Abstract
Ubiquitin mediated proteolysis is required for transition from one cell cycle phase to another. For instance, the mitosis inhibitor Wee1 is targeted for degradation during S phase and G2 to allow mitotic entry. Wee1 is an essential tyrosine kinase required for the G2/M transition and S-phase progression. Although several studies have concentrated on Wee1 regulation during mitosis, few have elucidated its degradation during interphase. Our prior studies have demonstrated that Wee1 is degraded via CK1δ dependent phosphorylation during the S and G2/M phases of the cell cycle. Here we demonstrate that GSK3β may work in concert with CK1δ to induce Wee1 destruction during interphase. We generated small molecules that specifically stabilized Wee1. We profiled these compounds against 296 kinases and found that they inhibit GSK3α and GSK3β, suggesting that Wee1 may be targeted for proteolysis by GSK3. Consistent with this notion, known GSK3 inhibitors stabilized Wee1 and GSK3β depletion reduced Wee1 turnover. Given Wee1's central role in cell cycle progression, we predicted that GSK3 inhibitors should limit cell proliferation. Indeed, we demonstrate that GSK3 inhibitors potently inhibited proliferation of the most abundant cell in the mammalian brain, the cerebellar granule cell progenitor (GCP). These studies identify a previously unappreciated role for GSK3β mediated regulation of Wee1 during the cell cycle and in neurogenesis. Furthermore, they suggest that pharmacological inhibition of Wee1 may be therapeutically attractive in some cancers where GSK-3β or Wee1 are dysregulated.
Abbreviations
| β-TrCP | = | β-transducin repeat containing |
| CDK | = | Cyclin-dependent kinase |
| GCP | = | Granule cell progenitor |
| GSK3 | = | Glycogen synthase kinase 3 |
| Plk1 | = | Polo-kinase 1 |
| SCF | = | Skp, Cullin, F-box containing complex |
| SHH | = | Sonic Hedgehog |
Introduction
Wee1 is a cell cycle kinase, which has recently emerged as a cancer target.Citation1-3 Wee1 controls cell cycle transitions by inactivating the mitosis-specific CDK1/cyclin B1 kinase during the S and G2 phases of the cell cycle.Citation4-6 Recent studies also suggest that Wee1 maintains the proper histone to DNA ratio by phosphorylating histone H2B at tyrosine 37 to terminate histone transcription at the end of S-phase.Citation7,8 Collectively these studies demonstrate that Wee1's dual role in CDK regulation and histone transcription is required for proper cell cycle progression.Citation4,7,8 Consistent with this notion, Wee1 small molecule inhibitors induce chromosome loss, apoptosis, and mitotic catastrophe.Citation6
Since Wee1 plays an essential cell cycle role, its protein levels are carefully controlled to ensure directional cell cycle transition. Wee1 is degraded via the ubiquitin proteasome pathway in a phosphorylation dependent manner.Citation4-6 Wee1 is phosphorylated during mitosis to enhance recognition by an SCF ubiquitin ligase containing the F-box protein β-TrCP. Similarly, Wee1 phosphorylation during S-phase and G2 is important for turnover. Our earlier studies utilized a chemical biology approach to identify kinases controlling Wee1 phosphorylation and destruction in interphase.Citation9 We generated a reporter of Wee1 turnover, K328M-Wee1-luciferase, and measured its levels in the presence of small molecules as we reasoned that some of these compounds may be kinase inhibitors. We incubated K328M-Wee1-luciferase expressing cells with 218,000 compounds from the NIH-MLPCN library and identified a small molecule, SR-6212901, which selectively stabilizes K328M-Wee1-luciferase.Citation9 In the current study, we generated SR-6212901 analogs to better understand the structural determinants mediating SR-6212901 stabilization of Wee1. We identified several analogs, which were more potent stabilizers of Wee1. When we profiled these analogs against 290 kinases, we found that they inhibited GSK3β , suggesting that this kinase may play a role in controlling Wee1 destruction. Consistent with this notion, known GSK3β inhibitors increased K328M-Wee1-luciferase levels. Further, GSK3β knockdown reduced turnover of endogenous Wee1. Taken together, these studies suggest that GSK3β may control cell cycle progession in part by controlling Wee1 levels.
To determine whether GSK3 inhibitors may affect cell proliferation, we tested their effect on the most abundant cell in the developing brain, the cerebellar granule cell progenitor (GCP). We chose this system since GCPs are essential for generating the cerebellar circuitry. They also give rise to the most common malignant pediatric brain tumor, medulloblastoma, Thus, studying GCP proliferation is important for understanding pathways that become dysregulated during tumorigenesis.Citation10,11 We found that GSK3β and Wee1 are expressed in GCPs allowing us to determine their role in GCP expansion. We report here that inhibition of GSK3 reduced GCP proliferation, which may be a promising therapeutic strategy in cancers where GSK3 or Wee1 are dysregulated.
