Abstract
For proper development, cells need to coordinate proliferation and cell cycle-exit. This is mediated by a cascade of proteins making sure that each phase of the cell cycle is controlled before the initiation of the next. Retinal progenitor cells divide during the process of interkinetic nuclear migration, where they undergo S-phase on the basal side, followed by mitoses on the apical side of the neuroepithelium. The final cell cycle of chicken retinal horizontal cells (HCs) is an exception to this general cell cycle behavior. Lim1 expressing (+) horizontal progenitor cells (HPCs) have a heterogenic final cell cycle, with some cells undergoing a terminal mitosis on the basal side of the retina. The results in this study show that this terminal basal mitosis of Lim1+ HPCs is not dependent on Chk1/2 for its regulation compared to retinal cells undergoing interkinetic nuclear migration. Neither activating nor blocking Chk1 had an effect on the basal mitosis of Lim1+ HPCs. Furthermore, the Lim1+ HPCs were not sensitive to cisplatin-induced DNA damage and were able to continue into mitosis in the presence of γ-H2AX without activation of caspase-3. However, Nutlin3a-induced expression of p21 did reduce the mitoses, suggesting the presence of a functional p53/p21 response in HPCs. In contrast, the apical mitoses were blocked upon activation of either Chk1/2 or p21, indicating the importance of these proteins during the process of interkinetic nuclear migration. Inhibiting Cdk1 blocked M-phase transition both for apical and basal mitoses. This confirmed that the cyclin B1-Cdk1 complex was active and functional during the basal mitosis of Lim1+ HPCs. The regulation of the final cell cycle of Lim1+ HPCs is of particular interest since it has been shown that the HCs are able to sustain persistent DNA damage, remain in the cell cycle for an extended period of time and, consequently, survive for months.
Abbreviations
| ATM | = | ataxia telangiectasia mutated |
| ATR | = | ataxia telangiectasia Rad-3 related protein |
| C-casp-3 | = | cleaved caspase 3 |
| Cdk1 | = | cyclin-dependent kinase 1 |
| Chk1 | = | checkpoint kinase 1 |
| Chk2 | = | checkpoint kinase 2 |
| E | = | Embryonic day; |
| γ-H2AX | = | phosphorylated histone H2AX |
| HCs | = | horizontal cells |
| HPCs | = | horizontal progenitor cells |
| INM | = | interkinetic nuclear migration |
| Mdm2 | = | murine double minute 2 |
| Mdm4/X | = | murine double minute 4/X |
| p21 | = | p21CIP1/waf1; |
| PH3 | = | PhosphoHistone 3 |
| st | = | stage |
| TBP | = | TATA binding protein |
Introduction
Neurogenesis in the retina is coordinated by control of proliferation, cell cycle exit and differentiation of retinal progenitor cells. This is fundamental for the generation of the 5 neuronal and one glia cell type that comprises the retina.Citation1 The phases of the cell cycle are distributed between the different layers of the developing retina. During the process of interkinetic nuclear migration (INM), cells undergo S-phase on the basal side, followed by mitoses on the apical side of the neuroepithelium.Citation2,3 Once the cells undergo the terminal/neurogenic mitosis, they migrate out and withdraw from the cell cycle.Citation4 While this is valid for several of the retinal neuronal cell types, some Lim1 expressing (+) horizontal cells (HCs) can be generated by delayed non-apical mitoses during their final cell cycle. In chicken, these terminal mitoses occur on the basal side of the retinaCitation5 and in zebrafish in the HC layer.Citation6
The coordination of INM and the cell cycle is critical and must be regulated carefully for proper transition between the different phases. The cell cycle is precisely controlled by checkpoints that allow monitoring of the successive events.Citation7 Fragel-Madeira and colleagues demonstrated, in the murine retina, that platelet activating factor regulates the cell cycle at the S/G2-phase transition through a transient checkpoint kinase 1 (Chk1)-dependent cell cycle arrest. This checkpoint is active during the unperturbed cell cycle to guarantee safe transition.Citation8 This is consistent with our results in which blocking Chk1 in the chicken retina resulted in an increase of apical mitoses. Surprisingly, the inhibitor did not affect the basal mitoses of the Lim1+ horizontal progenitor cells (HPCs),Citation9 indicating that the terminal basal mitosis is regulated in a different way compared to cells undergoing INM. This prompted us to further investigate the cell cycle regulation of Lim1+ HPCs undergoing terminal basal mitosis.
