal γH2AX focus that also co-localizes with MRE11. (B) Analysis of 53BP1 and γH2AX shows that, after irradiation, 53BP1 co-localizes with γH2AX foci at interphase nuclei (>) but not with γH2AX foci located on mitotic chromosomes, even when these foci are signaling chromatin breaks (*).
Discussion
Although it is accepted that the number of γH2AX foci reflects the number of DSBsCitation6,15,16 and that it is a powerful tool to evaluate DSB repair,Citation12-14 many studies have been performed using interphase nuclei, while relatively few studies have evaluated γH2AX dynamics in metaphase chromosomes. In the few that have, controversy remains over whether there is a link between the presence of γH2AX foci and DSB persistence in M phase. In line with these works, the present paper also shows that one must be careful when assuming a straightforward correlation between γH2AX foci and DSBs.
Direct evaluation of the presence of DNA DSBs during interphase is very difficult but it is achievable during mitosis, when chromosome and chromatid breaks are easily identified, thus providing a perfect tool to score the type of lesions underlying γH2AX foci. In this study, we show that mitotic chromosomes have many γH2AX foci with variable FI. According to their FI, these foci are classified as Small, Medium and Big and their position within the chromosome is evaluated. Before irradiation, most of the scored foci are Small or Medium, while a significant population of Big γH2AX foci arise after irradiation. Importantly, the residual chromosome and chromatid breaks that persist at long post-irradiation times in mitotic chromosomes are signaled with mostly Big, and sometimes Medium, γH2AX foci. Because the γH2AX foci that signal breaks appear as paired foci in both chromatids, they most probably arise from breaks generated during G1 phase, in which DNA has been replicated and the chromosome has reached M phase devoid of repair. These results demonstrate that cells can enter M phase even when complete repair has not been achieved. In this regard, Deckbar and colleagues described that G2 checkpoint release and entry into mitosis of irradiated cells occurs even when ∼10–20 DSBs identified as γH2AX foci remain.Citation34
While these Terminal Big γH2AX foci efficiently identify chromosome breaks, the rest of the Terminal γH2AX foci do not coincide with apparently damaged chromatin, but they indeed co-localize with MRE11, arguing for the presence of DNA damage. After ruling out p53 deficiency as the triggering event in the appearance of γH2AX foci at metaphase telomeres, the nature of these Terminal foci remains unknown. It has been recently described that T lymphocytes receiving γ-irradiation showed significant telomere shortening within only 48 hours.Citation35 In that scenario, those Terminal γH2AX foci may be signaling a slight degree of radiation-induced telomere erosion, which could not be counteracted before mitosis. Still, Terminal γH2AX foci are also scored in non-irradiated metaphases, suggesting that other events may contribute to Terminal γH2AX foci formation. A small number of metaphase TIFs (Telomere dysfunction-Induced Foci) commonly occur in telomerase-positive cells and wild-type p53 cell lines, and they most probably indicate that spontaneous defects during post-replicative telomere capping can occur at a low frequency.Citation36 In fact, the presence of phosphorylated H2AX at telomeric positions has been detected in pre-senescent cells with functional telomeresCitation35,37,38 and it has been proposed that they might represent telomeres in an intermediate state of deprotection, inherited from the G2-phase.Citation37 Consistently, no telomeric fusions indicative of severely shorted or uncapped telomeres were identified in the samples or in subsequent metaphase spreads from the same cell line. Nonetheless, further investigations are required to resolve the exact nature of this γH2AX signaling.
Similar to Terminal foci, a rough proportion of Interstitial foci lie on apparently intact chromatin, and they also co-localize with MRE11. It has been previously suggested that γH2AX foci clustered along apparently intact anaphase bridges could correspond to sites of illegitimate rejoining, as these bridges are most probably formed by dicentric chromosomes being pulled to both spindle poles.Citation22 By means of combining γH2AX immunofluorescence, telomeric FISH and mFISH, we show that none of the interstitial γH2AX foci scored on apparently intact chromosomes lay at the joining point of a misrejoined event. Moreover, illegitimately rejoined chromosomes such as translocations never had γH2AX foci at the joining site, demonstrating that resolved DSBs do not present γH2AX labeling scars in mitosis spotting the sites where repair has taken place.
