Abstract
At a wide range of doses, rapamycin extends life span in mice. It was shown that intraperitoneal injections (i.p.) of rapamycin prevent weight gain in mice on high-fat diet (HFD). We further investigated the effect of rapamycin on weight gain in female C57BL/6 mice on HFD started at the age of 7.5 months. By the age of 16 and 23 months, mice on HFD weighed significantly more (52 vs 33 g; p = 0.0001 and 70 vs 38 g; p < 0.0001, respectively) than mice on low fat diet (LFD). The i.p. administration of 1.5 mg/kg rapamycin, 3 times a week every other week, completely prevented weight gain, whereas administration of rapamycin by oral gavash did not. Rapamycin given in the drinking water slightly decreased weight gain by the age of 23 months. In addition, metabolic parameters were evaluated at the age of 16 and 23 months, 6 and 13 days after last rapamycin administration, respectively. Plasma leptin levels strongly correlated with body weight, (P < 0.0001, r=0.86), suggesting that the difference in weight was due to fat tissue mass. Levels of insulin, glucose, triglycerides and IGF1 were not statistically different in all groups, indicating that these courses of rapamycin treatment did not impair metabolic parameters at least after rapamycin discontinuation. Despite rapamycin discontinuation, cardiac levels of phospho-S6 and pAKT(S473) were low in the i.p.-treated group. This continuous effect of rapamycin can be explained by prevention of obesity in the i.p. group. We conclude that intermittent i.p. administration of rapamycin prevents weight gain without causing gross metabolic abnormalities. Intermittent gavash administration minimally affected weight gain. Potential clinical applications are discussed.
Keywords:
Introduction
In a wide range of doses and frequencies of administration, rapamycin extends life span in miceCitation1-26 As summarized by Anisimov et al, Citation26 rapamycin has been administered via intraperetoneal (ip) and subcutaneous (sc) injections, by oral gavage, in the drinking water and food (chow). It is difficult to compare the efficiency of these schedules, given a variety of murine strains used, including inbred, heterogeneous, progeric and cancer-prone mice. The emerging pattern is that the life-extension by rapamycin is more prominent at higher doses and frequencies.Citation15,14,26 Yet in high-dose daily administrations, rapamycin will unlikely be eagerly used to suppress aging in humans due to concerns of side effects. Also, in theory, pulse-treatment (intermittent treatment) may improve tissue regeneration.Citation27 Therefore, comprehensive comparison of doses and frequencies is needed for the development of effective and simultaneously safe anti-aging strategies.
Obesity, the most common age-related condition, accelerates aging and age-related diseases.Citation28-32 It was shown that rapamycin decreases obesity in rodents and humans.Citation33-41 If used properly, this “immunosuppressant” can stimulate immunity.Citation42
In some studies, rapamycin caused insulin resistance. Citation33,38,43 These metabolic changes are reversible.Citation44 Like so called “starvation-diabetes,” this condition may be benevolent and associated with longevity.Citation45 Also, “starvation-like” benevolent diabetes seems to be achieved only at high everyday doses and in some strains like C57BL/6NCr. In most other cases, rapamycin increases insulin sensitivity.Citation46-50
Here we sought to compare several routes of rapamycin administration using prevention of obesity on high fat diet (HFD) as a biomarker. In addition, we sought to investigate the effect of prolonged rapamycin treatment on glucose and lipid metabolism. To exclude direct effects of rapamycin, we measured these parameters after rapamycin discontinuation for 6-13 days (post-treatment levels). In addition, we compared rapamycin and metformin, which is known to improve metabolic parameters in humans.Citation32,51-55
