Abstract
Age-related macular degeneration (AMD), a neurodegenerative and vascular retinal disease, is the leading cause of blindness in the developed world. Accumulating evidence suggests that alterations in the expression of a small heat shock protein (αB-crystallin) are involved in the pathogeneses of AMD. Here we demonstrate that senescence-accelerated OXYS rats—an animal model of the dry form of AMD—develop spontaneous retinopathy against the background of reduced expression of αB-crystallin in the retina at the early preclinical stages of retinopathy (age 20 days) as well as at 4 and 24 months of age, during the progressive stage of the disease. The level of αA-crystallin expression in the retina of OXYS rats at all the ages examined was no different from that in disease-free Wistar rats. Treatment with the mitochondria-targeted antioxidant SkQ1 (plastoquinonyl-decyltriphenylphosphonium) from 1.5 to 4 months of age, 250 nmol/kg, increased the level of αB-crystallin expression in the retina of OXYS rats. SkQ1 slowed the development of retinopathy and reduced histological aberrations in retinal pigment epithelium cells. SkQ1 also attenuated neurodegenerative changes in the photoreceptors and facilitated circulation in choroid blood vessels in the retina of OXYS rats; this improvement was probably linked with the restoration of αB-crystallin expression.
Abbreviations
| AMD | = | age-related macular degeneration |
| RPE | = | retinal pigment epithelium |
| SkQ1 | = | 10-(6′ plastoquinonyl)decyltriphenylphosphonium |
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible visual impairment and blindness in industrialized countries. The prevalence of AMD is increasing dramatically as the proportion of the elderly in the population continues to rise. AMD is a multifactorial disease involving a complex interplay of genetic, environmental, metabolic, and functional factors. The main pathological changes that drive AMD are inflammation and oxidative and endoplasmic reticulum (ER) stress.Citation1,2
There is evidence that the development of AMD is associated with changes in expression and in functional activity of a small heat shock protein,Citation3 α-crystallin,Citation4 originally known as one of the structural proteins of the lens.Citation5 α-Crystallin is a soluble cytosolic protein, but it is also localized within subcellular organelles including mitochondria and ER.Citation6 α-Crystallin is expressed in many tissue types, including the retina, brain, muscle, spleen, lung, and skin, where α-crystallin is regulated by oxidative stress and angiogenesis and inhibits apoptosis and β-amyloid fibril formation.Citation7 An α-crystallin molecule is composed of 2 homologous subunits: αA- and αB-crystallin. Altered expression and/or accumulation of αB-crystallin are involved in a wide range of retinal diseases including AMD, diabetic retinopathy, uveitis, trauma, and ischemia.Citation4 Recently, αB-crystallin has been shown to have anti-inflammatory properties and was identified as an important regulator of mitochondria-mediated apoptosis; it inhibits apoptosis induced by oxidative stress in retinal pigment epithelium (RPE) and progression of retinal degeneration in animal models.Citation7,8 Thus, the contribution of alterations of molecular chaperones to the pathogenesis of AMD is obvious, but the mechanism behind this effect is poorly understood.
According to the clinical signs, there are two forms of AMD: dry (also known as nonexudative or atrophic AMD, ∼90% of all cases) and exudative (also known as wet or neovascular AMD, ∼10% of cases). There are effective treatments of vascular complications of neovascular AMD, but there is neither a treatment of the dry form of AMD nor preventive strategies against progression to the exudative form of AMD. Therefore, development of effective therapeutic and prophylactic agents against AMD is urgently needed. Recently, using senescence-accelerated OXYS rats as a model of AMD, we showed that the mitochondria-targeted antioxidant SkQ1 (plastoquinonyl-decyltriphenylphosphonium) is a promising pharmacological agent against the dry form of AMD. SkQ1 at nanomolar concentrations is capable of not only preventing the development of AMD-like retinopathy in OXYS rats but also reversing the existing pathological alterations in the retina.Citation9-12 It was also shown that SkQ1 has a therapeutic potential against other age-related diseasesCitation9,13-17 and increases the lifespan.Citation18,19
It is believed that the development of retinopathy in OXYS rats, just like AMD in humans, is associated with progressive mitochondrial dysfunctionCitation20,21 and with accumulation of β-amyloid.Citation22 Recently, by means of high-throughput RNA sequencing (RNA-Seq), we determined that the retinopathy in OXYS rats develops simultaneously with a decrease in the mRNA level of hundreds of genes including αB-crystallin (Cryab).Citation23 The aim of the present study was to analyze possible associations of protein expression of α-crystallin in the rat retina with the development of AMD-like retinopathy and with its response to SkQ1treatment.