We have previously demonstrated that the final cell cycle of Lim1+ HPCs is not regulated by the DNA damage response pathway.Citation9 However, phosphorylation of H2AX (γ-H2AX), a marker for DNA damage,Citation10 was induced in Lim1+ HPCs treated with DNA damaging agents indicates that the initial steps in the DNA damage response pathway is functional during the final cell cycle.Citation9 H2AX is phosphorylated by ataxia telangiectasia Rad-3 related protein (ATR) and ataxia telangiectasia mutated (ATM).Citation11,12 The ATM/ATR kinases activate downstream targets, Chk1, checkpoint kinase 2 (Chk2), and the tumor suppressor protein p53, leading to cell cycle arrest,Citation13 DNA repair, and apoptosis.Citation14 p53 is negatively regulated by murine double minute 2 (Mdm2) and murine double minute 4/X (Mdm4/X)Citation15,16 and regulates the cell cycle mainly via transcriptional activation of p21CIP1/waf1 (p21). p21 is a multifunctional protein that plays a main role as a cyclin-dependent kinase inhibitor,Citation17 as well as in the DNA damage-response and in the stress-response regulation.Citation18 p53 does not only have a function during a perturbed cell cycle, Vuong and colleagues demonstrated that p53 is expressed during the early development of the murine retina, where it may have a function in cell cycle progression without inducing apoptosis.Citation19
In this work we have studied the regulation of the basal mitoses with focus on the Chk1- and the DNA damage-response. We compare effects on the regulation of the basal and apical mitoses in st29 retina by cisplatin-induced DNA damage and inhibition or activation of the p53 system. The results confirmed that the terminal basal mitosis of Lim1+ HPCs is not dependent on Chk1 for its regulation, in contrast to the apical ones. Furthermore, the Lim1+ HPC basal mitoses were less sensitive, than apical mitoses, to cisplatin-induced DNA damage. The Lim1+ HPCs were also able to continue into mitosis despite the presence of γ-H2AX and without activating caspase-3. However, directly induced expression of p21, by the p53 activator Nutlin3a, did reduce both apical and basal mitoses. These data suggest that the HPC basal mitoses have a regulation that is less dependent on the Chk1/2 upon DNA damage but they have a functional p53/p21 system. Control experiments addressing the fundamental cell cycle machinery showed that the cyclin B1-Cdk1 complex was active and functional during the basal mitosis of Lim1+ HPCs. The HPCs seems to be able to circumvent cell cycle checkpoints and continue to divide despite activation of Chk1/2. This may equip the Lim1+ HPCs with a system for cell cycle progression that is more prone to tumor development, particularly in a defective genetic background.
Results
The terminal basal mitosis of HPCs is not regulated by Chk1
Proliferating retinal cells have a transient Chk1-dependent cell cycle arrest and we have previously analyzed the effect of blocking Chk1 during 2 developmental stages (st), st25 and st27.Citation20 These stages can be used to differentiate between the final cell cycle behaviors of the Lim1+ HPCs. At st25, HPCs have a final cell cycle with an S-phase that does not proceed into mitosis. At st27, the Lim1+ HPCs with basal mitoses can be observed in the very center of the retina. However, the peak in the number of basal mitoses is seen at st29.Citation5 To investigate the role of Chk1 in the regulation of the basal mitosis of Lim1+ HPCs, retina explants from st29 embryos were incubated with the Chk1 inhibitor SB218078Citation21 for 2 h. Cells in late G2/M-phase were identified with an antibody to phospho-histone 3 (PH3). We counted basal and apical mitoses separately in order to monitor Lim1+ HPCs (basal mitoses) and other retinal progenitor cells, which undergo INM (apical mitoses).
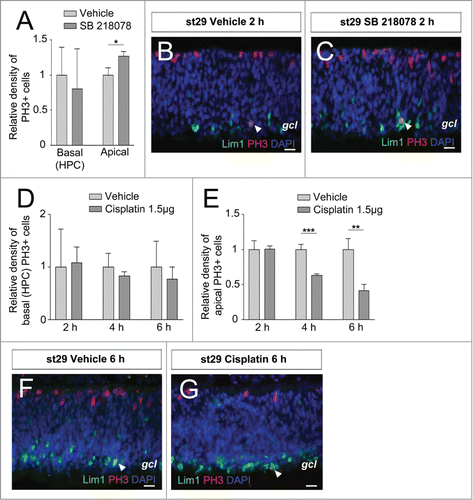
The number of Lim1, PH3 double-positive HPCs was similar after treatment with inhibitor as with vehicle indicating that Chk1 is not directly involved in regulating the terminal basal mitosis (). In contrast, an increase of cells entering apical mitoses was seen after treatment with Chk1 inhibitor compared with vehicle (). This result is consistent with our previous results, and with the results obtained by Fragel-Madeira and colleagues,Citation8 indicating that Chk1 influences the regulation of the cell cycle during INM at st29 but not during the basal mitosis of Lim1+ HPCs.
Figure 1. Effects on basal (HPCs) and apical mitoses in developing retina by inhibitor of Chk1 and DNA damage The relative density of mitoses (PH3+ cells/mm2) in the central region of inhibitor-treated (dark gray bars) compared with control (vehicle, light gray bars) st29 retinas. The basal mitoses are terminally dividing HPCs. Relative density of (A) basal (HPCs) PH3+ and apical PH3+ cells after treatment with the Chk1 inhibitor SB 218078 compared with vehicle. Fluorescence micrographs of Lim1, PH3 double-positive cells in st29 retinal explants after (B) vehicle or (C) SB 218078 treatment. (D) The relative density of basal (HPCs) PH3+ and (E) apical PH3+ cells after treatment with the DNA damaging agent, cisplatin, compared with vehicle. Fluorescence micrographs of Lim1, PH3 double-positive cells in st29 retinal explants after (F) vehicle or (G) cisplatin treatment. Arrowhead; double-positive HPC, gcl; ganglion cell layer, st; Hamburger and Hamilton stages. Student's t test, *P < 0.05, **P < 0.01, ***P < 0.001, n ≥ 4 treated eyes, 4 sections per eye, Scale bar is 10 μm.
Cisplatin does not block the terminal basal mitosis of HPCs
To further investigate the regulation of the basal mitosis of Lim1+ HPCs we triggered activation of Chk1/2 using the cytotoxic drug cisplatin. Cisplatin forms DNA adducts, triggering activation of the ATM/ATR kinases and activation of the downstream kinases Chk1 and Chk2.Citation22 However, cisplatin preferentially activates the ATR kinase and thereby Chk1, the main downstream target of ATR, resulting in a block in M-phase transition.Citation13,23
Stage 29 retinas were injected intraocularly with cisplatin and analyzed after 2 h, 4 h, or 6 h. The PH3+ cells were counted and there was no difference between the cisplatin- and vehicle-treated retinas with regard to PH3, Lim1 double-positive HPCs (). However, there was a reduction in the number of apical mitoses after 4 h and 6 h, verifying that the treatment was effective (). The results indicate that the basal mitosis of Lim1+ HPCs is not affected by cisplatin-induced DNA damage.