Results
Rapamycin by i.p. injections prevented weight gain and obesity-associated leptin in female mice on HFD
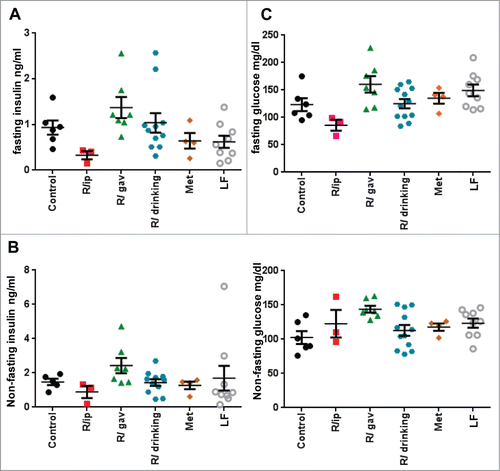
Eighty 7.5 months old female mice of C57BL/6NCr strain were divided in 6 groups. One group received regular low fat (LF) chow (LF): 5% fat. Other 5 groups were fed high (60%) fat diet (HFD): 60% fat. These HFD groups included: untreated (control); R/ip group, which was treated with rapamycin via i.p. 3 times per week every other week; R/gavage group received rapamycin through gavage 3 times per week every other week (gav.); R/drinking group received rapamycin in drinking water (R/d.w) and Metformin (Met) group was given metformin in drinking water (). Treatment began at the same time as mice were put on high fat diet (HFD). Weight was measured weekly and metabolic parameters were evaluated at the age of 16 and 23 months, 6-13 days after rapamycin discontinuation. As shown in , at age of 16 and 23 months mice on HFD weighed significantly more than mice on regular diet (52 vs 33 g and 70 vs 38 g, respectively). Rapamycin administered via i.p. completely prevented weight gain on HFD (). The body weight strongly correlated with blood levels of leptin, suggesting that the difference in weight was due to fat tissue mass (). 15-month administration of rapamycin or metformin in drinking water resulted in slight but statistically significant decrease in weight gain by the age of 23 months in mice on high fat diet (). Levels of insulin, glucose, triglycerides and IGF1 were not statistically different between all the groups, including treated and control mice on HFD and LFD (), indicating that prolonged administration of rapamycin did not have negative effects on metabolic parameters at the doses and schedules of treatment used. Notably, by the age 23 months (after 15-month treatment) mice in R/ip group showed statistically significant decrease in fasted insulin levels (p < 0.05), while glucose levels were similar to control mice, indicating their improved sensitivity to insulin ().
Figure 1. Body weight and leptin levels of female mice treated with different schedules of rapamycin or metformin. Weight (grams) of 16-month old mice, 8 months after beginning of the treatments. Low (5%) fat (LF) diet (LF); all other groups received high (60%) fat diet (HFD): 60% fat. These HFD groups included: untreated (control); R/ip group, which was treated with i.p. rapamycin 3 times per week every other week; R/gavage group received rapamycin through gavage 3 times per week every other week (gav.); R/drinking group received rapamycin in drinking water (R/d.w) and Metformin (Met) group was given metformin in drinking water. (B) Weights of 23-month old survived mice, 15 months after the beginning of treatments. (C) Leptin (ng/ml) concentration was determined in fasted blood of 16-month old mice 8 months after beginning treatments. Fasted blood was collected in the morning after overnight fasting. (D) Correlation between leptin levels and weight in 16-month old mice (Data presented in A and C). Data presented as mean ± SE; p values are provided for observed differences in comparison with control (HFD) group; r – Pearson coefficient.
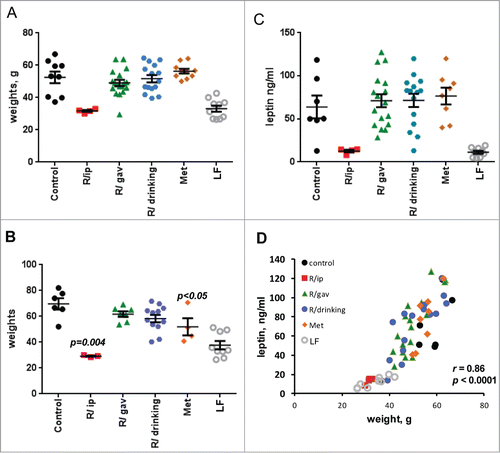
Figure 2. Metabolic profiles of 16 month-old mice on high fat diet 8 months from the beginning of the of treatment with different schedules of rapamycin or metformin. p values: the differences with control group (HFD). Glucose, insulin and triglycerides were determined in fasted serum. Data presented as mean ± SE. Fasted blood was collected in the morning after overnight fasting.