Results
Protein expression of αA- and αB-crystallin in the retina
Western blot analysis of α-crystallin levels in the retina of OXYS and Wistar rats (20-day-old and 4- and 24-month-old animals) are shown in . There was no difference between the level of αA-crystallin protein in the retina of OXYS rats and that in age-matched Wistar rats at all the ages examined. In contrast, expression of αB-crystallin, regardless of age, was lower in OXYS rats compared to Wistar rats (p < 0.001): at the age of 20 d by 60%, at 4 months by 51%, and at 24 months by 57% ().
Table 1. Protein expression of αA- and αB-crystallin in the retina of 20-day-old and 4- and 24-month-old senescence-accelerated OXYS rats
Effects of dietary supplementation with SkQ1
SkQ1 reduces clinical signs of retinopathy
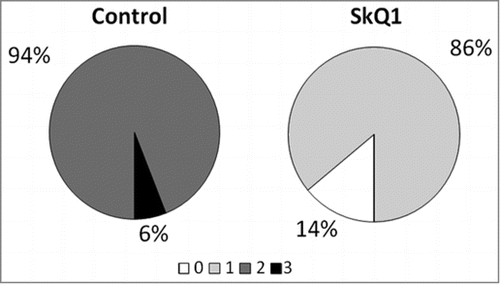
Both before and after supplementation with SkQ1, the animals were examined by an ophthalmologist (2 times total for each rat). The results of examination are shown in . The first (preliminary) examination of rats at the age of 1.5 months revealed that the same percentage of eyes in the experimental and the control group of OXYS rats had signs of the first stage of retinopathy (21% and 22% respectively). In this study, SkQ1 did not prevent completely but reduced the development of retinopathy in OXYS rats (). Therefore, at the age of 4 months, 86% of OXYS rats' eyes had signs of first-stage retinopathy, while in 14% of the eyes, the signs of the disease were not detectable. In contrast, in untreated OXYS rats, retinopathy did develop, and at the age of 4 months, in 94% and 6% of eyes we found signs corresponding to the second and third stage of the disease, respectively. Both the first and second ophthalmoscopic examination did not reveal pathological alterations in the retina of Wistar rats.
Figure 1. Treatment with 250 nmol/kg per day of SkQ1, starting at 1.5 months of age, attenuated the development of retinopathy in OXYS rats. The data are presented as stages (0, 1, 2, and 3) of retinopathy in 4-month-old control (untreated) and SkQ1-treated OXYS rats. In each group, 50 eyes of 25 animals were examined.
SkQ1 prevents aberrations of the RPE and choroid vasculature and reduces neurodegeneration as assessed using histological examination
We next compared the histological features of the retina in OXYS and Wistar rats. In OXYS rats, there were prominent aberrations of the choroidal vasculature, RPE cells, photoreceptors, associative and ganglion neurons, and radial glial cells. Unlike the choroid of Wistar rats (), the choroid of OXYS rats exhibited disturbances of blood flow: aggregation of blood cells, stasis, and thrombosis of small vessels (). Treatment with SkQ1 prevented the vessel problems in OXYS rats ().
Table 2. Morphometric parameters of the chorioretinal complex of 4-month-old Wistar and OXYS rats and SkQ1-treated OXYS rats
Figure 2. The morphology of the retina of 4-month-old rats. (A) In Wistar rats, RPE cells had a prismatic shape with oval nuclei (black arrows), normal retinal layers. (B) In OXYS rats, stasis, and sludge of the blood cells are visible in capillaries of the choroid, RPE cells were flat, with a variable size and shape of the nuclei (black arrows), pyknosis of nuclei of neurosensory cells. (C and D) In OXYS rats: treatment with SkQ1 prevented anomalies in the RPE cells (black arrows) and decreased the number of neurosensory cells with pyknotic nuclei in the outer and in the inner nuclear layer. The scale bar: 10 μm, staining: H&E; abbreviations: outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), and retinal pigment epithelium (RPE).
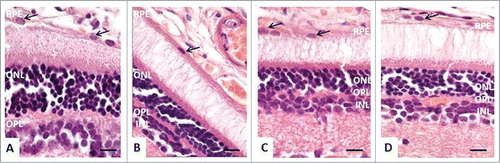
In Wistar rats, RPE cells had a prismatic shape with oval nuclei (). The cellular monolayer was dense, with normal contacts, suggestive of a functional blood–retina barrier. In OXYS rats, RPE cells were flat, with a variable size and shape of the nuclei (). Consequently, the specific area of retinal vessels with signs of partial occlusion was significantly greater in OXYS rats compared to the Wistar strain. Analysis of morphometric data showed that the average area of an RPE cell was 19% smaller in OXYS rats than in Wistar rats. SkQ1 increased this metric 2-fold. As a result, in SkQ1-treated OXYS rats, the average area of an RPE cell was greater than that in untreated Wistar rats; RPE cells retained a uniform shape and close contacts, consistent with the functional barrier. At the age of 4 months, the number of rows in the outer nuclear layer in retinas of OXYS rats () tended to decrease compared to Wistar rats. Dietary supplementation with SkQ1 increased this parameter in OXYS rats to the level of untreated Wistar rats (). In OXYS rats, there was nuclear pyknosis in photoreceptors, whereas supplementation with SkQ1 decreased the percentage of photoreceptors with pyknotic nuclei (C,D). In the retina of OXYS rats, ganglion and associative neurons degenerated, and radial glial cells showed chromatolysis and pyknotic nuclei (). There were approximately 2-fold more of such aberrations in OXYS rats compared to Wistar rats; this result pointed to a decline of the reserve capacity of those neurons. SkQ1 prevented the degeneration of the ganglion neurons, associative neurons, and of radial glial cells in the retina of OXYS rats ().
