Abstract
We previously identified and characterized TELO2 as a human protein that facilitates efficient DNA damage response (DDR) signaling. A subsequent yeast 2-hybrid screen identified LARG; Leukemia-Associated Rho Guanine Nucleotide Exchange Factor (also known as Arhgef12), as a potential novel TELO2 interactor. LARG was previously shown to interact with Pericentrin (PCNT), which, like TELO2, is required for efficient replication stress signaling. Here we confirm interactions between LARG, TELO2 and PCNT and show that a sub-set of LARG co-localizes with PCNT at the centrosome. LARG-deficient cells exhibit replication stress signaling defects as evidenced by; supernumerary centrosomes, reduced replication stress-induced γH2AX and RPA nuclear foci formation, and reduced activation of the replication stress signaling effector kinase Chk1 in response to hydroxyurea. As such, LARG-deficient cells are sensitive to replication stress-inducing agents such as hydroxyurea and mitomycin C. Conversely we also show that depletion of TELO2 and the replication stress signaling kinase ATR leads to RhoA signaling defects. These data therefore reveal a level of crosstalk between the RhoA and DDR signaling pathways. Given that mutations in both ATR and PCNT can give rise to the related primordial dwarfism disorders of Seckel Syndrome and Microcephalic osteodysplastic primordial dwarfism type II (MOPDII) respectively, which both exhibit defects in ATR-dependent checkpoint signaling, these data also raise the possibility that mutations in LARG or disruption to RhoA signaling may be contributory factors to the etiology of a sub-set of primordial dwarfism disorders.
Abbreviations
| DDR | = | DNA damage response |
| HU | = | hydroxyurea |
| PCNT | = | pericentrin |
Introduction
Arhgef12, otherwise known as Leukemia Associated Rho Guanine exchange factor 12 (LARG), activates the Rho family member RhoA by promoting the exchange of GDP for GTP and was originally identified in a patient with Acute Myeloid Leukemia.Citation1 LARG activates the ROCK pathway downstream of Gα12/13 signaling, leading to cytoskeletal reorganisation.Citation2 LARG performs this activity either as a homodimer or as a heterodimer with Arhgef11,Citation3 and while mouse knockout models of each gene are phenotypically normal (LARG knockout mice exhibit an sub-Mendelian birth rate), double knock-out mice exhibit developmental defects and embryonic lethality.Citation4 Previous work has described LARG as having the characteristics of a tumor suppressor, with reported under expression in breast and colorectal cancers, together with reduced proliferation, migration and colony formation in cells with forced over-expression.Citation5 Conversely, aberrant RhoA expression is strongly associated with cancer, with over-expressed RhoA reported in ovarian, testicular and gastric tumors.Citation6-8 Furthermore, elevated RhoA levels are associated with poor prognosis and increased venous cell invasion in hepatocellular carcinoma.Citation9 Indeed, it is thought that hyper-activation of RhoA in LARG-MLL cells facilitates their oncogenic potential to drive development of leukemia.Citation10 These data therefore suggest that changes in LARG expression may play an important role in various aspects of tumor biology.
Genome instability can be defined as a compromised ability to faithfully pass on genetic information to daughter cells. As such, genomic instability is a hallmark of nearly all cancers.Citation11 We previously demonstrated that TELO2/HCLK2 (the human homolog of the C.elegans protein RAD5/CLK2) is required for efficient induction of the intra-S-phase checkpoint in response to replication stress.Citation12 Further characterization of TELO2 determined that TELO2 depletion causes reduced protein levels of the related DNA Damage Response (DDR) kinases ATM, ATR and DNA-PK.Citation13,14 As part of studies to identify novel interacting partners of TELO2, we identified LARG as one such protein.
Although not extensively studied, previous research suggests that Rho pathways may be responsive to DNA damage. For example, the RhoA GTPase Net1 translocates to the nucleus and activates RhoA in response to ionizing radiation.Citation15 Furthermore, LARG has previously been shown to interact with the centrosomal protein PCNT,Citation16 mutations in which are associated with the autosomal recessive disorder MOPDII; a rare disorder marked by microcephaly and dwarfism, and characterized at a molecular level by supernumerary centrosomes and defective ATR-dependent checkpoint signaling.Citation17-19 Interestingly, the RhoGEF Arhgef10 has been shown to localize to centrosomes, and cells depleted of Arhgef10 also exhibit a supernumerary centrosome phenotype.Citation20 We therefore investigated if LARG (Arhegef12) is indeed a bona fide interacting partner of TELO2 and whether LARG is involved in cellular responses to replication stress. Such data may provide further evidence that these 2 pathways are functionally linked, and give further insight into how disruption to LARG function in cancer cells may impact on these signaling pathways.
Results
Identification of LARG as a novel interactor of TELO2
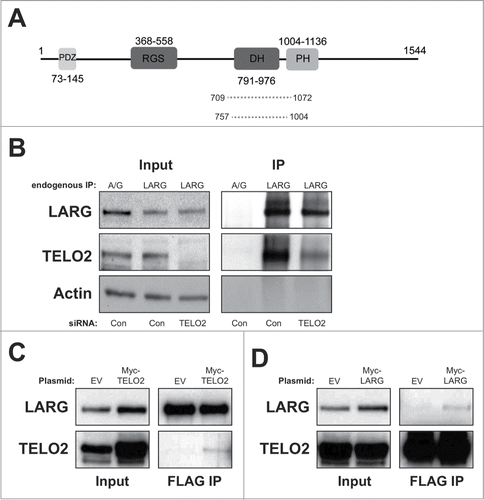
In an attempt to identify novel interactors of TELO2 in addition to those previously identified through proteomic-based approaches,Citation12,21 we conducted a yeast 2 hybrid screen using full-length TELO2 as bait. One of the putative interactors with a good confidence score was LARG, which has strong links to cancerCitation1,5,10 (). To confirm this interaction we immunoprecipitated endogenous LARG and probed for TELO2. Endogenous TELO2 co-immunoprecipitated with LARG, which was validated using TELO2-directed siRNA (). To further confirm this interaction, we next generated stable cell lines expressing N-terminally FLAG-tagged LARG and transfected them with a Myc-TELO2 plasmid. Myc-TELO2 co-purified with immunoprecipitated FLAG-LARG () further confirming their interaction. Additionally, we transfected FLAG-TELO2 stable cell linesCitation12,21 with a Myc-LARG expressing plasmid and observed that Myc-LARG weakly co-purified with immunoprecipitated FLAG-TELO2 (). Collectively, these data demonstrate that LARG and TELO2 interact in human cells.