Cisplatin-induced DNA damage triggers p53-dependent expression of p21
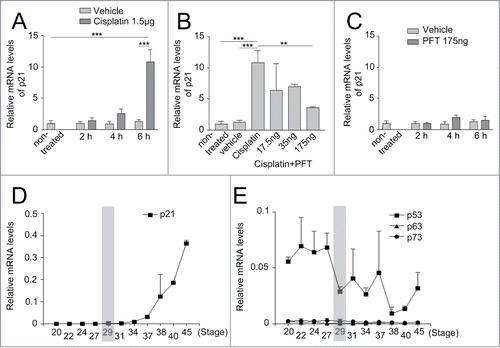
Cisplatin indirectly induces expression of the cell cycle regulator p21, which is able to arrest the cell cycle.Citation22 We investigated if cisplatin is able to induce the expression of p21 in the chicken retina. Cisplatin was injected intraocularly at st29 and p21 mRNA expression was analyzed by qRT-PCR after 2 h, 4 h, or 6 h. An increase in p21 mRNA was observed 6 h after treatment compared both to non-treated and to vehicle-treated retinas (). The result confirmed that p21 mRNA is induced in the retina after cisplatin treatment. The transcription level of p21 is regulated by p53Citation24 and to investigate if the cisplatin-induced increase of p21 mRNA was dependent on p53 we used cyclic Pifithrin-α, a specific p53 inhibitor.Citation25 Cisplatin and different amount of cyclic Pifithrin-α was injected intraocularly and p21 mRNA was analyzed by qRT-PCR after 6 h. Injection of 175 ng cyclic Pifithrin-α reduced the p21 expression (). These results indicate that cisplatin induces the canonical p53 pathway, leading to increased p21 expression that can be inhibited by blocking the activity of p53 with cyclic Pifithrin-α. We used cyclic Pifithrin-α to investigate the role of p53/p21 in the regulation of the basal mitosis of Lim1+ HPCs. Stage 29 retinas were treated intraocularly with 175 ng cyclic Pifithrin-α for 2 h, 4 h, and 6 h, and the basal and apical mitoses were counted. The number of both the apical PH3 positive and the basal Lim1, PH3 double-positive cells was similar after treatment with inhibitor or vehicle, indicating that canonical expression of p53 is not directly involved in regulating the mitoses during this developmental period (data not shown).
Figure 2. Expression of p21 after cisplatin, cyclic Pifithrin-α treatment, and during the normal development of the chicken retina. The relative mRNA levels of p21, at st29, after intraocular injections of (A) cisplatin, (B) cisplatin and different concentrations of cyclic Pifithrin-α (PFT), or (C) PFT treatment. Relative mRNA expression of (D) p21 and (E) p53, p63 and p73 in the developing retina from st20-45. St29 is marked with a gray bar. One-way ANOVA, Tukey's multiple comparison test, **P < 0.01, ***P < 0.001, n ≥ 4 treated eyes, st: Hamburger and Hamilton stages.
We used cyclic Pifithrin-α to indirectly reduce p21 expression in st29 retina. p21 mRNA has a half-life of 2.5 hCitation26 and we treated retinas with 175 ng cyclic Pifithrin-α for 2 h, 4 h, or 6 h. No reduction in p21 mRNA was observed (). We hypothesized that the absence of reduction in p21 mRNA after cyclic Pifithrin-α treatment may be a result of low endogenous expression of p21 and blocking p53 would thus have little effect on the p21 expression. The expression of p21, its regulator p53, and the p53-family members p63 and p73, were therefore studied in normal developing retina.
Expression of p21, its regulator p53 and family members during the normal development of the chicken retina
The analysis was restricted to the central part of embryonic chicken retinas, st20-45 (embryonic day [E3-19]). The mRNA levels of p21 were low in the normal retina until st34 (E8), thereafter increasing (). The low endogenous expression of p21 at st29 (E6) (, st29 is marked with a gray bar) is consistent with p21 not being actively transcribed during the early stages of retinal development.
The qRT-PCR analysis showed that the expression levels of p53 mRNA were elevated above background at all the investigated stages (). The p53 family, including p63 and p73,Citation27,28 have redundant functions and are able to activate p53 target genes, regulate the cell cycle, and mediate apoptosis.Citation29 Both p63 and p73 mRNA levels were low, at background levels, during all the stages investigated (), indicating that p53 is the functional paralogue in the developing retina during these stages.
Activation of p53 with Nutlin3a leads to increased expression of p21, which blocks the terminal basal mitosis of HPCs
The ability of Lim1+ HPCs to continue into mitosis after cisplatin treatment might be caused by an absence of a functional p53/p21 response in these cells. The transcriptional activity by p53 is negatively regulated by Mdm2 and Mdm4Citation15,16,30 and we analyzed the expression of these genes in normal developing retina. Mdm2 and Mdm4 mRNA levels were low, but above background levels in early retina thereafter increasing from st34 (E8) and reaching peak levels at st38 (E12) (). The results show that both Mdm2 and Mdm4 are expressed in the developing retina.