Thus, SkQ1 prevented anomalies in the RPE cells and in the retinal barrier and suppressed neurodegenerative changes in the inner retina. SkQ1 also significantly improved circulation in choroid blood vessels.
SkQ1 increased the level of αB-crystallin in the retina
Western blot data revealed no differences in the protein level of αA-crystallin in the retina of OXYS and Wistar rats (). Treatment with SkQ1 had no influence on its level in OXYS rats (p > 0.05; ).The level of αB-crystallin in the retina of the untreated OXYS rats was 2-fold lower than in Wistar rats (p < 0.001; ). After treatment with SkQ1, in the retina of OXYS rats we observed an increase of the level of αB-crystallin over and above the level in untreated OXYS and Wistar rats (p < 0.01; ).
Figure 3. Effects of treatment with SkQ1 on the protein levels of αА- and αВ-crystallin in the retina of OXYS rats. (A) Western blot analysis. (B) The protein gel blot results quantified as a percent of data from untreated age-matched Wistar rats (mean ± SEM), normalized to β-actin from 5 independent experiments. 1: Wistar rats, 2: OXYS rats, 3: OXYS rats treated with SkQ1. #Significant differences between untreated OXYS and Wistar rats (P < 0.05); *a significant effect of treatment with SkQ1 (P < 0.05). Confocal immunofluorescent images depict αB-crystallin (red signal) detected within the retina and RPE in OXYS rats (C) compared to disease-free Wistar (D) rats and (E) SkQ1-treated OXYS rats (250 nmol/kg per day from 1.5 to 4 months of age). Cell nuclei were stained with DAPI (blue). The scale bar: 50 μm. Abbreviations: outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), retinal pigment epithelium (RPE), and ganglion layer (GL).
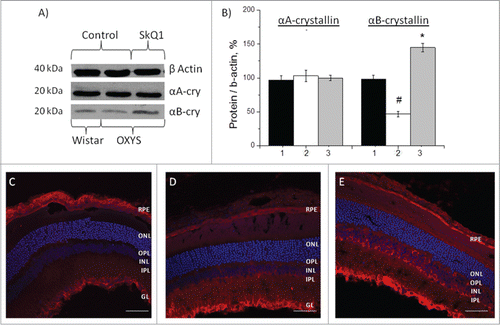
The data from western blotting were confirmed using immunohistochemical analysis. Immunostaining of retinal cryosections of 4-month-old rats revealed a decrease of αB-crystallin expression in all layers of retina of OXYS rats—RPE, the photoreceptor outer and inner segment—compared to disease-free controls (Wistar rats; ). Treatment with SkQ1 upregulated αB-crystallin within the retina of OXYS rats (p < 0.05) in RPE and in the inner nuclear layer compared to the untreated OXYS rats and untreated disease-free Wistar rats ().
Discussion
Degeneration and a loss of RPE and choroidal involution with a secondary loss of photoreceptors are cardinal features of the predominant form of AMD (dry form). OXYS rats develop retinopathy similar to the dry form of AMD;Citation24 the retinopathy in OXYS rats is associated with the decline of the amount of RPE cells and alterations of choroidal microcirculation.Citation12 The molecular mechanisms that lead to these atrophic changes have yet to be characterized.
There is evidence that changes in expression of α-crystallin are linked to AMD, and some authors suggested to use overexpression of αB-crystallin as a biomarker of the disease.Citation4,25 In contrast, using RNA-Seq, we showed recently that the level of αB-crystallin mRNA in the OXYS retina is considerably decreased compared to Wistar rats at the age of 3 and 18 months.Citation23 Here we demonstrated that OXYS rats develop AMD-like retinopathy simultaneously with reduced expression of the αB-crystallin protein. We also showed in this study that the mitochondria-targeted antioxidant SkQ1 inhibits the development of retinopathy and increases (restores) the protein level of αB-crystallin in the retina.