Contrary to those γH2AX foci that spot visible breaks, many of the Small and Medium foci located at interstitial and also at telomeric positions usually appeared as a single focus in one of the chromatids. This pattern is consistent with signatures of replication stress suffered during S-phase that have reached mitosis. It has been recently shown that fragile sites are particularly enriched with H2AX and phosphorylated H2AX, and that γH2AX enrichment may indicate the loci of stalled or broken replisomes.Citation39 Indeed, telomere and subtelomeric regions in mammalian cells resemble fragile sitesCitation40 that experience increased replisome stalling and DSB formation under replication stress.Citation39,40 Further research is required to unravel the exact nature of these cytogenetically invisible lesions as over- or under-estimation of DSBs may translate into dangerous biological consequences, such as misinterpretation of the actual efficiency regarding DNA damage infliction of several DNA damage agents used in therapy or misinterpretation of the repair capacity of the cell type evaluated.
Previous studies have related γH2AX foci on mitotic chromosomes with higher order chromatin condensation that prevents proper dephosphorylation or with a mitotic-specific signaling that is unrelated to DNA-damage.Citation20,21,23,25,41,42 Although incapability of mounting a complete DDR during mitosis could be due to chromatin condensation, one must consider that irradiation during mitosis is able to induce H2AX phosphorylationCitation18,43 and recruitment of some other signaling and repair factors,Citation44 thus, being able to access the condensed chromatin and start loading a DDR. The results presented here demonstrate that all γH2AX foci, those that co-localize with breaks, and those located at sites of apparently intact chromatin, strictly co-localize with MRE11 foci. The MRN complex is necessary for DSB sensing, stabilization and scaffolding,Citation45 as well as for ATM recruitment to the DSB.Citation46,47 In turn, once at the DSB, ATM amplifies the DNA damage response by phosphorylating H2AX, among other repair factors. In this study, we show that most of the γH2AX foci scored on mitotic chromosomes do not correspond with cytologically visible lesions. Although we cannot rule out that these foci are unrelated to the presence of DSBs, the co-localization of MRE11 with γH2AX suggests that, while not always microscopically visible, a detectable, DSB-related modification of chromatin exists. Although we could not detect 53BP1 presence in mitotic chromosomes, it was indeed scored co-localizing with most γH2AX foci in the interphase nuclei that surrounded the analyzed metaphase spreads, indicating that DDR is actively taking place. These results are consistent with previous descriptions of 53BP1 dissociation from endogenously arising DSBs at the G2/M boundaryCitation32,33 and with recent reports showing that lesions induced on mitotic chromosomes during M phase only trigger a primary DDR that will allow loading of MRE11, NBS1 and MDC1 components, while later repair and signaling factors, such as 53BP1 and RNF8 and RNF168 E3 ubiquitin ligases are absent from these mitotic IRIF.Citation43,44 Our data are consistent with this mechanism, indicating that not only DNA lesions induced during M phase, but also those lesions that reach mitosis devoid of proper repair are being correctly sensed. Although the absence of 53BP1 foci suggests that active DNA repair could be halted during M phase.
In summary, chromosome and chromatid breaks in mitosis are easily identified by the presence of Big γH2AX foci. Nevertheless, most of the γH2AX foci scored lie in apparently intact chromatin. These mitotic γH2AX foci always co-localize with early DDR factors, suggesting that, although not microscopically visible, a chromatin lesion exists that triggers activation of at least some part of the DNA damage response at those DNA sites. Indeed, rejoining events where repair has been completed do not present γH2AX scars, suggesting that persistent γH2AX foci may be an indication of unresolved DNA damage or chromatin modifications that require further processing.
Material and Methods
Cell culture and γ-ray exposure
The lymphoblastoid immortal cell line GM09622 has a normal and stable karyotype, and was obtained from the Coriell Cell Repositories. Cells were grown in suspension in RPMI 1640 (GIBCO, Life Technologies Corporation, CA, USA) medium and incubated at 37°C and in a 15% CO2 atmosphere. Cells were irradiated with 1Gy γ-rays while exponentially growing using a CSL 15 R-137Cs source (dose-rate: 5.7 Gy/min). Immediately after irradiation, BrdU (Sigma; Sigma Aldrich, Buchs SG, Switzerland) was added to the culture at a final concentration of 12 μg/ml and the protocol was followed as previously described.Citation27 Metaphase slides were obtained and UV irradiated at different post-irradiation times. Slides showing a majority of metaphases in first division after irradiation (78.5% of first divisions and 21.5% of second divisions at 48 h after irradiation) were chosen to avoid the loss of acentric fragments and other unrepaired events in further cell divisions.