Figure 3. Expression of p21 after Nutlin3a treatment and effect on mitoses in retinal explants. Relative mRNA expression of (A) Mdm2 and Mdm4 in the developing retina from st20-45. St29 (E6) is marked with a gray bar. (B) The relative mRNA levels of p21, at st29 retinal explants, after Nutlin3a 10 μM treatment. (C) The relative density (PH3+ cells/mm2) of basal (HPCs) PH3+ cells and (D) apical PH3+ after treatment with Nutlin3a compared with vehicle. Fluorescence micrographs of Lim1, PH3 double-positive cells in st29 retinal explants after (E) vehicle or (F) Nutlin3a treatment. Arrowhead: double-positive HPC, st: Hamburger and Hamilton stages, one-way ANOVA, Tukey's multiple comparison test or Student's t test, *P < 0.05, ***P < 0.001, n ≥ 4 treated eyes, 4 sections per eye. Scale bar is 10 μm.
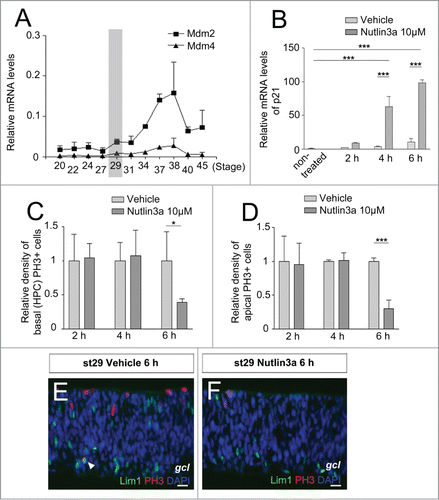
The inhibitory interactions between Mdm2/4 and p53 may be manipulated by Nutlin3a, which acts to stabilize and activate p53.Citation31,32 Several antibodies against p53 were tested in order to study the p53 protein expression or activity, but none of the antibodies tested gave any reproducible results. Instead, we used the p21 mRNA expression to indirectly monitor p53 activity. Nutlin3a was added to the retinal explants and p21 expression was analyzed. 10 μM Nutlin3a increased p21 mRNA levels both after 4 h and 6 h incubation (). The response was robust with a more than 10-fold higher p21 expression than that produced by cisplatin. This indicated that the p53 system is active in the retina and that Nutlin3a treatment stimulates p53 in the retinal explants. We then analyzed if the mitoses in st29 retinal explants were affected by Nutlin3a. The mitoses were analyzed by PH3 immunohistochemistry after 2 h, 4 h, or 6 h incubation. PH3+ cells on the basal (HPCs) and apical sides of the retina were counted. A clear reduction of the basal PH3+ HPCs was seen with Nutlin3a treatment after 6 h incubation compared to vehicle (). The same was observed for the apical PH3+ cells (). The results indicate that the regulation of the basal and apical mitoses displayed similar sensitivity to Nutlin3a treatment.
HPCs are able to enter mitosis in the presence of DNA damage
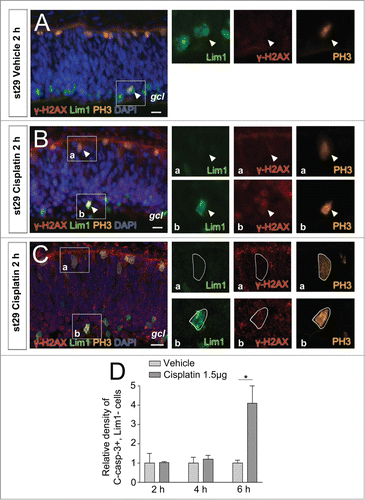
Our results indicate that the Lim1+ HPCs have a functional p53/p21 response. However, they do not arrest after cisplatin-induced DNA damage. We have previously shown that the Lim1+ HPCs have a functional response to DNA damage, including phosphorylation of H2AX (γ-H2AX).Citation9 We investigated the presence of γ-H2AX during the terminal basal mitosis of Lim1+ HPCs. Cisplatin was injected intraocularly at st29 and γ-H2AX immunoreactivity was analyzed after 2 h, 4 h, or 6 h. γ-H2AX immunoreactivity was neither observed in mitotic cells after vehicle treatment, nor in apical mitoses after cisplatin treatment (). However, some basal Lim1, PH3 double-positive HPCs were γ-H2AX positive after 2 h cisplatin treatment (). The presence of γ-H2AX immunoreactivity in basal Lim1, PH3 double-positive HPCs was confirmed by confocal microscopy (). Not all Lim1, PH3 double-positive HPCs were immunoreactive for γ-H2AX, indicating either absence of DNA damage or that the immunoreactivity was below detection. Furthermore, there was a decrease in the number of γ-H2AX+ cells after 6 h treatment, indicating an active DNA damage repair pathway (data not shown).
Figure 4. γ-H2AX and cleaved caspase-3 after cisplatin-induced DNA damage. Fluorescence micrographs of γ-H2AX, PH3, and/or Lim1 positive cells in st29 retinas after (A) vehicle or (B) cisplatin intraocular in ovo treatment. (C) Confocal image of . (D) The relative density of Lim1 negative, C-casp-3+ cells (C-casp-3+ cells/mm2) after treatment with cisplatin compared with vehicle. Arrowhead: positive cell, gcl: ganglion cell layer, st: Hamburger and Hamilton stages, Student's t test, *P < 0.05, n ≥ 4 treated eyes, 4 sections per eye. Scale bar is 10 μm.
Cisplatin does not activate caspase-3 in HPCs
Cisplatin treatment has been shown to activate an apoptotic response.Citation33 We analyzed cleaved caspase-3 (C-Casp-3) immunoreactivity in the ciplatin-treated retinas, to investigate if cisplatin triggered apoptosis in the Lim1+ HPCs. Incubation for up to 6 h was analyzed, but no difference to control was seen when C-Casp-3 was analyzed. None of the basal Lim1, PH3 double-positive HPCs were immunoreactive for C-Casp-3 when carefully inspected (73 Lim1, PH3 double-positive HPCs). Neither was any of the basal Lim1+, PH3 negative HPCs immunoreactive for C-Casp-3 during any of the time points (2223 Lim1+ HPCs). However, an increase in C-Casp-3 immunoreactivity was observed in Lim1 negative cells after cisplatin treatment compared to control retinas ().