Comparison of the levels of αA-crystallin expression in the retina of OXYS rats at the age of 20 d and 4 and 24 months showed no differences with Wistar rats. On the other hand, protein expression of αB-crystallin was reduced in the retina of OXYS rats at all the ages examined.
Recently, we reported that the development of retinopathy in OXYS rats is associated with alterations in RPE cells and choroid vessels at the age of 20 d.Citation12 In the present study, we detected downregulation of αB-crystallin in the retina of OXYS rats already at the age of 20 days, when any clinical signs of retinopathy are still absent. In support of the possible role of the αB-crystallin loss in the pathogenesis of retinopathy, it was shown by others that the pathological changes in retinal vasculature are associated with αB-crystallin downregulation.Citation7,26 Deficient vasculature could lead to hypoxia. Ischemia of the retina leads to metabolic changes in the early phases of degeneration, and these alterations contribute to further worsening of the loss of photoreceptor cells. It is possible that these pathological changes ultimately lead to a complete loss of photoreceptor cells in the OXYS retina by age 24 months.Citation12
An increase of α-crystallin expression is a normal response to stressors.Citation27-29 At the same time, chronic stress can downregulate αB-crystallin, and this change could promote degenerative processes.Citation4,30,31 We can hypothesize that the hereditary impairment of mRNA expression of αB-crystallin (Cryab) gene in OXYS ratsCitation23 and the same deficiency demonstrated here (the reduced level of the αB-crystallin protein in the OXYS retina)—in conjunction with the inability to fully respond to stress—can contribute to the development of AMD-like retinopathy in OXYS rats.
In the present study, in support of our previous reports,Citation10-11,21 we demonstrated that dietary supplementation with the mitochondria-targeted antioxidant SkQ1 is effective at suppressing the development of retinopathy in OXYS rats. Here we for the first time showed that the therapeutic action of SkQ1 involves restoration (increase) of expression of a small heat shock protein, αB-crystallin, in the retina of OXYS rats.
Within the retina, αB-crystallin expression has been well documented in the neural retina and in RPE.Citation4 A similar localization was also observed in our study. αB-Crystallin plays an important role in maintaining a neuroprotective environment in the retina. As shown by Bhat and Gangalum (32), exosome-mediated release of αB-crystallin from RPE cells can protect the photoreceptors from oxidative injury. The low level of αB-crystallin in RPE cells of OXYS rats renders them susceptible to oxidant-induced cell death and worsens their chances of survival. Our present data show that SkQ1: 1) prevents significant anomalies in RPE cells and neurodegenerative changes in photoreceptors and 2) normalizes/promotes circulation in choroid blood vessels, presumably, via the increase of αB-crystallin expression, as explained below.
Studies of RPE in αB-crystallin knockout mice have shown that αB-crystallin supports retinal and choroidal angiogenesis through the interaction with vascular endothelial growth factor (VEGF).Citation33,34 Recently, we reported a reduction of VEGF gene expression in the retina of OXYS rats compared to the parent Wistar strain (control) during retinopathy progressionCitation12; this effect may be linked to the early alterations in RPE cells and choroid vessels in OXYS rats. We supposed that such changes are prerequisite to the development of retinopathy and that their reversal is necessary for the prevention of the disease. Indeed, supplementation with SkQ1 restored the levels of VEGF gene expression in the retina of OXYS rats without neovascularization.Citation21
There is a growing body of evidence in support of an association of mitochondrial dysfunction with retinal degenerative diseases including AMD.Citation35 It is thought that the accelerated senescence and development of age-related diseases in OXYS rats is also linked with progressive mitochondrial dysfunction.Citation21,23,36 Thus, we can hypothesize that SkQ1 protects against stress and restores mitochondrial function in the retina of OXYS rats. In RPE cells, αB-crystallin provides critical protection of mitochondrial function, which prevents ER stress–mediated apoptosis.Citation8 In the present work, SkQ1 increased the protein expression of αB-crystallin in RPE and in the outer and inner segment of the OXYS retina. Successful treatment with melatonin,Citation37 SkQ1, and other antioxidantsCitation38 further support the idea that oxidative stress is directly linked to the age-associated neurodegenerative phenotype of OXYS rats, including AMD.
Some evidence exists that αB-crystallin is a potential therapeutic molecule for the treatment of β-amyloid (Aβ)–associated retinal diseases and other neurodegenerative diseases.Citation7 αB-Crystallin inhibits Aβ fibril formation.Citation39 Recently, we showed that Aβ accumulates with age in the retina and in the brain of OXYS rats;Citation22,40 however, the level of Aβ is not elevated at the early stages of retinopathy, that is, at the age of 3 months.Citation22 The Aβ level increases in the retina of middle-aged and old OXYS rats, when these rats present with severe stages of the disease accompanied by pronounced neurodegenerative changes.Citation22 In the present work, we did not evaluate the effect of SkQ1 on the level of Aβ in young OXYS rats.