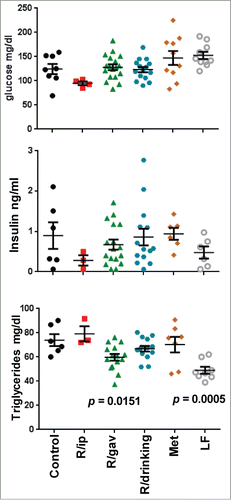
Figure 3. Blood glucose and insulin levels in 23 month-old mice on high fat diet 15 months from the beginning of the treatment with different schedules of rapamycin or metformin. Insulin (A and B) and glucose (C and D) concentrations were determined in fasting and non-fasting sera. Data presented as mean ± SE.
Prolonged treatment with rapamycin via i.p. resulted in sustained suppression of mTOR activity in murine hearts
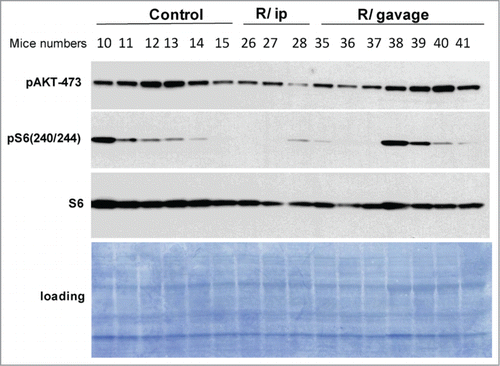
At the age of 23 months (after 15-month treatment) mice were sacrificed 13 days after last rapamycin administration. As shown in , levels of phospho-S6 and pAKT(S473) were low in the hearts of i.p. - treated mice compared with control (untreated mice on HFD) and gavage-treated group ().
Figure 4. Blood IGF1 and triglyceride levels in 23 month-old mice on high fat diet 15 months from the beginning of treatment with different schedules of rapamycin or metformin. IGF1 and triglyceride concentration. Data are mean ± SE.
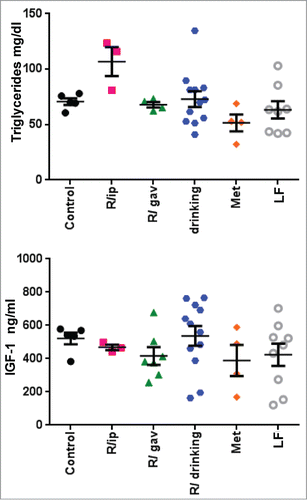
Figure 5. Cardiac levels of p-S6 in mice on HFD. Immunoblot analysis of protein lysates from the heart of 23 month-old female mice on high fat diet: control – untreated; R/ ip – treated with rapamycin i.p 3 times a week every other week; R /gavage – treated with rapamycin through gavage 3 times a week every other week. Mice were sacrificed after overnight fasting, 13 days after last treatment.
Discussion
Here we demonstrated that i.p. injections of rapamycin prevented weight gain on high fat diet, whereas rapamycin by gavash (also given 3 times per 2 weeks) did not. Orally administrated (gavash) rapamycin has poor bioavailability. The i.p. route of its administration ensures high peak of systemic levels of rapamycin. We can conclude that acute high levels of rapamycin may be necessary to prevent obesity. Noteworthy the i.p. therapy was effective even given 3 times per week every other week.
The i.p. administration of 2 mg/kg rapamycin just once a week to HFD-fed C57BL/6 mice decreased weight gain and protected against insulin resistance.Citation41
On the other hand, oral rapamycin in food daily did not prevent weight gain.Citation44
It may seem paradoxical that intermittent administration (by i.p.) is more effective than everyday administration (by oral gavash). One plausible explanation is that at high peak levels, rapamycin may affect cell types that are not sensitive to low concentrations of rapamycin. In fact, the effective concentrations of rapamycin vary broadly in cell lines in culture.