Cdk1/2 inhibitor III blocks mitotic entry
Our results show that the Lim1+ HPCs are able of progress into mitosis in the presence of DNA damage and the master regulatory complex of M-phase progression is the cyclin B1-Cdk1 complex. An active cyclin B1-Cdk1 complex in the nucleus will initiate M-phase transition.Citation34 Blocking the Cdk1-kinase activity will inhibit down-stream events that are necessary for cell cycle propagation into mitosis. Stage 29 retinal explants were incubated with the Cdk1/2 Inhibitor III for 1–6 h and Lim1, PH3 immunoreactivity was analyzed. The Cdk1/2 inhibitor reduced the number of PH3, Lim1 double-positive cells (), indicating a block of G2/M-phase transition. Similar results were seen for the apical mitoses ().
Figure 5. The effect of Cdk1/2 inhibition on mitoses in retinal explants. (A) The relative density (PH3+ cells/mm2) of basal (HPC) PH3+ cells at st29 after Cdk1/2 inhibitor III treatment for 1–6 h compared to vehicle and (B) the relative number of apical PH3+ cells at st29 after treatment for 1–6 h compared to vehicle. (C and D) Fluorescence micrographs of Lim1 and PH3 or (E) Lim1 and C-Casp-3 immunoreactivity in st29 retinal explants treated with the Cdk1/2 inhibitor or vehicle for 6 h. Arrowhead: double-positive HPC, st: Hamburger and Hamilton stages, Student's t test, *P < 0.05, **P < 0.01, ***P < 0.001, n ≥ 4 treated eyes, 4 sections per eye, gcl: ganglion cell layer. Scale bar is 10 μM.
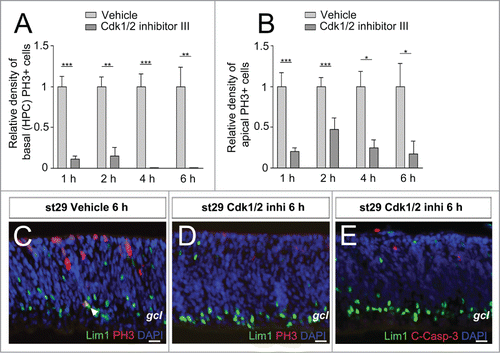
Cdk1/2 inhibitor III does not activate Caspase-3 in HPCs
Cdk1/2 inhibition in normal cells and in cells with replicative or cytotoxic stress has been shown to activate an apoptotic response.Citation35 To investigate if the Cdk1/2 inhibitor triggered apoptosis in the Lim1+ HPCs, we analyzed C-Casp-3 in the Cdk1/2 inhibitor-treated explants. Incubation for up to 6 h was analyzed, but no difference to control was seen when C-Casp-3 was analyzed (). Less than 0.2% of basal Lim1+ HPCs were immunoreactive for C-Casp-3 when carefully inspected at the 4 treatment time points (1012 Lim1+ HPCs).
Discussion
We have previously shown that the chicken Lim1+ HPCs have a heterogenic final cell cycle, where a proportion of the cells undergo a terminal basal mitosis. The heterogenic final cell cycle is neither regulated by the DNA damage response pathway nor active Chk1.Citation5,9 We have, in this study, followed up our previous results, showing that Chk1 is not directly involved in regulating the terminal basal mitosis. Neither blocking nor activating Chk1 had an effect on the basal mitosis of Lim1+ HPCs. This is in contrast to cells undergoing INM, where blocking Chk1 gave an increase in the number of apical mitoses. Chk1 dependent regulation of cell cycle progression has been demonstrated in mouse retinal progenitor cells.Citation8
The kinase inhibitor, p21 is involved in regulating cell cycle exit and DNA damage response. Inducing DNA damage with cisplatin resulted in a p53-dependent increase in p21 mRNA and a G2/M-phase transition arrest of cells undergoing INM. Cisplatin-induced DNA damage did not result in blockage of the G2/M-phase transition. This indicates that the Lim1+ HPCs are, to some degree, resistant to cisplatin-induced cell cycle arrest. Resistance can be acquired through several mechanisms, for example reduced intracellular drug accumulation and/or increase in DNA damage repair.Citation22 However, these mechanisms seem unlikely in our system as cisplatin induces intense γ-H2AX labeling in the Lim1+ HPCs, indicating the presence of substantial DNA damage.Citation9 Furthermore, there is cisplatin resistance in several tumor cell lines, when p21 expression is suppressed or when cytoplasmic expression of p21 is high.Citation36,37 We investigated the expression of p21 during development of the chicken retina. p21 mRNA levels were low during early retinal development, indicating that endogenous p21 does not have an active role in cell cycle regulation during the early stages of development. The mRNA levels of p21 increased from st34, suggesting that p21 may rather be important later during development. It has been shown that the p21 family members, p27 and p57, regulate proliferation of murine retinal progenitor cells and over-expression of p27 results in premature cell cycle exit.Citation38 This is consistent with our previous results showing high p27 protein expression in Lim1+ HPCs leaving the cell cycle, indicating that p27 may be the main regulator of cell cycle exit during the early development of the chicken retina.Citation5
The transcriptional regulation of p21 is mediated by p53 and we analyzed the expression levels of p53 and its family members. qRT-PCR revealed that p53 mRNA is expressed in the retina but p63 and p73 are not expressed. This suggests that p63 and p73 may play less important roles during retinal development than p53. These results are consistent with results from the developing murine retina where p63 and p73 levels were low and p53 levels were high.Citation19 p53 is negatively regulated by Mdm2 and Mdm4. The mRNA levels of Mdm2 and Mdm4 were low during development of the retina but peaked at the period when cell death is abundant in the developing retina. p53 regulates the mRNA levels of Mdm2 in a negative feedback loopCitation39 and there might be an increase in p53 protein levels, prior to developmental apoptosis, leading to transcription of Mdm2. We used Nutlin3a to block the inhibitors Mdm2 and Mdm4, thereby, indirectly activating p53. Within 4 h, an increase in p21 mRNA was observed, indicating an increase in p53 activity. Treatment with Nutlin3a for 6 h resulted in blockage of the G2/M-phase transition for both Lim1+ HPCs and cells with an apical mitosis. This indicats that the Lim1+ HPCs have a functional p53/p21 system.