Figure 1. Confirmation of an interaction between LARG and TELO2. (A). Schematic diagram showing LARG with highlighted functional domains; PDZ: protein interaction domain, RGS: regulator of G protein signaling, DH; Dbl homology domain, and PH; plextrin Homology domain. Dotted lines show fragments of LARG identified in the yeast 2-hybrid screen that interacted with TELO2, and numbers represent amino acid residues. (B). Immunoprecipitation of endogenous LARG from HEK 293 cells transfected with either control or TELO2-directed siRNA. Inputs and IPs were probed with the indicated antibody. Protein A/G beads were used as a negative control for LARG IPs. Note that longer exposures of input lanes (representing ∼4% of lysate by volume) are shown compared to IPs, in order to allow vizualization of appropriate bands. (C). Immunoprecipitation (M2 agarose) of FLAG-LARG from HeLa-FLAG-LARG cells transfected with either an empty vector (negative control) or Myc-TELO2 expressing plasmid. Inputs and IPs were probed with the indicated antibody. (D). Immunoprecipitation (M2 agarose) of FLAG-TELO2 from HeLa-FLAG-TELO2 cells transfected with either an empty vector (negative control) or Myc-LARG expressing plasmid. Inputs and IPs were probed with the indicated antibody.
LARG interacts and co-localizes with the centrosomal protein pericentrin
A previous study suggested that LARG interacts with the centrosomal protein pericentrin (PCNT),Citation16 although this was only confirmed with exogenous expression of LARG. This is particularly interesting given that, like TELO2, PCNT impacts on DDR pathways.Citation17 To confirm this interaction, we carried out endogenous immunoprecipitation of either LARG or PCNT. Endogenous LARG co-immunoprecipitated the previously reported isoforms of endogenous PCNT (). Moreover, PCNT weakly co-precipitated endogenous LARG (), which may be reflective of a small sub-population of PCNT interacting with LARG at the centrosome (see below). These data confirm an endogenous interaction between LARG and PCNT, and are consistent with previous findings that suggested co-localization with PCNT at centrosomes.Citation16 However, the reported co-localization was achieved using GFP-LARG microinjected into cells. In an attempt to visualize endogenous LARG at centrosomes, we employed 4 different commercial antibodies. However, none of these antibodies were able to detect endogenous LARG in immunofluorescence studies (data not shown). We therefore generated a tetracycline-inducible YFP-tagged LARG expressing cell line. As reported previously for microinjected GFP-LARG,Citation16 YFP-LARG was predominantly cytoplasmic with a localization that extended from the leading edge to the nuclear membrane (). Consistent with an interaction between LARG and PCNT (), YFP-LARG co-localized with PCNT at the centrosome in both interphase and mitotic cells ().
Figure 2. Interaction and co-localization between LARG and Pericentrin. (A). Endogenous co-immunoprecipitation of LARG and PCNT. Upper and lower panels show PCNT and LARG western blots on indicated inputs and IPs. Black arrows indicate the 2 reported isoforms of PCNT (∼380 KDa and ∼250 KDa). (B). Upper left panel shows stable T-REx-HeLa cells expressing N terminally tagged YFP-LARG (indicated by the black arrow) induced by the addition of 1 μg/ml tetracycline (Tet) for 24 hrs. Extracts were probed for LARG, with the endogenous band visible underneath the marked YFP-LARG band. To confirm that this higher MW band was indeed YFP-LARG, 2 separate LARG-directed siRNA were transfected 24 hrs prior to tetracycline addition leading to depletion of both the endogenous and YFP-LARG bands (lanes 3–6). Non-targeting siRNA was used as a negative control (lanes 1–2). Actin was used as a loading control. Lower left panel shows direct vizualization of YFP LARG in this stable cell line with or without the addition of 1 μg/ml tetracycline for 24 hrs. Right panel shows direct vizualization of YFP-LARG in this cell line as in the left panel, but with co-staining for endogenous PCNT. White arrows indicate YFP-LARG accumulation at PCNT-positive structures in both interphase (upper panel) and mitotic cells (lower panel). Inserts show enlarged images of co-localizing area. (C). HEK293 cells were transfected with 1 μg of Flag-PCNT plasmid and 3 μg Myc-LARG plasmids as indicated. Forty-8 hours after transfection FLAG IPs were performed and probed for either LARG (upper panels) or FLAG (lower panels). Inputs are shown in the left panels, with IPs shown on the right panels. Empty Myc-vector and Myc-LARG transfections were used as controls for non-specific binding to M2-FLAG beads. The regions of PCNT expressed by each plasmid are indicated on the left and in Figure S1A.
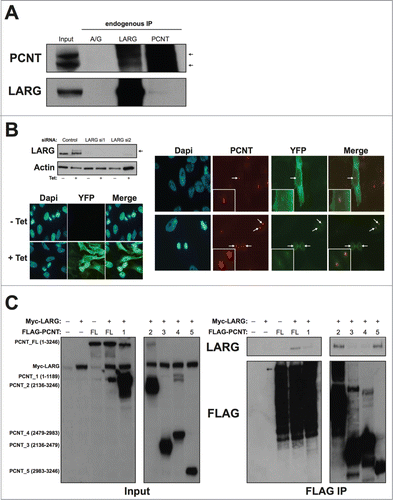
To further characterize the interaction between LARG and PCNT, and understand how LARG may co-localize with PCNT at centrosomes, we performed domain mapping to refine the interaction between LARG and PCNT using plasmids expressing various FLAG-tagged fragments of PCNTCitation22 (Fig. S1A). HEK293 cells were co-transfected with combinations of full-length Myc-LARG and various FLAG-tagged fragments of PCNT. As predicted from the endogenous co-IP data shown in , Myc-LARG co-immunoprecipitated with full-length FLAG-PCNT (). Interestingly, the only other fragments of PCNT that strongly interacted with LARG were those that contained the extreme C-terminal PACT domain (), which is responsible for a large proportion of the centrosomal localization of PCNT.Citation23 Indeed, PCNT fragment 5 (aa2983–3246) consists mainly of the PACT domain alone (Fig. S1A), and interacts with full length LARG (last lane of ). An interaction between LARG and the PACT domain of PCNT could therefore be a mechanism by which PCNT recruits LARG to the centrosome, and suggests that LARG and PCNT may be functionally linked.