Although i.p. injections are not suitable for prolonged treatment in humans, we suggest that the development of highly bioavailable oral preparations of rapamycin are warranted for obesity treatment in humans. Alternatively, existing formulations of rapamycin could be used in high doses (as single dose) to reach desired peak concentrations in humans. Although high doses of rapamycin can cause side effects if used daily, intermittent administration (one a week or 3 times per 2 weeks, for example) may be sufficient for anti-obesity effects.
In our study, chronic administration of rapamycin in drinking water, which was previously shown to extend life-span in mice, slightly decreased weight gain after 15 months of treatment and this effect was comparable with the effect of metformin. Importantly, levels of glucose and insulin were the same in all groups in the post-treatment period. We measured these parameters 6-13 days after last rapamycin administration (post treatment) to avoid direct effect of rapamycin on metabolism. It was important to evaluate the internal metabolic health in post treatment period. We found no alterations by rapamycin treatment at all schedules. Levels of triglycerides and IGF1 were also unchanged. Unexpectedly, levels of p-S6 were decreased in the i.p. group even after rapamycin discontinuation. This seemingly puzzling result can be explained by the prevention of obesity in the i.p. group. It was shown that obesityCitation56-59 and agingCitation60,61 can be associated with increased fasting p-S6 as a marker of increased mTOR activity. This suggests that prevention of obesity by rapamycin may decrease basal level of mTOR for a prolong time.
Rapamycin (and other agents and conditions that inhibit mTOR) suppress gero-conversion from quiescence to senescence.Citation62-70 In the organism, suppression of geroconversion prevents age-related diseases in mammalsCitation70-78 and pathological conditions in invertebrates, Citation79,80 extending healthy lifespan.Citation81-83
Methods
Mice
Animal studies were conducted in accordance with the regulations of the Committee of Animal Care and Use at Roswell Park Cancer Institute. 80 female mice of C57BL/6NCr strain (7.5 month old) were divided into groups (10 mice per group and 2 groups: 20 mice per group): one group received standard laboratory chow (5% fat, Regular Diet – RD or low fat diet LFD). All other groups were fed on 60% fat chow (High fat diet - HFD) (Reaserch Diets, Inc., Cat # D12492 Rodent Diet 60% kCal fat; New Brunswick, NJ, USA). These groups on HFD were as follows: control – untreated, R/ ip group (N = 10 initially) was treated with rapamycin (LC Laboratories, Woburn, MA) via i.p. injections 1.5 mg/kg 3 times a week every other week; R/gavage group received 1.5 mg/kg rapamune (Sirolimus, rapamycin) (Cardinal Health, Syracuse, NY, USA) as gavage 3 times a week every other week; R/ drinking group received rapamycin in drinking water; Metformin group received metformin(Sigma-Aldrich, St. Louis, MO, USA) in drinking water.
Mice were sacrificed, if they lose weight more than 15% (according to the institution guidelines) or developed sickness such as dermatitis.
At 8 months from the beginning of treatment mice (reached 16 months of age) were fasted overnight and fasting blood was collected for biochemical analysis. After 15 months of treatment, mice (reached 23 months of age) were fasted overnight and sacrificed 13 days after the last treatment. Blood was collected at the end of the day before food was removed for overnight fasting. Next morning, fasted blood was collected. Non-fasted and fasted sera were prepared for biochemical analysis.
Glucose concentration in blood sera was measured using Accu-Chek Aviva strips (MaKesson, Atlanta, GA).
Insulin, IGF1, leptin and triglyceride concentration in sera were measured using Insulin (Mouse) Ultrasensitive ELISA kit (Alpco Diagnostics, Salem, NH), IGF1 (Mouse/Rat) ELISA kit (Alpco), Mouse leptin ELISA kit (Crystal Chem Inc., Downers Grove, IL) and Triglyceride Colorimetric Assay kit (Cayman Chemical Company, Ann Arbor, MI), respectively, as previously described Citation23,35,65,84
Data were analyzed using range of standards and 4 parameter logistic fit or linear regression.
Statistical analysis
T test and correlation analyses (Pearson r coefficient and p value (2-tailed) were performed using GraphPad Prism version 6 for Windows, GraphPad Software (San Diego, CA; www.graphpad.com).