Fluorescent in situ hybridization (FISH)
Telomeric hybridization was performed following the manufacturer's instructions using a Cy3-(CCCTAA)3 PNA-probe for telomeres (PE Biosystems; Life Technologies Corporation, CA, USA), and the samples were counterstained with DAPI. Chromosome breaks and acentric fragments are unequivocally identified by the absence of telomeric signals at one of their ends. After PNA-FISH, the same slides were used to perform multiplex FISH (mFISH) in order to obtain the differential paintings of the whole set of chromosomes. The mFISH probe (Vysis; Vysis Inc., IL, USA), containing 5 fluorochromes (Spectrum GoldTM, Spectrum AquaTM, Spectrum FRedTM, Spectrum GreenTM and Spectrum RedTM) was applied following the manufacturer's instructions. After mFISH hybridization and DAPI counterstaining, metaphases were relocated and recaptured. All fluorescent signals were visualized under an Olympus BX 51 microscope using a 100X (Olympus, PA, USA) U Plan Apochromat lens (1.35 NA). The microscope was equipped with epifluorescence optics and a CV-M300 camera (MetaSystems GmbH). Images were captured and analyzed using Isis V5.0 software (FISH Imaging System, MetaSystems; Heidelberg, Germany).
Immunofluorescence
After irradiation, cells were cultured for 48 h and chromosome spreads were obtained as described by Jeppesen (2000).Citation48 Briefly, cells were centrifuged and resuspended to a final concentration of 5 × 104 cells/ml in a 1:1 proportion of cell culture medium and hypotonic solution (KCl 0.075M) supplemented with 0.1% Tween 20. The cells were subsequently cytocentrifuged, and the slides were washed with potassium chromosome medium (KCM) for 15 min at room temperature (RT). After air drying, blocking solution (2% (v/v) foetal bovine serum in KCM) was applied to the slides for 1 h at RT. Both primary and secondary antibodies were diluted in KCM containing 10% (v/v) serum. Slides were removed from KCM and a 1:800-diluted mouse anti-phospho-histone H2A.X (ser139) (Upstate; Millipore, MA, USA) antibody was applied alone or together with a 1:200-diluted rabbit anti-MRE11 antibody (Abcam; Cambridge, UK). Incubation was performed for 1 h at 37°C. Before secondary antibody incubation, the preparations were washed twice in KCM (5 min each) and the 1:400-diluted Alexa 488 and 1:1000-diluted Alexa 568 secondary antibodies (Molecular Probes; Life Technologies Corporation, CA, USA) were applied. After 40 minutes incubation at RT, the slides were washed for 2 × 5 min in KCM and fixed in KCM containing 4% (w/v) formaldehyde for 15 min. Finally, the preparations were briefly rinsed in distilled water and left to air dry. DAPI counterstain was applied to the slides and chromosome spreads were visualized and captured. A telomeric-FISH and an mFISH probe were consecutively applied to the same slides, and metaphases were relocated and re-captured.
Foci counting and classification
The number of γH2AX foci and their mean fluorescence intensity (FI) was obtained by using the free software ImageJ (http:/rsbweb.nih.gov/ij/) and its plugin FociPicker 3D (http://rsb.info.nih.gov/ij/plugins/foci-picker3d/index.html) which also provides measures of Fluorescence Intensity (FI) for each foci. To determine the frequency of these FI, foci were classified into 6 initial categories that were later grouped into 3 final categories: Big, Medium and Small γH2AX foci (Supplemental ). The FociPicker 3D program calculates the mean FI of each γH2AX foci, which is measured using AU (Arbitrary Units). Small foci presented FI ranging between 25 to 92.9 AU; Medium foci presented FI ranging between 93 to 158.9 AU and Big foci presented the highest values of FI, ranging between 159 and 225 AU. The scoring of foci and the calculations for the mean FI and standard deviation (SD) of Small, Medium and Big foci was calculated out of 5 slides corresponding to 5 different experiments ().
Statistical analysis
All statistical analysis in the work was performed using GraphPad InStat Software (GraphPad Software, CA, USA).