LARG-depleted cells exhibit defective ATR signaling in response to replication stress
The data presented in establish LARG as a novel interacting partner of TELO2 and PCNT, both of which are required for efficient replication stress signaling through ATR-mediated signaling events.Citation12,17 We therefore hypothesized that LARG-deficient cells may also have similar ATR signaling defects. The presence of supernumerary centrosomes (> 2 centrosome structures) is a common phenotype of cells derived from Seckel Syndrome/MOPDII patients, which exhibit ATR checkpoint signaling defects due to causative mutations in ATR or PCNT, as well as several other genes.Citation17,24 We therefore used 2 independent LARG-directed siRNAs to deplete endogenous LARG in cells expressing GFP-tagged centrin-2 (a centriole marker),Citation25 and assessed centrosome numbers in LARG-depleted cells compared to those transfected with non-targeting control siRNA. Depletion of LARG led to a 3–5-fold increase in the number of cells displaying supernumerary centrosomes (; Fig. S1C). Comparable data (4-5% in control versus 10-12% in LARG-depleted cells) was obtained in both HeLa and U2OS cells (data not shown) further confirming this finding.
Figure 3. LARG depleted cells exhibit supernumerary centrosomes and aberrant cellular responses to replication stress. (A). Upper left panel shows an example of depletion of endogenous LARG following siRNA treatments in HeLa GFP-Centrin2 cells. Further examples in other cell lines are shown in Figure S1B. Upper right panel shows representative images of cells exhibiting normal (2) and abnormal (>2 ) centrosomes. Lower left panel shows quantification of supernumerary centrosomes in Control siRNA and LARG siRNA transfected cells. Data represents the means from 3 independent experiments with errors bars representing the standard deviation of the means. Asterisks denote a P-value of <0 .05 using a paired twin tailed students t-test assuming equal variances. A more detailed breakdown of the numbers of centrosomes is shown in Figure S1C. (B). Left panel shows representative γH2AX staining in untreated, HU (2 hrs post-3 mM) and IR (1 hr post-5 Gy) treated RPE-1 cells. Right panel show quantification of γH2AX in control and LARG siRNA transfected cells either untreated, 2 hrs following 3 mM HU or 1 hr following 5 Gy IR treatments. Data shown is the mean calculated from 3 independent experiments with error bars and asterisks as described in (A). A similar defective in γH2AX foci formation was observed in LARG-depleted cells following UV light induced replication stress (data not shown). (C). Left panel shows representative pRPA foci in untreated and HU treated HCT116 cells. Right panel show quantification of pRPA foci in control and LARG siRNA transfected cells either untreated, or 2 hrs following 3 mM HU treatments. Data shown is the mean calculated from 3 independent experiments with error bars and asterisks as described in (A).
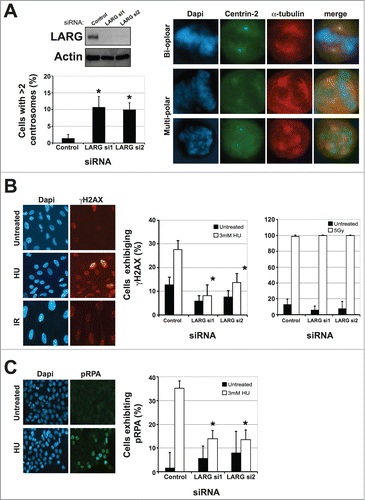
The phosphorylation of the histone variant H2AX on Ser139 (termed γH2AX) is an early DDR signaling event that is mediated by ATR in response to replication stress.Citation26 We therefore assessed the appearance of γH2AX in response to the replication stress-inducing agent hydroxyurea (HU), which depletes the cell of dNTPs. Cells transfected with non-targeting control siRNA exhibited a robust increase in γH2AX foci following a 3 hr exposure to 3 mM HU (). However, LARG depleted cells failed to efficiently induce γH2AX foci in response to HU-mediated replication stress (). A similar defect in LARG-depleted cells was also observed in response to ultraviolet light treatments (data not shown). Importantly, no defects in γH2AX were observed in LARG-depleted cells in response to ionizing radiation (), indicating that these defects were not a consequence of a general DDR signaling defect, but rather a specific defect associated with replication stress signaling. In order to gain further insight into the role of LARG in the replication stress response, we assessed phosphorylation of the replication protein RPA; an early event in cellular responses to replication stress.Citation12,27,28 Following hydroxyurea treatment, control siRNA transfected cells exhibited a robust increase in the number of cells exhibiting pRPA nuclear accumulation/foci (). However, LARG depleted cells showed a marked reduction in pRPA following replication stress (). Importantly, such differences cannot be ascribed to changes in cell cycle distributions following LARG depletion either in the absence or presence of HU (Fig. S1D), further supporting a specific replication stress-signaling defect in LARG deficient cells.
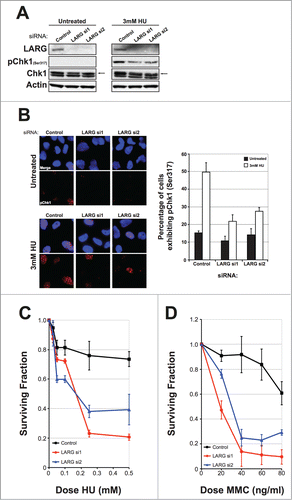
A key target for ATR in response to replication stress is the phosphorylation and activation of the effector kinase Chk1 on Serine 317.Citation29 To further characterize replication stress signaling defects in LARG-deficient cells, we assessed phosphorylation of Chk1 on Ser317 (pChk1) in control siRNA or LARG-depleted cells. Control siRNA treated cells exhibited a rapid phosphorylation of Chk1 in response to hydroxyurea treatments (). However, LARG-depleted cells exhibited reduced pChk1 under the same conditions (). This was also confirmed by immunofluorescence detection/quantification of pChk1 in control and LARG siRNA-transfected cells (). A consequence of defective ATR signaling is an increased sensitivity to replication stress-inducing agents.Citation12 Given the confirmed ATR signaling defects, we hypothesized that LARG deficient cells would be rendered sensitive to such agents. Indeed, depletion of LARG led to increased sensitivity to the ribonucleotide reductase inhibitor hydroxyurea (), and the DNA inter-strand crosslinking agent mitomycin C (). Collectively these data establish that ATR-mediated replication stress signaling is compromised in LARG-deficient cells.