We further investigated the Lim1+ HPCs that progressed into mitosis after cisplatin treatment and some Lim1, PH3 double-positive HPCs that were γ-H2AX positive were observed. The Lim1+ HPCs did not show immunoreactivity for cleaved caspase-3, indicating that the basal mitoses were not followed by apoptosis. The ability of Lim1+ HPCs to continue into mitosis in the presence of DNA damage is consistent with results obtained in the conditional Rb1-inactivated mouse retina, where HCs with DNA damage were able to remain in the cell cycle due to increased expression of the transcription factor E2f1 and its target genes.Citation40 However, further investigation is needed to clarify whether this is the case in chicken retinal Lim1+ HPCs. Furthermore, blocking Cdk1 reduced both basal and apical mitoses, verifying the well established fact that active Cdk1 is necessary for M-phase transition.
The results indicate that the terminal basal mitosis of Lim1+ HPCs is less sensitive to cisplatin-induced cell cycle arrest compared to cells dividing with an apical mitosis and that Lim1+ HPCs are able to enter mitosis in the presence of DNA damage. However, direct activation of p53/p21 may arrest the terminal mitosis.
Materials and Methods
Animals
Fertilized White Leghorn eggs (Gallus gallus) were obtained from Ova Production AB and incubated at 38°C in a humidified incubator. Embryos were classified into stages (st) or the corresponding embryonic age in days (E) according to Hamburger and Hamilton.Citation20
The Lim1+ HPCs exhibit 3 different behaviors during their development. They are generated by 1) an INM with an apical mitosis (between st19-31), 2) by a final cell cycle with an S-phase that is not followed by any mitosis (between st19-31), such cells remain with a replicated genome, or 3) by non-apical (basal) mitoses (between st26-31). We have used st29 (E6) retinas for our experiment because there is a peak of basal mitoses during this developmental stage.Citation5
Whole retinal explants
Eyes from st29 embryos were dissected in 37°C PBS. The pigment epithelium was removed, leaving the lens and the entire neuronal retina attached to the vitreous body. The eyes were cultured at 37°C in 35 mm dishes on a rotator shaker, with a constant speed of 50 rpm, inside an incubator with 5% CO2. The retinas were cultured 60 min before adding the chemical or vehicle. The medium was 1:1 DMEM:F12 Nutrient mix, 10% FCS, 10 U/ml penicillin streptomycin, 5 μg/ml Insulin, and 2 mM l-glutamine. The control eye and the treated eye were dissected from the same embryo. After treatment for 1–6 h (depending on the experiment), the eyes were analyzed by immunohistochemistry.
Three different chemicals were administered to the retinal explants (). Chemicals were resuspended in either DMSO or EtOH and their final concentration in the medium is given. SB 218078 (2560, Tocris), an inhibitor of Chk1, was used at a concentration of 1 μM in 0.09% DMSO. Nutlin3a (18585, Cayman chemical), an inhibitor of Mdm2 and Mdm4 interaction with p53, was used at a concentration of 10 μM in 0.1% EtOH. Cdk1/2 inhibitor III (217714, Calbiochem) an ATP-competitive inhibitor of cyclin B1-Cdk1 and cyclin A-Cdk2, was used at a concentration of 300 nM in 0.01% DMSO. The concentration of DMSO and EtOH in the vehicle-treated controls was always identical to the experimental eye.
Table 1. The chemicals used in the study and their modes of action
Intraocular injections
Stage 29 eyes were injected with 1.5 μg cisplatin followed by 2 h, 4 h, or 6 h incubation prior to analysis. Stage 29 retinas were treated simultaneously with 1.5 μg cisplatin and 17.5 ng, 35 ng, or 175 ng cyclic Pifithrin-α for 6 h incubation prior to analysis. Stage 29 eyes were injected with 175 ng Pifithrin-α followed by 2 h, 4 h, or 6 h incubation prior to analysis. The retinas were treated according to the immunohistochemical protocol.
Immunohistochemistry
Immunohistochemistry was performed as described previously.Citation5 Tissue was fixed in 4% paraformaldehyde in PBS. The following antibodies were used: the transcription factor Lim1/2 (1:20, mouse, 4F2-s, Developmental studies hybridoma bank), PhosphoHistone 3 (PH3) (1:4000, rabbit, 06-570, Millipore; 1:400, goat, sc-12927, Santa Cruz), γ-H2AX (1:4000, rabbit, ab11174, Abcam), Caspase-3, cleaved (1:4000, rabbit, #9661, Cell Signaling). Secondary antibodies were obtained from Invitrogen. Samples were analyzed using a Zeiss Axioplan 2 microscope or a Zeiss LSM 510 confocal microscope, equipped with an AxioCam C camera and Axiovision software. Images were formatted, resized, enhanced and arranged using Axiovision and Adobe Photoshop CS4.