Immunoblot analysis
Tissues were homogenized and immunoblotting was performed as previously described.Citation23,35,65,84
Rabbit anti-phospho-S6 (Ser 240/244), anti-phospho-AKT(Ser 473) and anti-S6 were purchased from Cell Signaling Biotechnology (Danvers, MA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal 2009; 2:ra75; PMID:19934433
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogenous mice. Nature 2009; 460:392-396; PMID:19587680
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 2011; 66:191-201; PMID:20974732; http://dx.doi.org/10.1093/gerona/glq178
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol 2010; 176:2092-2097; PMID:20363920; http://dx.doi.org/10.2353/ajpath.2010.091050
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 2011; 10:4230-4236; PMID:22107964; http://dx.doi.org/10.4161/cc.10.24.18486
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell 2012; 11:675-682; PMID:22587563; http://dx.doi.org/10.1111/j.1474-9726.2012.00832.x
- Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, MacKay VL, An EH, Strong R, Ladiges WC, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med 2012; 4:144ra103; PMID:22837538; http://dx.doi.org/10.1126/scitranslmed.3003802
- Comas M, Toshkov I, Kuropatwinski KK, Chernova OB, Polinsky A, Blagosklonny MV, Gudkov AV, Antoch MP. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53-/- mice by delaying carcinogenesis. Aging (Albany NY) 2012; 4:715-722; PMID:23117593
- Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, Blagosklonny MV, Gudkov AV. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/- mice. Aging (Albany NY) 2012; 4:709-714; PMID:23123616
- Donehower LA. Rapamycin as longevity enhancer and cancer preventative agent in the context of p53 deficiency. Aging (Albany NY) 2012; 4:660-661; PMID:23128359
- Livi CB, Hardman RL, Christy BA, Dodds SG, Jones D, Williams C, Strong R, Bokov A, Javors MA, Ikeno Y, et al. Rapamycin extends life span of Rb1+/- mice by inhibiting neuroendocrine tumors. Aging (Albany NY) 2013; 5:100-110; PMID:23454836
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci 2014; 69:119-130; PMID:23682161; http://dx.doi.org/10.1093/gerona/glt056
- Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest 2013; 123:3272-91; PMID:23863708; http://dx.doi.org/10.1172/JCI67674
- Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 2013; 342:1524-8; PMID:24231806; http://dx.doi.org/10.1126/science.1244360
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, et al. Rapamycin-ediated lifespan increase in mice is dose and sex-dependent and appears metabolically distinct from dietary restriction. Aging Cell 2013; 13(3):468-77; PMID:24341993; http://dx.doi.org/10.1111/acel.12194
- Ye L, Widlund AL, Sims CA, Lamming DW, Guan Y, Davis JG, Sabatini DM, Harrison DE, Vang O, Baur JA. Rapamycin doses sufficient to extend lifespan do not compromise muscle mitochondrial content or endurance. Aging (Albany NY) 2013; 5:539-50; PMID:23929887
- Fok WC, Chen Y, Bokov A, Zhang Y, Salmon AB, Diaz V, Javors M, Wood WH 3rd, Zhang Y, Becker KG, et al. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One 2013; 9:e83988.