Figure 4. ATR signaling is defective in LARG depleted cells rendering them sensitive to replication stress-inducing agents. (A). Western blot showing Chk1 activation as measured by phosphorylation of Chk1 on Ser317 in control and LARG siRNA transfected HeLa cells. Cells were either untreated or treated with 3 mM hydroxyurea (HU) for 2 hrs prior to preparation of protein extracts. Total Chk1 and actin levels are used as loading controls. (B). Left panel shows representative images of pChk1 (Ser317) staining in control and LARG siRNA transfected HeLa cells as described in (A). Right panel shows quantification of the percentage of cells exhibiting pChk1 (Ser317). Data shown is derived from 2 independent experiments (>250 cells scored per experiment) with their respective standard deviations. (C). Cell survival curves of control and LARG siRNA transfected HeLa cells treated with the indicated doses of hydroxyurea (HU) for 5 d Data shown is the mean generated from at least 3 independent experiments with their respective standard errors. (D). Cell survival curves of control and LARG siRNA transfected HeLa cells treated with the indicated doses of mitomycin C (MMC) for 5 d Data shown is the mean generated from at least 3 independent experiments with their respective standard errors.
ATR-deficient cells exhibit Rho signaling defects
Given that perturbation to LARG impacts on ATR signaling, we speculated that disruption to ATR signaling might likewise affect RhoA signaling, and point toward some level of functional crosstalk between these 2 pathways. LARG activation can be measured in a number of ways, including the phosphorylation of myosin Light Chain 2 on Thr18 and Ser19 in response to lysophosphatidic acid (LPA).Citation30 RPE-1 cells (which exhibit a robust pMLC response to LPA) were transfected with control, LARG, TELO2 or ATR siRNA. As previously shown by others,Citation30 LARG depleted cells exhibited a reduction in the phosphorylation of myosin Light Chain 2 (). Interestingly, cells transfected with previously validated TELO2 and ATR siRNACitation12 transfected cells exhibited a reproducible comparable defect in pMLC in response to LPA treatments (). Additionally, preliminary data showed that treatment of cells with the ATR inhibitor VE-821 confers a reduction in LPA-induced pMLC that is comparable with that observed in ATR siRNA treated cells (data not shown).
Figure 5. ATR and TELO2 deficient cells exhibit defective RhoA signaling. (A). Assessment of Rho activity by vizualization/quantification of pMLC (Thr18/Ser19) following LPA induction in cells treated with indicated siRNA. Left panel; 3 independent examples of pMLC protein gel blots following LPA induction (50 μM LPA for 10 minutes) of cells treated with indicated siRNA. Due to the nature of lysis (directly into SDS loading buffer to preserve the labile phosphorylation sites), there is some variation in loading levels. Middle panel; quantification of induction of pMLC from the western blot data shown in left panel. Fold induction was calculated by comparing normalized pMLC levels in untreated and LPA treated lanes for each respective siRNA. Right panel; graph showing mean fold induction and standard error of each siRNA compared to control siRNA from the 3 experiments shown in the left panel. (B). Quantification of Rho-GTP levels in cells treated with indicated siRNA (note: TELO2 and ATR siRNA were previously validated).Citation12 60 hours after transfection cells were serum starved for 16 hours before LPA induction with 50 μM LPA for 10 minutes. Levels of RhoA-GTP correlate with higher absorbance values at 450 nM and purified constitutively active Rho is used as a positive control for each experiment. Data shown represents the mean from 2 independent experiments (each consisting of 3 replicates) with their associated standard errors. Individual data sets for these Rho-GTP ELISA assays are shown in Figure S1E.
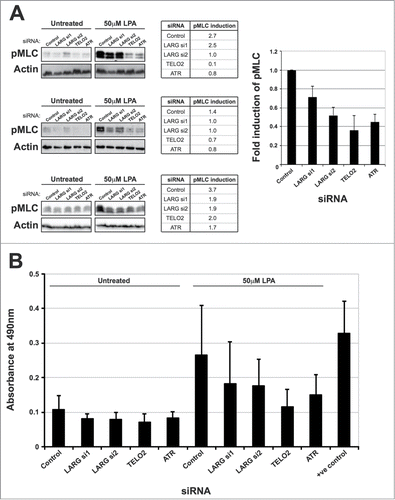
As pMLC is a downstream event in RhoA activation mediated by RhoGEFs such as LARG, these data suggest that RhoA signaling may be compromised in cells with defective ATR signaling. In order to determine whether the pMLC defect in observed in TELO2 and ATR-depleted cells is due to a reduction in RhoA signaling or due to downstream events such as ROCK activation, GTP-bound active RhoA following LPA stimulation was examined using an established ELISA-based assay. Consistent with the pMLC data in , depletion of LARG led to a modest reduction in cellular levels of active RhoA (). As with pMLC levels, depletion of either TELO2 or ATR led to a more pronounced reduction in cellular levels of active RhoA (). We attempted to validate these data further by assessing pMLC and active Rho levels in lymphoblasts from patients with Seckel syndrome, who harbour genetic loss of ATR.Citation31 However, technical difficulties in obtaining robust/reproducible response to LPA stimulation in these cells prevented such analyses. Collectively, our data show that the ATR-mediated replication stress is defective in LARG-deficient cells and that Rho signaling pathway is compromised in cells with ATR signaling defects. These data therefore suggest that these 2 pathways may be functionally connected to co-ordinate cellular responses to various cellular stresses.
Discussion
The data presented here describe a novel interaction between LARG and TELO2, and provide evidence that the Rho and replication stress signaling pathways may be functionally linked. We also show that LARG interacts with PCNT and that a sub-fraction of LARG may reside at the centrosome, where it co-localizes with PCNT. The mammalian centrosome is a major site of microtubule nucleation and organization during interphase, and it is also important for establishing a mitotic spindle to ensure efficient chromosome segregation and genome stability. Interesting, several recent studies have uncovered a previously unappreciated connection between centrosomal proteins and genome stability, with several DDR-related proteins residing at centrosomes, and several centrosome-associated proteins having functional roles in cellular responses to DNA damage.Citation17,25,32-35 Centrosome amplification has also recently been shown to promote cancer cell invasion.Citation36 Our findings are therefore consistent with these data, and LARG can be added to the expanding list of centriolar-localized proteins that impact on DDR signaling.