Western blotting
Cells were trypsinised, counted, and an equal number collected (0.5 × 106 cells) by centrifugation. Pellets were resuspended in SDS sample buffer (100 μl) and sonicated in an ultrasonic water bath at 75°C. Whole-cell extracts were loaded onto a 12% SDS-polyacrylamide gel that was run at 180 V for 50 minutes in a Bio-Rad mini-gel system. Proteins were transferred to a PVDF membrane (100 V, 1 h) and blocked for 1 h in 0.1% Tween 20, 5% non-fat milk at room temperature. A rabbit polyclonal phospho-p53 (Ser15) antibody was used (Cell Signaling) at 1:1000 dilution and was detected by a donkey anti-rabbit antibody (Amersham-Pharmacia) conjugated to horseradish peroxidase. Proteins were visualized using the ECL-Plus kit (GE Healthcare) and the signal was captured with the VersaDoc (Bio-Rad).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
947786_Supplemental_Files.zip
Download Zip (4.3 MB)Acknowledgments
We thank the radiological Protection Unit at Universitat Autònoma de Barcelona for sample irradiation.
Additional information
Funding
References
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM Phosphorylates Histone H2AX in response to DNA double-strand breaks. J Biol Chem 2001; 276:42462-7; PMID:11571274; http://dx.doi.org/10.1074/jbc.C100466200
- Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson S. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 2005; 123:1213-26; PMID:16377563; http://dx.doi.org/10.1016/j.cell.2005.09.038
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on Serine 139. J Biol Chem 1998; 273:5858-68; PMID:9488723; http://dx.doi.org/10.1074/jbc.273.10.5858
- Cleaver JE. γH2Ax: Biomarker of damage or functional participant in DNA repair “All that Glitters is not Gold!” Photochem Photobiol 2011; 87:1230-9; PMID:21883247; http://dx.doi.org/10.1111/j.1751-1097.2011.00995.x
- Chen HT, Bhandoola A, Difilippantonio MJ, Zhu J, Brown MJ, Tai X, Rogakou EP, Brotz TM, Bonner WM, Ried Tet al. Response to RAG-mediated V(D)J cleavage by NBS1 and g-H2AX. Science 2000; 290:1962-4; PMID:11110662; http://dx.doi.org/10.1126/science.290.5498.1962
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 1999; 146:905-16; PMID:10477747; http://dx.doi.org/10.1083/jcb.146.5.905
- Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at Serine 139. J Biol Chem 2000; 275:9390-5; PMID:10734083; http://dx.doi.org/10.1074/jbc.275.13.9390
- Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM, Nussenzweig A. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol 2003; 5:675-9; PMID:12792649; http://dx.doi.org/10.1038/ncb1004
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 2000; 10:886-95; PMID:10959836; http://dx.doi.org/10.1016/S0960-9822(00)00610-2
- Bassing CH, Chua KF, Sekiguchi J, Suh H, Whitlow SR, Fleming JC, Monroe BC, Ciccone DN, Yan C, Vlasakova K, et al. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc Nat Acad Sci U S A 2002; 99:8173-8; PMID:12034884; http://dx.doi.org/10.1073/pnas.122228699
- Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, et al. Genomic instability in mice lacking histone H2AX. Science 2002; 296:922-7; PMID:11934988; http://dx.doi.org/10.1126/science.1069398
- Löbrich M, Shibata A, Beucher A, Fisher A, Ensminger M, Goodarzi AA, Barton O, Jeggo PA. γH2AX foci analysis for monitoring DNA double-strand break repair: Strengths, limitations and optimization. Cell Cycle 2010; 9:662-9; PMID:20139725; http://dx.doi.org/10.4161/cc.9.4.10764
- Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. gammaH2AX and cancer. Nat Rev Cancer 2008; 8:957-67; PMID:19005492; http://dx.doi.org/10.1038/nrc2523
- Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucl Acids Res 2008; 36:5678-94; PMID:18772227; http://dx.doi.org/10.1093/nar/gkn550
- Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Nat Acad Sci 2003; 100:5057-62; PMID:12679524; http://dx.doi.org/10.1073/pnas.0830918100
- Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of 125IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res 2002:486-92; PMID:12236816; http://dx.doi.org/10.1667/0033-7587(2002)158[0486:QDOIID]2.0.CO;2
- Foster ER, Downs JA. Histone H2A phosphorylation in DNA double-strand break repair. FEBS J 2005; 272:3231-40; PMID:15978030; http://dx.doi.org/10.1111/j.1742-4658.2005.04741.x
- Forand A, Dutrillaux B, Bernardino-Sgherri J. Gamma-H2AX expression pattern in non-irradiated neonatal mouse germ cells and after low-dose gamma-radiation: relationships between chromatid breaks and DNA double-strand breaks. Biol Reprod 2004; 71:643-9; PMID:15115728; http://dx.doi.org/10.1095/biolreprod.104.027466
- Kato TA, Nagasawa H, Weil MM, Little JB, Bedford JS. Levels of gamma-H2AX Foci after low-dose-rate irradiation reveal a DNA DSB rejoining defect in cells from human ATM heterozygotes in two at families and in another apparently normal individual. Radiat Res 2006; 166:443-53; PMID:18179804; PMID:16953663; http://dx.doi.org/10.1667/RR3604.1
- Kato TA, Okayasu R, Bedford JS. Comparison of the induction and disappearance of DNA double strand breaks and γ-H2AX foci after irradiation of chromosomes in G1-phase or in condensed metaphase cells. Mutat Res/Fund Mol Mech Mutagen 2008; 639:108-12; http://dx.doi.org/10.1016/j.mrfmmm.2007.11.006
- McManus KJ, Hendzel MJ. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol Biol Cell 2005; 16:5013-25; PMID:16030261; http://dx.doi.org/10.1091/mbc.E05-01-0065
- Suzuki M, Suzuki K, Kodama S, Watanabe M. Phosphorylated histone H2AX foci persist on rejoined mitotic chromosomes in normal human diploid cells exposed to ionizing radiation. Radiat Res 2006; 165:269-76; PMID:16494514; http://dx.doi.org/10.1667/RR3508.1
- Ichijima Y, Sakasai R, Okita N, Asahina K, Mizutani S, Teraoka H. Phosphorylation of histone H2AX at M phase in human cells without DNA damage response. Biochem Biophys Res Commun 2005; 336:807-12; PMID:16153602; http://dx.doi.org/10.1016/j.bbrc.2005.08.164
- Kato TA, Okayasu R, Bedford JS. Signatures of DNA double strand breaks produced in irradiated G1 and G2 cells persist into mitosis. J Cell Physiol 2009; 219:760-5; PMID:19206160; http://dx.doi.org/10.1002/jcp.21726
- Svetlova MP, Solovjeva LV, Tomilin NV. Mechanism of elimination of phosphorylated histone H2AX from chromatin after repair of DNA double-strand breaks. Mutat Res/Fund Mol Mech Mutagen 2010; 685:54-60; PMID:19682466; http://dx.doi.org/10.1016/j.mrfmmm.2009.08.001
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem 2001; 276:47759-62; PMID:11673449; http://dx.doi.org/10.1074/jbc.M009785200
- Martín M, Genescà A, Latre L, Ribas M, Miró R, Egozcue J, Tusell L. Radiation-induced chromosome breaks in ataxia-telangiectasia cells remain open. Int J Radiat Biol 2003; 79:203-10; PMID:12745885; http://dx.doi.org/10.1080/0955300031000089601
- Martín M, Terradas M, Iliakis G, Tusell L, Genescà A. Breaks invisible to the DNA damage response machinery accumulate in ATM-deficient cells. Genes, Chromosome Cancer 2009; 48:745-59; PMID:19455703; http://dx.doi.org/10.1002/gcc.20679
- Thanasoula M, Escandell JM, Martinez P, Badie S, Muñoz P, Blasco MA, Tarsounas M. p53 prevents entry into mitosis with uncapped telomeres. Curr Biol 2010; 20:521-6; PMID:20226664; http://dx.doi.org/10.1016/j.cub.2010.01.046
- Williams RS, Moncalian G, Williams JS, Yamada Y, Limbo O, Shin DS, Groocock LM, Cahill D, Hitomi C, Guenther G, et al. Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair. Cell 2008; 135:97-109; PMID:18854158; http://dx.doi.org/10.1016/j.cell.2008.08.017
