Primary cilia (PC) are primordial, thin, hair-like organelles that protrude from the surface of vertebrate cells acting as a nexus that sense and transduce various extracellular signals, particularly that of Hedgehog (Hh) signallingCitation1. PC have been implicated in development and cancer as well as in a group of genetic disorders termed ciliopathies that affect a diverse range of organs in which the underlying phenomenon is structural or functional abnormalities of ciliaCitation1. PC have a dual role in cancer as they promote or prevent tumour development depending on whether Hh signalling is activated at receptor level or downstreamCitation2. Many tumour cells, such as those from pancreatic tumours, often lack cilia, highlighting that PC may be growth-inhibitoryCitation3. However, the association depends on tumour type, as PC are present in human basal cell carcinomas (BCCs) as well as in distinct molecular subgroups of medulloblastomasCitation2.
Uncontrolled cell proliferation is a hallmark of cancer. Therefore, senescence, a stable cell cycle arrest induced by replicative exhaustion or in response to stresses such as oncogene activation, is a potent barrier against tumorigenesisCitation4. The percentage of PC-containing cells increases in response to quiescence, a transient form of cell cycle arrest, but whether senescence promotes ciliogenesis and if so, what role PC plays in senescence remains to be addressed.
The current manuscript by Morrison and colleaguesCitation5 shows that senescent (late passage) human fibroblasts display both increased frequency and length of PC, compared to proliferating (young passage) fibroblasts. Importantly, the expression of Hh signalling components were lower in fibroblasts undergoing replicative senescence in comparison to proliferating cells. Inhibition of Hh signalling decreased cell proliferation and extended the length of PC in proliferating young fibroblasts, suggesting that Hh signalling inhibits ciliogenesis and replicative senescence in human fibroblasts (). Additionally, promoting ciliogenesis by depleting CP110, a negative regulator of ciliogenesis, was sufficient to reduce the proliferative capacity of young cells. However, overexpressing CP110 failed to reinstitute the proliferation of senescent cells. These findings would fit with the growth-inhibitory functions of PC and would suggest that the increase in PC observed in senescent cells could contribute to tumour suppression and that there is a need to suppress PC during cancer progression.
Figure 1. Relationship between senescence and the primary cilia. Frequency of primary cilia increases during senescence in human fibroblasts (middle) but not in human mammary epithelial cells (HMECs, right). During pancreatic tumorigenesis (left), primary cilia that are observed in normal ducts are lost in pancreatic intraepithelial neoplasias (PanIN) and pancreatic ductal adenocarcinomas (PDAC). PanIN lesions are enriched in senescent cells.
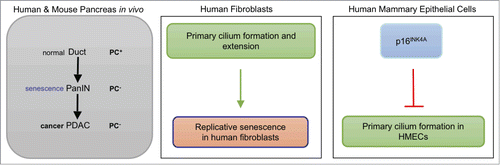
However, as it seems to be the case with PC and tumorigenesis, the relation between PC and senescence is not clear-cut. It was previously reported that only a small percentage of primary human mammary epithelial cells (HMECs) exhibited PC and these PC-containing HMECs displayed low levels of p16INKCitation4A, a marker of senescenceCitation6. In addition, depletion of p16INK4A or increased Hh signalling via IHH promoted the formation of PC6. In the same direction, cilia were absent both from pancreatic intraepithelial neoplasias (PanINs), which are preneoplastic lesions enriched in senescent cells, and in the resulting pancreatic ductal adenocarcinomas (PDACs)Citation3. Therefore, an alternative explanation could be that loss of PC is a cancer-related phenotype that is acquired early in tumour progression (at the senescent stage), as it happens with global alterations in DNA methylation.
The differences observed between the two studiesCitation5, 6 could be due to different gene expression patterns, ciliary signalling or the frequency of basal PC present in human senescing fibroblasts and HMECs, as Morrison and colleagues suggestedCitation5. There are also molecular differences between replicative senescence in human fibroblasts and the M0-barrier observed in primary HMECs. Some similarities were also noted in the two reports, as mitogenesis was impaired by the inhibition of Hh signalling through the GLI2 transcription factor in both human fibroblasts and HMECsCitation5, 6.
More importantly, if conclusions are to be drawn on how the relation between senescence and ciliogenesis impacts cancer, the fate of PC needs to be studied in response to oncogene-induced senescence (OIS) and compared with the existing observations during replicative senescence. It is also tempting to speculate that primary cilia may play a role in developmental senescenceCitation7, especially knowing the importance of these structures in transducing Hh signalling during vertebrate development. Importantly, as the list of roles that primary cilia play in physiology and pathology is ever growing, the finding that ciliogenesis increases in senescent cells may present a novel therapeutic target for cancer and ageing as well as help in understanding the molecular basis of ciliopathies.
REFERENCES
- Goetz, S.C, et al. Nat Rev 2010: 11:331–344.
- Toftgård, R. Nat Med 2009: 15:994–996.
- Seeley, E.S, et al. Cancer Res 2009; 69:422–430.
- Kuilman, T, et al. Genes Dev 2010: 24:2463–2479.
- Breslin, L, et al. Cell Cycle 2014:
- Bishop, CL, et al. Mol Cell 2010: 40:533–547.
- Muñoz-Espin et al. Nat Rev Mol Cel Biol 2014: 15:482–496.