The mammalian centrosome is also the origin of primary cilia formation and recent genetic and cell-based studies have revealed emerging links between the DDR and cilia biology.Citation37-41 Pertinent to the work presented here, is the finding that ATR localizes to the photoreceptor connecting cilium.Citation39 Consistent with a putative functional role for ATR at the cilia, ATR-deficient mice exhibit ciliary defects and photoreceptor degeneration.Citation39 Furthermore, it has been recently demonstrated that the renal ciliopathy-associated kinase Nek8 also facilitates ATR signaling as part of the cellular responses to replication stress to help preserve genomic integrity.Citation38 Additionally, mutations in several DNA replication-licensing factors impair ciliogenesis and are causal for Meier-Gorlin syndrome.Citation42 During the course of our studies, we found that LARG-depleted cells exhibit both increased cilia formation and cilia growth (data not shown). This may be somewhat counter-intuitive given that LARG-depleted cells exhibit ATR signaling defects. However, RhoA has previously been implicated in cilia formation where it is required for early stages of ciliogenesis during differentiation.Citation43 Similarly, activation of the Rho family member Rac1 has been shown to be perturbed in Lowe Syndrome, which is caused by mutations in the cilia-associated phosphatase OCRL, leading to cilia formation.Citation44 Furthermore, recent work has demonstrated that the RhoA GTPase Net1 translocates to the nucleus and activates RhoA in response DNA damage.Citation15 Thus, in LARG depleted cells, disruption to both RhoA and ATR-mediated signaling pathways could potentially influence subsequent effects on cilia formation and growth. ATR may therefore modulate replication stress signaling and nutrient stress signaling in order to coordinate these cellular responses. Indeed, ATR has been postulated to phosphorylate a number of centrosome/cilia-associated proteins, which may in turn regulate their activities at these structures.Citation45,46 Related to this, the ATM/ATR substrate screen reported by Matsuoka et al. suggested that LARG might be phosphorylated on Ser1288 in an ATR-dependent manner.Citation46 However, we were unable to detect any such phosphorylation at this site (data not shown), although this could be due to the limitations of the reagents used, and may be worthy of further study in light of our findings.
As mentioned previously, genetic disruption of ATR and other genes that cause ATR-signaling defects can give rise to Seckel Syndrome, which shares similar clinical characteristics with several related disorders.Citation47 One of the hallmarks of Seckel Syndrome is microcephaly, and research into these disorders over the last few years has uncovered functional links between several pathways which may account for such shared clinical manifestations, including microcephaly and mental retardation.Citation48 Due to technical difficulties, we were unable to ascertain if lymphoblast derived from Seckel Syndrome patients harbour RhoA signaling defects (data not shown), however, our data suggest that mutations in LARG, or disruption to RhoA signaling pathways may contribute to the etiology of a sub-set of currently genetically uncharacterized Seckel Syndrome disorders.
Materials and Methods
Cell Culture
HCT116, HeLa, U2OS and HEK293 cells were maintained as an adherent monolayers in DMEM media containing 10% FBS at 37°C in a humidified atmosphere of 5% carbon dioxide. HeLa Flp-in T-Rex and HEK293 Flp-In T-Rex cells (Invitrogen) were maintained in DMEM media containing 10% FBS and 1% penicillin/streptomycin, supplemented with 4 μg/ml Blasticidin S (Melford) and 100 μg/ml Zeocin (Invitrogen).
Stable cell line generation
LARG was PCR cloned from human cDNA (Open Biosystems) using Gateway compatible primers, inserted into the donor vector p221 (Invitrogen) and fully sequence verified. LARG was then sub-cloned into various destination vectors to generate N-terminally tagged LARG derivatives as described in the manufactures’ protocol. Stable tetracycline-inducible HEK293 Flp-In cell lines expressing FLAG-tagged LARG and HeLa Flp-In cell lines expressing YFP- or FLAG-tagged LARG were created by co-transfection of these cell lines with pPGKFLPobpA-Flp recombinase and either empty pDEST-Flag/FRT/TO or pDEST-Flag/FRT/TO-LARG according to the Flp-In manufacturer's protocol. Recombinants were then selected in media containing 4 μg/ml Blasticidin S and 150 μg/ml Hygromycin B (Invitrogen). All transient transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
RNAi and drug treatments
HEK293, U2OS, RPE-1 and HeLa cells were transfected with between 30–100 nM siRNA using Lipofectamine 2000 (Invitrogen), RNAiMAX (Invitrogen) or Dharmafect 1 (Dharmacon) according to the manufacturer's instructions. Cells were collected, lysed or fixed for analysis after 48 hrs unless otherwise indicated. Cells were treated with hydroxyurea or mitomycin C as the doses indicated in the Figure legends. For immunofluorescence and western blot-based studies, cells were treated for 3–16 hrs as indicated, and for up to 5 d for cytotoxicity assays. For pMLC2 experiments, cells were serum starved for 16 hrs before being treated with 50 μM LPA for 10 mins, after which cells were directly lysed in protein loading buffer to retain the labile phosphorylation status on MLC2.
GLISA RhoA Activity Assay
The GLISA assay kit from Cytoskeleton (Cat. No. BK124) was used to quantify the relative activation of RhoA-GTP between cell samples and therefore measure RhoA activation following serum starvation and subsequent stimulation by 50μM LPA for 10 mins. The manufacturer's protocol was followed, including a 16 hr incubation of the cells in serum-free media to reduced background activation of RhoA, and absorbance at 490 nm determined using a plate reader (Multiskan FC, Thermo Scientific 51119000).
Yeast 2-hybrid screen
This was carried out by Hybrigenics Inc. Briefly, TELO2 was used as bait for a genome-wide yeast 2-hybrid screen carried with c-terminal tagged preys derived from human breast tumor epithelial cells RP1. Over 0.9×107 interactions were analyzed at a 3-AT concentration of 20 mM and scored/ranked into sub-categories of confidence based on proprietary algorithms.