Previously, we showed that long-term treatment with SkQ1 starting at age 1.5 months inhibits the development of retinopathy in OXYS rats up to 24 months of age.Citation9 Moreover, SkQ1 increases the αB-crystallin level and decreases the Aβ level in the retina (manuscript in preparation) as well as in the brain.Citation40 These findings are consistent with the present study and support the view that αB-crystallin is involved in degeneration of the retina. In conclusion, our results may lead to the identification of new therapeutic targets in AMD or at least will advance the understanding of the pathophysiology of this disease.
Methods
Animals, diet, and ophthalmoscopic examination
Male senescence-accelerated OXYS and age-matched male Wistar rats were obtained from the Breeding Experimental Animal Laboratory of the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences (SB RAS; Novosibirsk, Russia).
All animal procedures were in compliance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research as well as the European Communities Council Directive No. 86/609/EES. At the age of 4 weeks, the pups were weaned and housed in groups of 5 animals per cage (57 × 36 × 20 cm) and kept under standard laboratory conditions (at 22 ± 2°C, 60% relative humidity and natural light), provided with standard rodent feed, PK-120-1, Ltd. (Laboratorsnab, Russia) and given water ad libitum.
To estimate the age-related changes of the α-crystallin level in the rat retina, we used 20-day-old and 4- and 24-month-old OXYS rats and age-matched Wistar rats as a control (7 rats per group).
To assess the influence of treatment with SkQ1 (from the age of 1.5 months to the age of 4 months) on retinopathy development, 1.5-month-old male OXYS rats were randomly assigned to 1 of the 2 groups: the standard (control) diet or the diet supplemented with 250 nmol SkQ1 per kilogram of body weight per day (15 rats per group). The age-matched Wistar rats (standard diet) served as a control (15 rats in this group). The weight gain was measured in the course of the experiment. Body weight was significantly higher in Wistar rats than in OXYS rats before the start of SkQ1 treatment at the age of 1.5 months (208 ± 3.3 g and 183 ± 6.0 g respectively, p < 0.05) and at the age of 4 months (465 ± 7.8 g and 365 ± 5.4 g, respectively, p < 0.05). SkQ1 treatment did not affect body weight of OXYS rats (p > 0.05).
Ophthalmoscopic examination (after dilatation with 1% tropicamide) of rats' eyes was carried out using a Betta direct ophthalmoscope (Heine, Germany) twice: before (at the age of 1.5 months) and after SkQ1 supplementation (age 4 months). Assessment of stages of retinopathy corresponding to stages of AMD in humans was carried out according to the Age-Related Eye Disease Study (AREDS) grade protocol (http://eyephoto.ophth.wisc.edu). Wistar rats were used as a control strain (disease-free, not treated with SkQ1).
The rats were euthanized using CO2 inhalation and decapitated 5 d after the last examination of eyes. The retinas were removed, frozen, and stored at −80°C until analysis.
Immunoblot and antibodies
Total protein was isolated from retinas using RIPA buffer (50 mmol Tris-HCl pH 7.4, 150 mmol NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and 1 mmol EDTA) supplemented with a protease inhibitor cocktail (cat. #P8340, Sigma–Aldrich). After incubation for 30 minutes on ice, the protein samples were centrifuged at 12,000 g for 30 minutes at 4°C. The total protein in the samples was measured using a Bio-Rad Bradford kit (Bio-Rad Laboratories, USA) and was separated by means of 15% (w/v) SDS-polyacrylamide gel electrophoresis (50 μg protein per lane) and then transferred to a nitrocellulose membrane using a liquid transfer system (Bio-Rad Laboratories, USA). The membranes were blocked in BSA (5% w/v in PBS with Tween 0.1%) for 1 h at room temperature (RT), then incubated with primary antibodies (anti–αA- or anti–αB-crystallin or anti–β-actin) for 1 h at RT (1:1,000 dilution; #14821, #5577, Abcam, USA), followed by a secondary antibody: either an anti–rabbit IgG or an anti–mouse IgG antibody (1:5,000 dilution; # 6721, #6808, Abcam, USA). The signals were scanned and the intensity of the emission bands was measured using the ImageJ 1.44 software (NIH, Bethesda, MD) and normalized to β-actin (loading control).