Quantification of mitoses and cleaved caspase-3
Cells in late G2/M-phase were identified using a PH3 antibody and cells in the early phases of apoptosis were identified using a C-casp-3 antibody. At least 4 sections per eye from 4 different embryos per treatment and antibody-combination were used for cell counting (cells/mm2). Only the central part of the retina was analyzed to avoid bias imposed by the temporal and centro-peripherial aspects of retinal development. Both ventricular (apical) and vitreal (basal) mitotic cells were counted. The mean number (+/− SD) for each combination of labeling and stage was calculated and the data analyzed in GraphPad Prism (v3.02, GraphPad software Inc.). Analysis of variance was done with Student's t test and statistical significance was set to P < 0.05.
Quantitative reverse transcription PCR
Retinas from different embryonic stages: st20 (E3), st22 (E3 ½), st24 (E4), st27 (E5), st29 (E6), st31 (E7), st34 (E8), st36 (E10), st38 (E12), st40 (E14) and st45 (E19), were stripped of pigmented epithelium before being collected for the quantitative reverse transcription PCR (qRT-PCR). For st29 and older embryos, the central part of the retina was collected to avoid bias imposed by the centro-peripherial aspects of retinal development. For st34 and younger, a minimum of 2 animals per batch was collected, to ensure that the amount of mRNA would be sufficient for the qRT-PCR. For all stages, a minimum of 2 batches were used. The mRNA was extracted with Trizol reagent (Invitrogen). The mRNA batches were treated with DNase for 30 min at 37°C before 1 μg of mRNA from each batch was used to prepare cDNA with the high capacity RNA-to-cDNA kit (Applied biosystem). For the qRT-PCR each batch was run in duplicates using IQ SyBr Green Supermix (Bio-Rad laboratories AB). The primers () were designed with either Primer Express v2.0 (Applied biosystem) or Primer3 Input version 0.4.0. The initial mRNA levels were normalized to β-actin and TATA box binding protein (TBP). Control reactions containing primers but no cDNA were analyzed in parallel. The data was analyzed with one-way ANOVA followed by Tukey's multiple comparison test.
Table 2. Primers used for qRT-PCR
Ethics statement
This study was performed in accordance with the recommendations in the “Guide for the Care and Use of Laboratory Animals of the Association for research in vision and ophthalmology.”
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The work was supported by Barncancerfonden (Proj 11/86, PR 2013-0104), Swedish Research Council (20859-01-3, 12187-15-3), Ögonfonden, Kronprinsessan Margaretas arbetsnämnd för synskadade, Synfrämjandets forskningsfond, and St Eriks Ögonsjukhus forskningsstipendier.
References
- Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron 1990; 4:833-45; PMID:2163263; http://dx.doi.org/10.1016/0896-6273(90)90136-4
- Baye LM, Link BA. Interkinetic nuclear migration and the selection of neurogenic cell divisions during vertebrate retinogenesis. J Neurosci 2007; 27:10143-52; PMID:17881520; http://dx.doi.org/10.1523/JNEUROSCI.2754-07.2007
- Sauer F. Mitosis in the neural tube. J Comp Neurol 1935; 62:377-405; http://dx.doi.org/10.1002/cne.900620207
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol 2005; 6:777-88; PMID:16314867; http://dx.doi.org/10.1038/nrm1739
- Shirazi Fard S, Jarrin M, Boije H, Fillon V, All-Eriksson C, Hallbook F. Heterogenic final cell cycle by chicken retinal Lim1 horizontal progenitor cells leads to heteroploid cells with a remaining replicated genome. PLoS One 2013; 8:e59133; PMID:23527113; http://dx.doi.org/10.1371/journal.pone.0059133
- Godinho L, Williams PR, Claassen Y, Provost E, Leach SD, Kamermans M, Wong RO. Nonapical symmetric divisions underlie horizontal cell layer formation in the developing retina in vivo. Neuron 2007; 56:597-603; PMID:18031679
- Murray A. Cell cycle checkpoints. Curr Opin Cell Biol 1994; 6:872-6; PMID:7880536; http://dx.doi.org/10.1016/0955-0674(94)90059-0
- Fragel-Madeira L, Meletti T, Mariante RM, Monteiro RQ, Einicker-Lamas M, Bernardo RR, Lopes AH, Linden R. Platelet activating factor blocks interkinetic nuclear migration in retinal progenitors through an arrest of the cell cycle at the SG2 transition. PLoS One 2011; 6:e16058; PMID:21298035; http://dx.doi.org/10.1371/journal.pone.0016058
- Shirazi Fard S, All-Ericsson C, Hallbook F. The heterogenic final cell cycle of chicken retinal Lim1 horizontal cells is not regulated by the DNA damage response pathway. Cell Cycle 2014; 13:408-17; PMID:24247150; http://dx.doi.org/10.4161/cc.27200
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 1998; 273:5858-68; PMID:9488723; http://dx.doi.org/10.1074/jbc.273.10.5858
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 2001; 276:42462-7; PMID:11571274; http://dx.doi.org/10.1074/jbc.C100466200
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem 2001; 276:47759-62; PMID:11673449; http://dx.doi.org/10.1074/jbc.M009785200
- Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol 2008; 9:616-27; PMID:18594563; http://dx.doi.org/10.1038/nrm2450