Antibodies
The following primary antibodies were used in this study: α-Tubulin (Abcam ab7792, 1:1000), ATR (Santa Cruz sc1887, 1:1000), β-actin (Abcam ab8226, 1:5000), BrdU (DAKO clone BU20a), Chk1 (Sigma C9358, 1:1000), FITC DAKO, F0232), FLAG (Sigma F3165, 1:1000), FLAG-HRP (Sigma A8592, 1:2000), GFP (Abcam ab290, 1:1000), LARG (Santa Cruz sc25638, 1:1000, and sc15439, 1:1000), TELO2 (Sigma, SAB1100719, 1:1000), pChk1 (Cell Signaling ♯2344, 1:1000), pH2AX (Cell Signaling ♯25775, 1:500), MLC2 (Abcam ab89594, 1:1000), pMLC2 (Cell Signaling ♯3671 and ♯3674, 1:1000), pRPA (Abcam ab61065, 1:500), Myc (Cell Signaling ♯2276, 1:2000), PCNT (Abcam ab4448 1:500–1:1000), RPA (Calbiochem NA19L, 1:500). For Western blotting, primary antibodies were visualised using HRP-conjugated anti-rabbit or anti-mouse secondary antibodies at 1:5000 (DAKO P0399 and P0447 respectively). For immunofluorescence, anti-mouse Alexa-488 or anti-rabbit Alexa-594 (Invitrogen) were used at 1:1000.
Cell lysis and protein gel blotting
For whole-cell extracts, cells were solubilized on ice for 20 minutes in lysis buffer; 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM DTT and 1 mM EDTA supplemented with 50 U/μl benzonase (Novagen), protease and phosphatase inhibitors (Sigma). Cleared lysates were produced by centrifugation of the resulting samples at 16,000 × g for 15 min at 4°C. Gel electrophoresis was performed using the NuPAGE system (Invitrogen). Briefly, samples were resolved on 4–12% Bis-Tris gels in MOPS buffer, transferred to a PVDF membrane which was then probed for the protein of interest using antibodies diluted in TBS containing 5% Marvel and 0.1% Tween-20 (Sigma). Quantification from western blot data was carried out using Image J image analysis software.
Immunofluorescence
Cells were grown on glass coverslips and treated as indicated, then fixed with 4% buffered paraformaldehyde for 10 min at RT, and permeabilised in PBS containing 0.5% Triton X-100 for 5 min at RT. Cells were then incubated with primary antibody for 1 hr at RT, and detected with a secondary Alexa-488 or Alexa-594 conjugated goat anti-rabbit or anti-mouse IgG. Antibody dilutions and washes after incubations were performed in TBS containing 3% BSA. DNA was stained with DAPI (1 μg/ml) and coverslips were mounted in Shandon Immu-Mount medium (Thermo). Fluorescence microscopy was performed on a Nikon Eclipse T200 inverted microscope (Melville), equipped with a Hamamatsu Orca ER camera and a 200 W metal arc lamp (Prior Scientific, United Kingdom), with a 60× and 100× objective lens. Images were captured and analyzed using Velocity software (Improvision).
Flow Cytometric Analyses
Cells were collected using trypsin, pelleted, washed with PBS, fixed in 70% ice-cold ethanol, and stored at −20°C for up to 2 weeks. After thoroughly washing with PBS, to remove any residual ethanol, cells were stained with a propidium iodide solution (50 μg/ml) containing RNase A (25 μg/ml) for 30 min before flow cytometry was performed on a Becton Dickinson FACScalibur instrument. The percentage of cells in each cell cycle phase was subsequently calculated using FloJo analysis software. For BrdU analyses, cells were pulsed with 10 μM BrdU (Sigma) for 20 min, then collected using trypsin. Pellets were washed with PBS, fixed in 70% ice-cold ethanol, and stored at −20°C for up to 2 weeks. To denature DNA, fixed cells were re-suspended in 2N HCl and incubated for 30 min at RT. After thoroughly washing with PBS, to remove any residual acid, cells were incubated with a mouse monoclonal anti-BrdU antibody diluted at a ratio of 1:50 in PBS-T (PBS containing 0.1% BSA and 0.2% Tween 20, pH 7.4) for 20 min at RT. Cells were then processed for PI staining and FACS analyses as described above.
Cytotoxicity assays
Cells were plated at a density of between 1000–2000 cells/well in 96-well plates (depending on cell line), and the following day transfected with appropriate siRNA, and drug added 2 d later at various concentrations. After 5 d of growth, MTT reagent was added to the cells at a final concentration of 3 mg/ml, and incubated at 37°C for 3 hrs. The media was removed and replaced with 200 μl DMSO to solubilise the formazan product, which was quantified by determining optical density at 540 nm using a spectrophotometric microtitre plate reader. Cytotoxicity was calculated for each treatment by normalization to appropriate vehicle only controls for each set of transfectants.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
956529_Supplementary_Materials.zip
Download Zip (1.1 MB)Acknowledgments
We thank Mikiko Takahashi, Teikyo Heisei University for kind gift of FLAG-PCNT expressing vectors. HeLa cells expressing GFP-centrin-2 were a kind gift from Dr. Fanni Gergely (Cambridge Research Institute, UK). We also respectively thank Prof. Mark O'Driscoll & Prof. Penny Jeggo (University of Sussex) and Dr. Chryso Kanthou (University of Sheffield) for technical advice regarding lymphoblast cell lines and pMLC protein gel blot techniques.
Funding
SJC is funded by a Cancer Research UK (CR-UK) Senior Cancer Research Fellowship (SCaRF; #C36435/A12102), which also supports KNM and CJS. RDDB was funded by a YCR PhD studentship (S001PhD), with consumables support and lab resources funded by SJC's CR-UK SCaRF.
Supplemental Materials
Supplemental materials for this article can be found on the publisher's website.