Immunohistochemistry
The eyes were removed and fixed in fresh 4% paraformaldehyde in PBS, for 1 h, washed 3 times in PBS, then cryopreserved in graded sucrose solutions. Posterior eyecups were embedded in Killik (Bio-Optica, Italy), frozen, and stored at −80°C. The sections (14 μm thick) were made on a Microm HM-505 N cryostat (Microm, Germany) at −20°C, transferred onto Polysine glass slides (Menzel-Glaser, Braunschweig, Germany) and stored at −20°C. The sections were incubated for 1 h in the buffer containing 5% BSA and 0.3% Triton X-100 in PBS, followed by an overnight incubation at +4°C with a rabbit polyclonal antibody to αB-crystallin (1:50 dilution; #5577, Abcam, USA). After washing in PBS, the sections were incubated for 1 h with a secondary antibody: a Cy3-conjugated donkey anti–rabbit IgG antibody (Millipore, USA) at a dilution of 1:100. In negative controls, the primary antibody was omitted. The sections were washed in PBS and coverslipped with the Fluoroshield mounting medium containing DAPI (Abcam, USA). Confocal images were acquired using a Zeiss LSM780 confocal microscope (Carl Zeiss, Germany). Gain settings (when taking pictures) were the same for all samples. No signals were detected in the control without a primary antibody. The images were processed using the Axiovision 4.8 software (Carl Zeiss Vision, Hallbergmoos, Germany).
Histological analysis
The posterior wall of the eye was collected, fixed in 12% neutral formalin during the day, washed in distilled water, dehydrated in ascending alcohol concentrations, and was embedded in paraffin. Serial frontal sections (4 to 5 μm thick) were made, stained with hematoxylin and eosin (H&E), and examined under a microscope (Carl Zeiss Axiostar plus; Germany). The morphometric parameters were measured using quantitative analysis of the images in the Axiovision 4.8 software (Carl Zeiss Vision, Hallbergmoos, Germany). Estimation was performed by examining 5 random fields of view for each retina, with magnification of 10 × 100 using frame area of 900 μm. The specific area of choroid vessels (open, with stasis, or with thrombosis) and the specific area of RPE cells were calculated. The number of ganglion neurons with central and total chromatolysis and nuclear pyknosis was calculated separately. The percentage of photoreceptors with nuclear pyknosis was calculated per 1000 photoreceptors, percentage of radial glial cells, and neurons in the inner nuclear and ganglionar layer per 200 corresponding cells of the retina.
Statistical analysis
The data were analyzed using repeated-measures ANOVA (analysis of variance) and nonparametric tests using the statistical package Statistica 6.0. ANOVA was used to evaluate the differences between OXYS and Wistar rats and the effects of dietary supplementation with SkQ1. The Newman–Keuls post hoc test was applied to significant main effects and interactions in order to estimate the differences between those sets of means. One-way ANOVA was used for pairwise group comparisons. The data were presented as mean ± SEM. Comparison of means was conducted using either one-way or repeated-measures ANOVA, when appropriate. The differences were considered statistically significant if p < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Microscopy was performed at the Microscopy Center of the Institute of Cytology and Genetics, SB RAS, Russia.
Funding
This study was supported by the Russian Foundation for Basic Research (Grant # 14-04-00376A) and partially by Grants of the Government of the Russian Federation N 2012-220-03-435 and N 14.B25.31.0033.
References
- Kaarniranta K, Salminen A, Haapasalo A, Soininen H, Hiltunen M. Age-related macular degeneration (AMD): Alzheimer's disease in the eye? J Alzheimers Dis 2011; 24(4):615-31; PMID:21297256; http://dx.doi.org/10.3233/JAD-2011-101908
- Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta 2013; 1833(12):3460-70; PMID:23850759; http://dx.doi.org/10.1016/j.bbamcr.2013.06.028
- Landis G, Shen J, Tower J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging (Albany NY) 2012; 4(11):768-89; PMID:23211361
- Fort PE, Lampi KJ. New focus on alpha-crystallins in retinal neurodegenerative diseases. Exp Eye Res 2011; 92(2):98-103; PMID:21115004; http://dx.doi.org/10.1016/j.exer.2010.11.008