- Hasty P, Livi CB, Dodds SG, Jones D, Strong R, Javors M, et al. eRapa Restores a Normal Life Span in a FAP Mouse Model. Cancer Prev Res (Phila) 2014; 7:169-78; PMID:24282255
- Fang Y, Bartke A. Prolonged rapamycin treatment led to beneficial metabolic switch. Aging (Albany NY) 2013; 5:328-9; PMID:23645164
- Luo LL, Xu JJ, Fu YC. Rapamycin prolongs female reproductive lifespan. Cell Cycle 2013; 12:3353-4; PMID:24091532
- Spong A, Bartke A. Rapamycin slows aging in mice. Cell Cycle 2012; 11; PMID:22356747
- Selman C, Partridge L. A double whammy for aging? Rapamycin extends lifespan and inhibits cancer in inbred female mice. Cell Cycle 2012; 11:17-8; PMID:22186783; http://dx.doi.org/10.4161/cc.11.1.18736
- Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell 2014; 13:616-22; PMID:24655348
- Khapre RV, Kondratova AA, Patel S, Dubrovsky Y, Wrobel M, Antoch MP, Kondratov RV. BMAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging (Albany NY) 2014; 6:48-57; PMID:24481314
- Kondratov RV, Kondratova AA. Rapamycin in preventive (very low) doses. Aging (Albany NY) 2014; 6:158-9; PMID:24670390
- Popovich IG, Anisimov VN, Zabezhinski MA, Semenchenko AV, Tyndyk ML, Yurova MN, Blagosklonny MV. Lifespan extension and cancer prevention in HER-2/neu transgenic mice treated with low intermittent doses of rapamycin. Cancer Biol Ther 2014; 15:586-92; PMID:24556924; http://dx.doi.org/10.4161/cbt.28164
- Blagosklonny MV. Aging, stem cells, and mammalian target of rapamycin: a prospect of pharmacologic rejuvenation of aging stem cells. Rejuvenation Res 2008; 11:801-8; PMID:18729812; http://dx.doi.org/10.1089/rej.2008.0722
- Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys Acta 2009; 1790:1117-23; PMID:19364483; http://dx.doi.org/10.1016/j.bbagen.2009.01.008
- Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev 2013; 23:53-62.
- Blagosklonny MV. Common drugs and treatments for cancer and age-related diseases: revitalizing answers to NCI's provocative questions. Oncotarget 2012; 3:1711-1724; PMID:23565531
- Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY) 2012; 4:535-546; PMID:22935594
- Berstein LM. Metformin in obesity, cancer and aging: addressing controversies. Aging (Albany NY) 2012; 4:320-9; PMID:22589237
- Chang GR, Wu YY, Chiu YS, Chen WY, Liao JW, Hsu HM, Chao TH, Hung SW, Mao FC. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol 2009; 105:188-98; PMID:19496779; http://dx.doi.org/10.1111/j.1742-7843.2009.00427.x
- Chang GR, Chiu YS, Wu YY, Chen WY, Liao JW, Chao TH, Mao FC. Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci 2009; 109:496-503; PMID:19372632; http://dx.doi.org/10.1254/jphs.08215FP
- Leontieva OV, Paszkiewicz G, Demidenko ZN, Blagosklonny MV. Resveratrol potentiates rapamycin to prevent hyperinsulinemia and obesity in male mice on high fat diet. Cell Death Dis 2013; 4:e472; PMID:23348586
- Rovira J, E MA, Burke JT, Brault Y, Moya-Rull D, Ba—–n-Maneus E, Ramírez-Bajo MJ, Gutiérrez-Dalmau A, Revuelta I, Quintana LF, et al. Effect of mTOR inhibitor on body weight: from an experimental rat model to human transplant patients. Transpl Int 2008; 21:992-8; PMID:18657090; http://dx.doi.org/10.1111/j.1432-2277.2008.00710.x
- Houde VP, Brule S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, Marette A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes 2010; 59:1338-48; PMID:20299475; http://dx.doi.org/10.2337/db09-1324
- Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, Maeder C, Fournier M, Montet X, Rohner-Jeanrenaud F, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol 2012; 165:2325-40; PMID:22014210; http://dx.doi.org/10.1111/j.1476-5381.2011.01716.x
- Diekmann F, Campistol JM, Rovira J, Budde K, Neumayer HH, Oppenheimer F, Flechner SM. Treatment with sirolimus is associated with less weight gain after kidney transplantation. Transplantation 2013; 96:480-6; PMID:23912169; http://dx.doi.org/10.1097/TP.0b013e31829a9231
- Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron 75:425-36.
- Makki K, Taront S, Molendi-Coste O, Bouchaert E, Neve B, Eury E, Lobbens S, Labalette M, Duez H, Staels B, et al. Beneficial metabolic effects of rapamycin are associated with enhanced regulatory cells in diet-induced obese mice. PLoS One 2014; 9:e92684; http://dx.doi.org/10.1371/journal.pone.0092684
- Bravo-San Pedro JM, Senovilla L. Immunostimulatory activity of lifespan-extending agents. Aging (Albany NY) 2013; 5:793-801; PMID:24389041
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 2012; 335:1638-43; PMID:22461615; http://dx.doi.org/10.1126/science.1215135
- Liu Y, Diaz V, Fernandez E, Strong R, Ye L, Baur JA, Lamming DW, Richardson A, Salmon AB. Rapamycin-induced metabolic defects are reversible in both lean and OBESE mice. Aging (Albany NY) 2014; 6:742-54.
- Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging (Albany NY) 2012; 4:350-8; PMID:22683661
- Vodenik B, Rovira J, Campistol JM. Mammalian target of rapamycin and diabetes: what does the current evidence tell us? Transplant Proc 2009; 41:S31-38; PMID:19651294; http://dx.doi.org/10.1016/j.transproceed.2009.06.159
- Mordier S, Iynedjian PB. Activation of mammalian target of rapamycin complex 1 and insulin resistance induced by palmitate in hepatocytes. Biochem Biophys Res Commun 2007; 362:206-11; PMID:17698034; http://dx.doi.org/10.1016/j.bbrc.2007.08.004
- Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhäusl W, Marette A, Roden M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005; 54:2674-84; PMID:16123357; http://dx.doi.org/10.2337/diabetes.54.9.2674
- Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, Fürnsinn C, Promintzer M, Anderwald C, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes 2007; 56:1600-7; PMID:17329620; http://dx.doi.org/10.2337/db06-1016
- Leontieva OV, Demidenko ZN, Blagosklonny MV. Rapamycin reverses insulin resistance (IR) in high-glucose medium without causing IR in normoglycemic medium. Cell Death Dis 2014; 5:e1214; PMID:24810050; http://dx.doi.org/10.1038/cddis.2014.178
- Moiseeva O, Deschenes-Simard X, Pollak M, Ferbeyre G. Metformin, aging and cancer. Aging (Albany NY). 2013; 5:330-1; PMID:23660016
- Anisimov VN. Metformin: do we finally have an anti-aging drug? Cell Cycle 2013; 12:3483-9; PMID:24189526; http://dx.doi.org/10.4161/cc.26928
- Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany NY) 2010; 2(11):760-74; PMID:21084729
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun 2013; 4:2192; PMID:23900241; http://dx.doi.org/10.1038/ncomms3192
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev 2012; 11:390-8; PMID:22210414; http://dx.doi.org/10.1016/j.arr.2011.11.005
- Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 2005; 146:1473-81; PMID:15604215; http://dx.doi.org/10.1210/en.2004-0921
- Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med 2007; 13:252-259; PMID:17452018; http://dx.doi.org/10.1016/j.molmed.2007.04.002
- Wang CY, Kim HH, Hiroi Y, Sawada N, Salomone S, Benjamin LE, Walsh K, Moskowitz MA, Liao JK. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal 2009; 2:ra11; PMID:19293429
- Wang N, Luo LL, Xu JJ, Xu MY, Zhang XM, Zhou XL, Liu WJ, Fu YC. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism 2013; 63:94-103; PMID:24135502; http://dx.doi.org/10.1016/j.metabol.2013.09.001
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 2010; 468:1100-4; PMID:21179166; http://dx.doi.org/10.1038/nature09584
- Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Fasting levels of hepatic p-S6 are increased in old mice. Cell Cycle 2014; 13: 2656-9; http://dx.doi.org/10.4161/cc.27973
- Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle 2008; 7:3355-61; PMID:18948731; http://dx.doi.org/10.4161/cc.7.21.6919
- Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci U S A 2010; 107:9660-4; PMID:20457898; http://dx.doi.org/10.1073/pnas.1002298107
- Leontieva OV, Natarajan V, Demidenko ZN, Burdelya LG, Gudkov AV, Blagosklonny MV. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc Natl Acad Sci U S A 2012; 109:13314-8; PMID:22847439; http://dx.doi.org/10.1073/pnas.1205690109
- Leontieva OV, Demidenko ZN, Blagosklonny MV. Contact inhibition and high cell density deactivate the mammalian target of rapamycin pathway, thus suppressing the senescence program. Proc Natl Acad Sci U S A 2014; 111:8832-7; PMID:24889617; http://dx.doi.org/10.1073/pnas.1405723111
- Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle 2012; 11:2391-401; PMID:22627671; http://dx.doi.org/10.4161/cc.20683
- Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012; 4:159-65; PMID:22394614
- Luo Y, Li L, Zou P, Wang J, Shao L, Zhou D, Liu L. Rapamycin enhances long-term hematopoietic reconstitution of ex vivo expanded mouse hematopoietic stem cells by inhibiting senescence. Transplantation 2014; 97:20-9; PMID:24092377; http://dx.doi.org/10.1097/TP.0b013e3182a7fcf8
- Halicka HD, Zhao H, Li J, Lee YS, Hsieh TC, Wu JM, Darzynkiewicz Z. Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling. Aging (Albany NY) 2012; 4:952-65; PMID:23363784
- Hinojosa CA, Mgbemena V, Van Roekel S, Austad SN, Miller RA, Bose S, Orihuela CJ. Enteric-delivered rapamycin enhances resistance of aged mice to pneumococcal pneumonia through reduced cellular senescence. Exp Gerontol 2012; 47:958-65; PMID:22981852; http://dx.doi.org/10.1016/j.exger.2012.08.013
- Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle 2006; 5:2087-102; PMID:17012837; http://dx.doi.org/10.4161/cc.5.18.3288
- Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging 2009; 1:357-62; PMID:20157523
- Mercier I, Camacho J, Titchen K, Gonzales DM, Quann K, Bryant KG, Molchansky A, Milliman JN, Whitaker-Menezes D, Sotgia F, et al. Caveolin-1 and accelerated host aging in the breast tumor microenvironment: chemoprevention with rapamycin, an mTOR inhibitor and anti-aging drug. Am J Pathol 2012; 181:278-93; PMID:22698676; http://dx.doi.org/10.1016/j.ajpath.2012.03.017
- Perkey E, Fingar D, Miller RA, Garcia GG. Increased mammalian target of rapamycin complex 2 signaling promotes age-related decline in CD4 T cell signaling and function. J Immunol 2013; 191:4648-55; PMID:24078700; http://dx.doi.org/10.4049/jimmunol.1300750
- Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R, Richardson A, Hart MJ, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience 2012; 223:102-13; PMID:22750207; http://dx.doi.org/10.1016/j.neuroscience.2012.06.054
- Flynn JM, O'Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, et al. Late life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 2013; 12(5):851-62; PMID:23734717; http://dx.doi.org/10.1111/acel.12109
- Paoletti E, Cannella G. Regression of left ventricular hypertrophy in kidney transplant recipients: the potential role for inhibition of mammalian target of rapamycin. Transplant Proc 2010; 42:S41-43.
- Zheng XF. Chemoprevention of age-related macular regeneration (AMD) with rapamycin. Aging (Albany NY) 2012; 4:375-376; PMID:22796653
- Gems DH, de la Guardia YI. Alternative Perspectives on Aging in C. elegans: Reactive Oxygen Species or Hyperfunction? Antioxid Redox Signal 2013; 19:321-9; PMID:22870907; http://dx.doi.org/10.1089/ars.2012.4840
- Gems D, Partridge L. Genetics of Longevity in Model Organisms: Debates and Paradigm Shifts. Annu Rev Physiol 2013; 75:621-644; PMID:23190075; http://dx.doi.org/10.1146/annurev-physiol-030212-183712
- Blagosklonny MV. M(o)TOR of aging: MTOR as a universal molecular hypothalamus. Aging (Albany NY) 2013; 5:490-4; PMID:23872658
- Blagosklonny MV. How to save Medicare: the anti-aging remedy. Aging (Albany NY) 2012; 4:547-52; PMID:22915707
- Blagosklonny MV. Rapamycin extends life- and health span because it slows aging. Aging (Albany NY) 2013; 5:592-8; PMID:23934728
- Leontieva OV, Geraldine M. Paszkiewicz GM, Blagosklonny MV. Mechanistic or mammalian target of rapamycin (mTOR) may determine robustness in young male mice at the cost of accelerated aging. Aging (Albany NY) 2012; 4:899-916; PMID:23443503