References
- Kourlas PJ, Strout MP, Becknell B, Veronese ML, Croce CM, Theil KS, Krahe R, Ruutu T, Knuutila S, Bloomfield CD, et al. Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: evidence for its fusion with MLL in acute myeloid leukemia. Proc Natl Acad Sci U S A 2000; 97:2145-50; PMID:10681437; http://dx.doi.org/10.1073/pnas.040569197
- Suzuki N, Nakamura S, Mano H, Kozasa T. Galpha 12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc Natl Acad Sci U S A 2003; 100:733-8; PMID:12515866; http://dx.doi.org/10.1073/pnas.0234057100
- Chikumi H, Barac A, Behbahani B, Gao Y, Teramoto H, Zheng Y, Gutkind JS. Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene 2004; 23:233-40; PMID:14712228; http://dx.doi.org/10.1038/sj.onc.1207012
- Mikelis CM, Palmby TR, Simaan M, Li W, Szabo R, Lyons R, Martin D, Yagi H, Fukuhara S, Chikumi H, et al. PDZ-RhoGEF and LARG are essential for embryonic development and provide a link between thrombin and LPA receptors and Rho activation. J Biol Chem 2013; 288:12232-43; PMID:23467409; http://dx.doi.org/10.1074/jbc.M112.428599
- Ong DC, Ho YM, Rudduck C, Chin K, Kuo WL, Lie DK, Chua CL, Tan PH, Eu KW, Seow-Choen F, et al. LARG at chromosome 11q23 has functional characteristics of a tumor suppressor in human breast and colorectal cancer. Oncogene 2009; 28:4189-200; PMID:19734946; http://dx.doi.org/10.1038/onc.2009.266
- Horiuchi A, Imai T, Wang C, Ohira S, Feng Y, Nikaido T, Konishi I. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest 2003; 83:861-70; PMID:12808121; http://dx.doi.org/10.1097/01.LAB.0000073128.16098.31
- Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y, Yoshida K. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res 2004; 10:4799-805; PMID:15269155; http://dx.doi.org/10.1158/1078-0432.CCR-0436-03
- Pan Y, Bi F, Liu N, Xue Y, Yao X, Zheng Y, Fan D. Expression of seven main Rho family members in gastric carcinoma. Biochem Biophys Res Commun 2004; 315:686-91; PMID:14975755; http://dx.doi.org/10.1016/j.bbrc.2004.01.108
- Li XR, Ji F, Ouyang J, Wu W, Qian LY, Yang KY. Overexpression of RhoA is associated with poor prognosis in hepatocellular carcinoma. Eur J Sur Oncol 2006; 32:1130-4; PMID:16806792; http://dx.doi.org/10.1016/j.ejso.2006.05.012
- Reuther GW, Lambert QT, Booden MA, Wennerberg K, Becknell B, Marcucci G, Sondek J, Caligiuri MA, Der CJ. Leukemia-associated Rho guanine nucleotide exchange factor, a Dbl family protein found mutated in leukemia, causes transformation by activation of RhoA. J Biol Chem 2001; 276:27145-51; PMID:11373293; http://dx.doi.org/10.1074/jbc.M103565200
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/10.1016/j.cell.2011.02.013
- Collis SJ, Barber LJ, Clark AJ, Martin JS, Ward JD, Boulton SJ. HCLK2 is essential for the mammalian S-phase checkpoint and impacts on Chk1 stability. Nat Cell Biol 2007; 9:391-401; PMID:17384638; http://dx.doi.org/10.1038/ncb1555
- Horejsi Z, Takai H, Adelman CA, Collis SJ, Flynn H, Maslen S, Skehel JM, de Lange T, Boulton SJ. CK2 phospho-dependent binding of R2TP complex to TEL2 is essential for mTOR and SMG1 stability. Mol Cell 2010; 39:839-50; PMID:20864032; http://dx.doi.org/10.1016/j.molcel.2010.08.037
- Takai H, Wang RC, Takai KK, Yang H, de Lange T. Tel2 regulates the stability of PI3K-related protein kinases. Cell 2007; 131:1248-59; PMID:18160036; http://dx.doi.org/10.1016/j.cell.2007.10.052
- Dubash AD, Guilluy C, Srougi MC, Boulter E, Burridge K, Garcia-Mata R. The small GTPase RhoA localizes to the nucleus and is activated by Net1 and DNA damage signals. PLoS One 2011; 6:e17380; PMID:21390328; http://dx.doi.org/10.1371/journal.pone.0017380
- Goulimari P, Knieling H, Engel U, Grosse R. LARG and mDia1 link Galpha12/13 to cell polarity and microtubule dynamics. Mol Biol Cell 2008; 19:30-40; PMID:17959834; http://dx.doi.org/10.1091/mbc.E06-11-1045
- Griffith E, Walker S, Martin CA, Vagnarelli P, Stiff T, Vernay B, Al Sanna N, Saggar A, Hamel B, Earnshaw WC, et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet 2008; 40:232-6; PMID:18157127; http://dx.doi.org/10.1038/ng.2007.80
- Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, Ekici AB, van Essen AJ, Goecke TO, Al-Gazali L, Chrzanowska KH, et al. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science 2008; 319:816-9; PMID:18174396; http://dx.doi.org/10.1126/science.1151174
- Willems M, Genevieve D, Borck G, Baumann C, Baujat G, Bieth E, Edery P, Farra C, Gerard M, Heron D, et al. Molecular analysis of pericentrin gene (PCNT) in a series of 24 Seckel/microcephalic osteodysplastic primordial dwarfism type II (MOPD II) families. J Med Genet 2010; 47:797-802; PMID:19643772; http://dx.doi.org/10.1136/jmg.2009.067298
- Aoki T, Ueda S, Kataoka T, Satoh T. Regulation of mitotic spindle formation by the RhoA guanine nucleotide exchange factor ARHGEF10. BMC Cell Biol 2009; 10:56; PMID:19635168; http://dx.doi.org/10.1186/1471-2121-10-56
- Collis SJ, Ciccia A, Deans AJ, Horejsi Z, Martin JS, Maslen SL, Skehel JM, Elledge SJ, West SC, Boulton SJ. FANCM and FAAP24 function in ATR-mediated checkpoint signaling independently of the Fanconi anemia core complex. Molecular cell 2008; 32:313-24; PMID:18995830; http://dx.doi.org/10.1016/j.molcel.2008.10.014
- Matsuo K, Nishimura T, Hayakawa A, Ono Y, Takahashi M. Involvement of a centrosomal protein kendrin in the maintenance of centrosome cohesion by modulating Nek2A kinase activity. Biochem Biophys Res Commun 2010; 398:217-23; PMID:20599736; http://dx.doi.org/10.1016/j.bbrc.2010.06.063