- Bloemendal H. Lens proteins. CRC Critical Reviews in Biochem 1982; 12:1-38; http://dx.doi.org/10.3109/10409238209105849
- Yaung J, Jin M, Barron E, Spee C, Wawrousek EF, Kannan R, Hinton DR. Alpha-crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol Vis 2007; 13:566e577
- Kannan R, Sreekumar PG, Hinton DR. Novel roles for α-crystallins in retinal function and disease. Prog Retin Eye Res 2012; 31(6):576-604; PMID:22721717; http://dx.doi.org/10.1016/j.preteyeres.2012.06.001
- Dou G, Sreekumar PG, Spee C, He S, Ryan SJ, Kannan R, Hinton DR. Deficiency of αB crystallin augments ER stress-induced apoptosis by enhancing mitochondrial dysfunction. Free Radic Biol Med 2012; 1;53(5):1111-22; PMID:22781655; http://dx.doi.org/10.1016/j.freeradbiomed.2012.06.042
- Neroev VV, Archipova MM, Bakeeva LE, Fursova AZh, Grigorian EN, Grishanova AY, Iomdina EN, Ivashchenko ZhN, Katargina LA, Khoroshilova-Maslova IP, et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 4. Age-related eye disease. SkQ1 returns vision to blind animals. Biochem (Mosc) 2008; 73(12):1317-28; PMID:19120017; http://dx.doi.org/10.1134/S0006297908120043
- Saprunova VB, Pilipenko DI, Alexeevsky AV, Fursova AZh, Kolosova NG, Bakeeva LE. Lipofuscin granule dynamics during development of age-related macular degeneration. Biochem (Mosc) 2010; 75(2):130-8; PMID:20367599; http://dx.doi.org/10.1134/S0006297910020021
- Saprunova VB, Lelekova MA, Kolosova NG, Bakeeva LE. SkQ1 slows development of age-dependent destructive processes in retina and vascular layer of eyes of wistar and OXYS rats. Biochem (Mosc) 2012; 77(6):648-58; PMID:22817465; http://dx.doi.org/10.1134/S0006297912060120
- Markovets AM, Saprunova VB, Zhdankina AA, Fursova AZh, Bakeeva LE, Kolosova NG. Alterations of retinal pigment epithelium cause AMD-like retinopathy in senescence-accelerated OXYS rats. Aging (Albany NY) 2011; 3:44-54; PMID:21191149
- Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, et al. An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta 2009; 1787(5):437-61; PMID:19159610; http://dx.doi.org/10.1016/j.bbabio.2008.12.008
- Kolosova NG, Stefanova NA, Muraleva NA, Skulachev VP. The mitochondria-targeted antioxidant SkQ1 but not N-acetylcysteine reverses aging-related biomarkers in rats. Aging (Albany NY) 2012; 4(10):686-94; PMID:23104863
- Vays VB, Eldarov CM, Vangely IM, Kolosova NG, Bakeeva LE, Skulachev VP. Antioxidant SkQ1 delays sarcopenia-associated damage of mitochondrial ultrastructure. Aging (Albany NY) 2014; 6(2):140-8; PMID:24519884
- Kolosova NG, Vitovtov AO, Muraleva NA, Akulov AE, Stefanova NA, Blagosklonny MV. Rapamycin suppresses brain aging in senescence-accelerated OXYS rats. Aging (Albany NY) 2013; 5(6):474-84; PMID:23817674
- Obukhova LA, Skulachev VP, Kolosova NG. Mitochondria-targeted antioxidant SkQ1 inhibits age-dependent involution of the thymus in normal and senescence-prone rats. Aging (Albany NY) 2009; 1(4):389-401; PMID:20195490
- Skulachev VP. SkQ1 treatment and food restriction–two ways to retard an aging program of organisms. Aging (Albany NY) 2011; 3(11):1045-50; PMID:22170754
- Anisimov VN, Egorov MV, Krasilshchikova MS, Lyamzaev KG, Manskikh VN, Moshkin MP, Novikov EA, Popovich IG, Rogovin KA, Shabalina IG, et al. Effects of the mitochondria-targeted antioxidant SkQ1 on lifespan of rodents. Aging (Albany NY) 2011; 3(11):1110-9; PMID:22166671
- Zhdankina AA, Fursova AZh, Logvinov SV, Kolosova NG. Clinical and Morphological Characteristics of Chorioretinal Degeneration in Early Aging OXYS Rats. Bull Exp Biol Med 2008; 146(4):455-8; PMID:19489319; http://dx.doi.org/10.1007/s10517-009-0298-4
- Markovets AM, Fursova AZ, Kolosova NG. Therapeutic action of the mitochondria-targeted antioxidant SkQ1 on retinopathy in OXYS rats linked with improvement of VEGF and PEDF gene expression. PLoS One 2011; 6:e21682; PMID:21750722; http://dx.doi.org/10.1371/journal.pone.0021682
- Kozhevnikova OS, Korbolina EE, Stefanova NA, Muraleva NA, Orlov YL, Kolosova NG. Association of AMD-like retinopathy development with an Alzheimer's disease metabolic pathway in OXYS rats. Biogerontology 2013; 14(6):753-62; PMID:23959258; http://dx.doi.org/10.1007/s10522-013-9439-2