- Bohlig L, Rother K. One function–multiple mechanisms: the manifold activities of p53 as a transcriptional repressor. J Biomed Biotechnol 2011; 2011:464916; PMID:21436991; http://dx.doi.org/10.1155/2011/464916
- Zdzalik M, Pustelny K, Kedracka-Krok S, Huben K, Pecak A, Wladyka B, Jankowski S, Dubin A, Potempa J, Dubin G. Interaction of regulators Mdm2 and Mdmx with transcription factors p53, p63 and p73. Cell Cycle 2010; 9:4584-91; PMID:21088494; http://dx.doi.org/10.4161/cc.9.22.13871
- Marine JC, Lozano G. Mdm2-mediated ubiquitylation: p53 and beyond. Cell Death Differ 2010; 17:93-102; PMID:19498444; http://dx.doi.org/10.1038/cdd.2009.68
- Taylor WR, Stark GR. Regulation of the G2M transition by p53. Oncogene 2001; 20:1803-15; PMID:11313928; http://dx.doi.org/10.1038/sj.onc.1204252
- Bartek J, Lukas J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol 2001; 13:738-47; PMID:11698191; http://dx.doi.org/10.1016/S0955-0674(00)00280-5
- Vuong L, Conley SM, Al-Ubaidi MR. Expression and role of p53 in the retina. Invest Ophthalmol Vis Sci 2012; 53:1362-71; PMID:22427613; http://dx.doi.org/10.1167/iovs.11-8909
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951; 88:49-92; PMID:24539719
- Jackson JR, Gilmartin A, Imburgia C, Winkler JD, Marshall LA, Roshak A. An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res 2000; 60:566-72; PMID:10676638
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 2003; 22:7265-79; PMID:14576837; http://dx.doi.org/10.1038/sj.onc.1206933
- Damia G, Filiberti L, Vikhanskaya F, Carrassa L, Taya Y, D’Incalci M, D'incalci M, Broggini M. Cisplatinum and taxol induce different patterns of p53 phosphorylation. Neoplasia 2001; 3:10-6; PMID:11326311; http://dx.doi.org/10.1038/sj.neo.7900122
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature 1993; 366:701-4.
- Pietrancosta N, Maina F, Dono R, Moumen A, Garino C, Laras Y, Burlet S, Quéléver G, Kraus JL. Novel cyclized Pifithrin-alpha p53 inactivators: synthesis and biological studies. Bioorg Med Chem Lett 2005; 15:1561-4; PMID:15745797; http://dx.doi.org/10.1016/j.bmcl.2005.01.075
- Scoumanne A, Cho SJ, Zhang J, Chen X. The cyclin-dependent kinase inhibitor p21 is regulated by RNA-binding protein PCBP4 via mRNA stability. Nucleic Acids Res 2011; 39:213-24; PMID:20817677; http://dx.doi.org/10.1093/nar/gkq778
- Yang A, McKeon F. P63 and P73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol 2000; 1:199-207; PMID:11252895; http://dx.doi.org/10.1038/35043127
- Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G. The p53p63p73 family of transcription factors: overlapping and distinct functions. J Cell Sci 2000; 113 (Pt 10):1661-70; PMID:10769197
- Sheikh MS, Fornace AJ Jr. Role of p53 family members in apoptosis. J Cell Physiol 2000; 182:171-81; PMID:10623880; http://dx.doi.org/10.1002/(SICI)1097-4652(200002)182:2%3c171::AID-JCP5%3e3.0.CO;2-3
- Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene 2006; 25:1125-42; PMID:16314846; http://dx.doi.org/10.1038/sj.onc.1209080
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004; 303:844-8; PMID:14704432; http://dx.doi.org/10.1126/science.1092472
- Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C, Teunisse A, Lam S, Ramos Y, Mohan A, et al. Inactivation of the p53 pathway in retinoblastoma. Nature 2006; 444:61-6; PMID:17080083; http://dx.doi.org/10.1038/nature05194
- Sedletska Y, Giraud-Panis MJ, Malinge JM. Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: importance of apoptotic pathways. Curr Med Chem Anticancer Agents 2005; 5:251-65; PMID:15992353; http://dx.doi.org/10.2174/1568011053765967
- Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell 2010; 18:533-43; PMID:20412769; http://dx.doi.org/10.1016/j.devcel.2010.02.013
- Castedo M, Perfettini JL, Roumier T, Kroemer G. Cyclin-dependent kinase-1: linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ 2002; 9:1287-93; PMID:12478465; http://dx.doi.org/10.1038/sj.cdd.4401130
- Chang LJ, Eastman A. Decreased translation of p21waf1 mRNA causes attenuated p53 signaling in some p53 wild-type tumors. Cell Cycle 2012; 11:1818-26; PMID:22510560; http://dx.doi.org/10.4161/cc.20208
- Koster R, di Pietro A, Timmer-Bosscha H, Gibcus JH, van den Berg A, Suurmeijer AJ, Bischoff R, Gietema JA, de Jong S. Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J Clin Invest 2010; 120:3594-605; PMID:20811155; http://dx.doi.org/10.1172/JCI41939
- Dyer MA, Cepko CL. p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J Neurosci 2001; 21:4259-71; PMID:11404411
- Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res 2003; 1:1001-8; PMID:14707283
- Donovan SL, Corbo JC. Retinal horizontal cells lacking Rb1 sustain persistent DNA damage and survive as polyploid giant cells. Mol Biol Cell 2012; 23:4362-72; PMID:23015754; http://dx.doi.org/10.1091/mbc.E12-04-0293
- Gavet O, Pines J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J Cell Biol 2010; 189:247-59; PMID:20404109; http://dx.doi.org/10.1083/jcb.200909144
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 1999; 285:1733-7; PMID:10481009; http://dx.doi.org/10.1126/science.285.5434.1733