- Gillingham AK, Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep 2000; 1:524-9; PMID:11263498; http://dx.doi.org/10.1093/embo-reports/kvd105
- Alderton GK, Joenje H, Varon R, Borglum AD, Jeggo PA, O'Driscoll M. Seckel syndrome exhibits cellular features demonstrating defects in the ATR-signalling pathway. Hum Mol Genet 2004; 13:3127-38; PMID:15496423; http://dx.doi.org/10.1093/hmg/ddh335
- Staples CJ, Myers KN, Beveridge RD, Patil AA, Lee AJ, Swanton C, Howell M, Boulton SJ, Collis SJ. The centriolar satellite protein Cep131 is important for genome stability. J Cell Sci 2012; 125:4770-9; PMID:22797915; http://dx.doi.org/10.1242/jcs.104059
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem 2001; 276:47759-62; PMID:11673449; http://dx.doi.org/10.1074/jbc.M009785200
- Block WD, Yu Y, Lees-Miller SP. Phosphatidyl inositol 3-kinase-like serine/threonine protein kinases (PIKKs) are required for DNA damage-induced phosphorylation of the 32 kDa subunit of replication protein A at threonine 21. Nucleic Acids Res 2004; 32:997-1005; PMID:14872059; http://dx.doi.org/10.1093/nar/gkh265
- Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 2003; 300:1542-8; PMID:12791985; http://dx.doi.org/10.1126/science.1083430
- Zeman MK, Cimprich KA. Causes and consequences of replication stress. Nat Cell Biol 2014; 16:2-9; PMID:24366029; http://dx.doi.org/10.1038/ncb2897
- Kitzing TM, Sahadevan AS, Brandt DT, Knieling H, Hannemann S, Fackler OT, Grosshans J, Grosse R. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev 2007; 21:1478-83; PMID:17575049; http://dx.doi.org/10.1101/gad.424807
- O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet 2003; 33:497-501; PMID:12640452; http://dx.doi.org/10.1038/ng1129
- Kalay E, Yigit G, Aslan Y, Brown KE, Pohl E, Bicknell LS, Kayserili H, Li Y, Tuysuz B, Nurnberg G, et al. CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat Genet 2011; 43:23-6; PMID:21131973; http://dx.doi.org/10.1038/ng.725
- Sivasubramaniam S, Sun X, Pan YR, Wang S, Lee EY. Cep164 is a mediator protein required for the maintenance of genomic stability through modulation of MDC1, RPA, and CHK1. Genes Dev 2008; 22:587-600; PMID:18283122; http://dx.doi.org/10.1101/gad.1627708
- Staples CJ, Myers KN, Beveridge RD, Patil AA, Howard AE, Barone G, Lee AJ, Swanton C, Howell M, Maslen S, et al. Ccdc13; a novel human centriolar satellite protein required for ciliogenesis and genome stability. J Cell Sci 2014; 127(Pt 13):2910-9; PMID:24816561; http://dx.doi.org/10.1242/jcs.147785
- Alderton GK, Galbiati L, Griffith E, Surinya KH, Neitzel H, Jackson AP, Jeggo PA, O'Driscoll M. Regulation of mitotic entry by microcephalin and its overlap with ATR signalling. Nat Cell Biol 2006; 8:725-33; PMID:16783362; http://dx.doi.org/10.1038/ncb1431
- Godinho SA, Picone R, Burute M, Dagher R, Su Y, Leung CT, Polyak K, Brugge JS, Thery M, Pellman D. Oncogene-like induction of cellular invasion from centrosome amplification. Nature 2014; 510:167-71; PMID:24739973; http://dx.doi.org/10.1038/nature13277
- Chaki M, Airik R, Ghosh AK, Giles RH, Chen R, Slaats GG, Wang H, Hurd TW, Zhou W, Cluckey A, et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell 2012; 150:533-48; PMID:22863007; http://dx.doi.org/10.1016/j.cell.2012.06.028
- Choi HJ, Lin JR, Vannier JB, Slaats GG, Kile AC, Paulsen RD, Manning DK, Beier DR, Giles RH, Boulton SJ, et al. NEK8 Links the ATR-Regulated Replication Stress Response and S Phase CDK Activity to Renal Ciliopathies. Mol Cell 2013; 51:423-39; PMID:23973373; http://dx.doi.org/10.1016/j.molcel.2013.08.006
- Valdes-Sanchez L, De la Cerda B, Diaz-Corrales FJ, Massalini S, Chakarova CF, Wright AF, Bhattacharya SS. ATR localizes to the photoreceptor connecting cilium and deficiency leads to severe photoreceptor degeneration in mice. Hum Mol Genet 2013; 22:1507-15; PMID:23297361; http://dx.doi.org/10.1093/hmg/dds563
- Zhou W, Otto EA, Cluckey A, Airik R, Hurd TW, Chaki M, Diaz K, Lach FP, Bennett GR, Gee HY, et al. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat Genet 2012; 44:910-5; PMID:22772369; http://dx.doi.org/10.1038/ng.2347
- Chavali PL, Gergely F. Cilia born out of shock and stress. Embo J 2013; 32:3011-3; PMID:24185901; http://dx.doi.org/10.1038/emboj.2013.241
- Stiff T, Alagoz M, Alcantara D, Outwin E, Brunner HG, Bongers EM, O'Driscoll M, Jeggo PA. Deficiency in origin licensing proteins impairs cilia formation: implications for the aetiology of Meier-Gorlin syndrome. PLoS Genet 2013; 9:e1003360; PMID:23516378; http://dx.doi.org/10.1371/journal.pgen.1003360
- Pan J, You Y, Huang T, Brody SL. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J Cell Sci 2007; 120:1868-76; PMID:17488776; http://dx.doi.org/10.1242/jcs.005306
- Madhivanan K, Mukherjee D, Aguilar RC. Lowe syndrome: Between primary cilia assembly and Rac1-mediated membrane remodeling. Commun Integr Biol 2012; 5:641-4; PMID:23739214; http://dx.doi.org/10.4161/cib.21952
- Cheung HC, San Lucas FA, Hicks S, Chang K, Bertuch AA, Ribes-Zamora A. An S/T-Q cluster domain census unveils new putative targets under Tel1/Mec1 control. BMC Genomics 2012; 13:664; PMID:23176708; http://dx.doi.org/10.1186/1471-2164-13-664
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007; 316:1160-6; PMID:17525332; http://dx.doi.org/10.1126/science.1140321
- O'Driscoll M. Diseases associated with defective responses to DNA damage. Cold Spring Harb Perspect Biol 2012; 4:1-25; PMID:23209155; http://dx.doi.org/10.1101/cshperspect.a012773
- Alcantara D, O'Driscoll M. Congenital microcephaly. Am J Med Genet C Semin Med Genet 2014; 166C(2):124-39; PMID:24816482