- Kozhevnikova OS, Korbolina EE, Ershov NI, Kolosova NG. Rat retinal transcriptome: effects of aging and AMD-like retinopathy. Cell Cycle 2013; 12(11):1745-61; PMID:23656783; http://dx.doi.org/10.4161/cc.24825
- Korbolina EE, Kozhevnikova OS, Stefanova NA, Kolosova NG. Quantitative trait loci on chromosome 1 for cataract and AMD-like retinopathy in senescence-accelerated OXYS rats. Aging (Albany NY) 2012; 4(1):49-59; PMID:22300709
- De S, Rabin DM, Salero E, Lederman PL, Temple S, Stern JH. Human retinal pigment epithelium cell changes and expression of alphaB-crystallin: a biomarker for retinal pigment epithelium cell change in age-related macular degeneration. Arch Ophthalmol 2007; 125(5):641-5; PMID:17502503; http://dx.doi.org/10.1001/archopht.125.5.641
- Kase S, He S, Sonoda S, Kitamura M, Spee C, Wawrousek E, Ryan SJ, Kannan R, Hinton DR. AlphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood 2010; 22; 115(16):3398-406; PMID:20023214; http://dx.doi.org/10.1182/blood-2009-01-197095
- Beatty S, Koh H, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000; 45(2):115-34; PMID:11033038; http://dx.doi.org/10.1016/S0039-6257(00)00140-5
- Yaung J, Kannan R, Wawrousek EF, Spee C, Sreekumar PG, Hinton DR. Exacerbation of retinal degeneration in the absence of alpha crystallins in an in vivo model of chemically induced hypoxia. Exp Eye Res 2008; 86:355e365; http://dx.doi.org/10.1016/j.exer.2007.11.007
- He S, Yaung J, Kim YH, Barron E, Ryan SJ, Hinton DR. Endoplasmic reticulum stress induced by oxidative stress in retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol 2008; 246(5):677-83; PMID:18278507; http://dx.doi.org/10.1007/s00417-008-0770-2
- Rabin DM, Rabin RL, Blenkinsop TA, Temple S, Stern JH. Chronic oxidative stress upregulates Drusen-related protein expression in adult human RPE stem cell-derived RPE cells: a novel culture model for dry AMD. Aging (Albany NY) 2013; 5(1):51-66; PMID:23257616
- Kourtis N, Nikoletopoulou V, Tavernarakis N. Heat shock response and ionstasis: axis against neurodegeneration. Aging (Albany NY) 2012; 4(12):856-8; PMID:23257629
- Bhat SP, Gangalum RK. Secretion of αB-Crystallin via exosomes: new clues to the function of human retinal pigment epithelium. Commun Integr Biol 2011; 1;4(6):739-41; PMID:22446542; http://dx.doi.org/10.4161/cib.17610
- Bhutto IA, McLeod DS, Hasegawa T, Kim SY, Merges C, Tong P, Lutty GA. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res 2006; 82(1):99-110; PMID:16019000; http://dx.doi.org/10.1016/j.exer.2005.05.007
- Binz N, Ali Rahman IS, Chinnery HR, McKeone R, Simpson KM, Speed TP, Lai CM, Rakoczy PE. Effect of vascular endothelial growth factor upregulation on retinal gene expression in the Kimba mouse. Clin Experiment Ophthalmol 2013; 41(3):251-62; PMID:22788671; http://dx.doi.org/10.1111/j.1442-9071.2012.02845.x
- Barot M, Gokulgandhi MR, Mitra AK. Mitochondrial dysfunction in retinal diseases. Curr Eye Res 2011; 36(12):1069-77; PMID:21978133; http://dx.doi.org/10.3109/02713683.2011.607536
- Kolosova NG, Aidagulova SV, Nepomnyashchikh GI, Shabalina IG, Shalbueva NI. Dynamics of structural and functional changes in hepatocyte mitochondria of senescence-accelerated OXYS rats. Bull Exp Biol Med 2001; 132(2):814-9; PMID:11713575; http://dx.doi.org/10.1023/A:1013014919721
- Stefanova NA, Zhdankina AA, Fursova AZh, Kolosova NG. Potential of melatonin for prevention of age-related macular degeneration: experimental study. Adv Gerontol 2013; 26(1):122-9; PMID:24003738
- Kolosova NG, Shcheglova TV, Sergeeva SV, Loskutova LV. Long-term antioxidant supplementation attenuates oxidative stress markers and cognitive deficits in senescent-accelerated OXYS rats. Neurobiol Aging 2006; 27(9):1289-97; PMID:16246464; http://dx.doi.org/10.1016/j.neurobiolaging.2005.07.022
- Dehle FC, Ecroyd H, Musgrave IF, Carver JA. aB-Crystallin inhibits the cell toxicity associated with amyloid fibril formation by k-casein and the amyloidb peptide. Cell Stress Chaperones 2010; 15:1013e1026; http://dx.doi.org/10.1007/s12192-010-0212-z
- Stefanova NA, Kozhevnikova OS, Vitovtov AO, Maksimova KY, Logvinov SV, Rudnitskaya EA, Korbolina EE, Muraleva NA, Kolosova NG. Senescence-accelerated OXYS rats: a model of age-related cognitive decline with relevance to abnormalities in Alzheimer disease. Cell Cycle 2014; 13(6):898-909; PMID:24552807; http://dx.doi.org/10.4161/cc.28255