- Schultz LB, Chehab NH, Malikzay A, Halazonetis TD. P53 binding protein 1 (53bp1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol 2000; 151:1381-90; PMID:11134068; http://dx.doi.org/10.1083/jcb.151.7.1381
- Jullien D, Vagnarelli P, Earnshaw WC, Adachi Y. Kinetochore localisation of the DNA damage response component 53BP1 during mitosis. J Cell Sci 2002; 115:71-9; PMID:11801725
- Nelson G, Buhmann M, von Zglinicki T. DNA damage foci in mitosis are devoid of 53BP1. Cell Cycle 2009; 8:3379-83; PMID:19806024; http://dx.doi.org/10.4161/cc.8.20.9857
- Deckbar D, Birraux J, Krempler A, Tchouandong L, Beucher A, Walker S, Stiff T, Jeggo P, Löbrich M. Chromosome breakage after G2 checkpoint release. J Cell Biol 2007; 176:749-55; PMID:17353355; http://dx.doi.org/10.1083/jcb.200612047
- Li P, Hou M, Lou F, Björkholm M, Xu D. Telomere dysfunction induced by chemotherapeutic agents and radiation in normal human cells. Int J Biochem Cell Biol 2012; 44:1531-40; PMID:22728163; http://dx.doi.org/10.1016/j.biocel.2012.06.020
- Cesare AJ, Kaul Z, Cohen SB, Napier CE, Pickett HA, Neumann AA, Reddel RR. Spontaneous occurrence of telomeric DNA damage response in the absence of chromosome fusions. Nat Struct Mol Biol 2009; 16:1244-51; PMID:19935685; http://dx.doi.org/10.1038/nsmb.1725
- Cesare Anthony J, Hayashi Makoto T, Crabbe L, Karlseder J. The telomere deprotection response is functionally distinct from the genomic DNA damage response. Mol Cell 2013; 51:141-55; PMID:23850488; http://dx.doi.org/10.1016/j.molcel.2013.06.006
- Jullien L, Mestre M, Roux P, Gire V. Eroded human telomeres are more prone to remain uncapped and to trigger a G2 checkpoint response. Nucl Acids Res 2013; 41:900-11; PMID:23193277; http://dx.doi.org/10.1093/nar/gks1121
- Seo J, Kim K, Chang D-Y, Kang H-B, Shin E-C, Kwon J, Choi JK. Genome-wide reorganization of histone H2AX toward particular fragile sites on cell activation. Nucl Acids Res 2014; 42:1016-25; PMID:24163101; http://dx.doi.org/10.1093/nar/gkt951
- Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 2009; 138:90-103; PMID:19596237; http://dx.doi.org/10.1016/j.cell.2009.06.021
- MacPhail SH, Banáth JP, Yu Y, Chu E, Olive PL. Cell cycle-dependent expression of phosphorylated histone H2AX: Reduced expression in unirradiated but not X-irradiated G1-phase cells. Radiat Res 2003:759-67; PMID:12751958; http://dx.doi.org/10.1667/RR3003
- Tu W-Z, Li B, Huang B, Wang Y, Liu X-D, Guan H, Zhang SM, Tang Y, Rang WQ, Zhou PK, et al. γH2AX foci formation in the absence of DNA damage: Mitotic H2AX phosphorylation is mediated by the DNA-PKcs/CHK2 pathway. FEBS Lett 2013; 587:3437-43; PMID:24021642; http://dx.doi.org/10.1016/j.febslet.2013.08.028
- Zhang W, Peng G, Lin S-Y, Zhang P. DNA damage response is suppressed by the high cyclin-dependent kinase 1 activity in mitotic mammalian cells. J Biol Chem 2011; 286:35899-905; PMID:21878640; http://dx.doi.org/10.1074/jbc.M111.267690
- Giunta S, Belotserkovskaya R, Jackson SP. DNA damage signaling in response to double-strand breaks during mitosis. J Cell Biol 2010; 190:197-207; PMID:20660628; http://dx.doi.org/10.1083/jcb.200911156
- Williams RS, Williams JS, Tainera JA. Mre11–Rad50–Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template. Biochem Cell Biol 2007; 85:509-20; PMID:17713585; http://dx.doi.org/10.1139/O07-069
- Lee J-H, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 2005; 308:551-4; PMID:15790808; http://dx.doi.org/10.1126/science.1108297
- Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J 2003; 22:5612-21; PMID:14532133; http://dx.doi.org/10.1093/emboj/cdg541
- Jeppesen P. Immunofluorescence in cytogenetic analysis: method and applications. Genet Mol Biol 2000; 23 1107-14; http://dx.doi.org/10.1590/S1415-47572000000400059