Results
SR-621291 and its analogs stabilize K328M-Wee1-luciferase
Our earlier studies identified and characterized a luciferase fusion of Wee1 termed K328M-Wee1-luciferase.Citation9,12 The K to M mutation at position 328 in human Wee1 is well known to inhibit kinase activity. The K328M-Wee1 mutant has been used by several groups to measure Wee1 turnover in cells and cell extracts since overexpression of wild-type human Wee1 causes cell cycle arrest. We have shown that turnover of K328M-Wee1 or K328M-Wee1-luciferase is comparable to wild-type Wee1. We have also utilized the K328M-Wee1-luciferase assay to identify CK1δ as a mediator of Wee1 turnover during interphase.Citation13,14 We incubated K328M-Wee1-luciferase expressing cells with a kinase scaffold library and measured the steady-state levels of the Wee1 reporter. The most potent small molecules identified were CK1δ inhibitors.Citation13,14 Subsequent biochemical and genetic experiments suggested that CK1δ phosphorylates Wee1 on its N-terminus to induce Wee1 turnover.Citation13 Thus, collectively our prior studies validated the K328M-Wee1-luciferase assay as an important means of identifying pathways controlling Wee1 turnover.
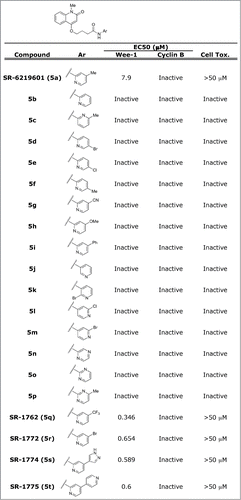
We identified SR-621291 (SID4243143) after screening approximately 218,000 compounds for K328M-Wee1-luciferase stabilization.Citation9 To better understand the structural determinants mediating K328M-Wee1-luciferase stabilization, SR-621291 analogs were synthesized and tested in K328M-Wee1-luciferase assays (see Supplemental information, ). Compounds were first tested at a concentration of 10 μM on HeLa cells expressing K328M-Wee1-luciferase or another protein degraded via the ubiquitin proteasome pathway, N-cyclin B-luciferase. Those compounds showing comparable activity and selectivity as SR-621291 were further tested in a 10-point dose response curve. Among the analogs tested, SR-1772, SR-1774, SR-1775, and SR-1762, were more potent stabilizers of K328M-Wee1-luciferase than SR-621291. Furthermore, these compounds did not affect cell viability as measured via a CellTiter-Glo assay ().
Figure 1. SR-621291 analogs specifically stabilize K328M-Wee1-luciferase. SR-621291 and the analogs increase the steady-state levels of K328M-Wee1-luciferase with respect to DMSO control. 10 μM of the compounds were incubated with K328M-Wee1-Luciferase expressing HeLa cells and the extent of stabilization measured after adding britelite. The EC50s were calculated by performing a 10 dose response curve in the presence of K328M-Wee1-luciferase expressing cells as previously described.Citation46 Similar studies were performed for cyclin B1-luciferase expressing cells. Cytotoxicity was measured using a CellTiter-Glo assay to measure ATP content.
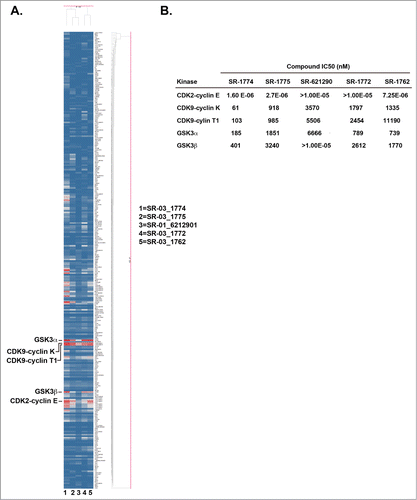
SR-621291 analogs target GSK3β, GSK3α, CDK9 and CDK2 kinases
Wee1 degradation is regulated by complex pathways involving kinases and ubqiutin ligase.Citation15,16 In an attempt to identify novel kinases controlling Wee1 phosphorylation, we profiled the most potent SR-621291 analogs (SR-1772, SR-1774, SR-1775, and SR-1762) against a panel of kinases (). In a single point assay, the main kinases inhibited included GSK3β, GSK3α, CDK9-cyclin K, CDK9-cylin T1 and CDK2-cyclin E (). When tested in a 10-point dose response curve, the IC50s for inhibition of these kinases were comparable to one another with the exception that SR-1772 was inactive toward CDK2-cyclin E. The specifically highlighted SR-621291 analogs were more potent inhibitors of GSK3β than SR-621291 (Figure 2B).
GSK3β and CDK2 knockdown increase Wee1 protein steady-state levels
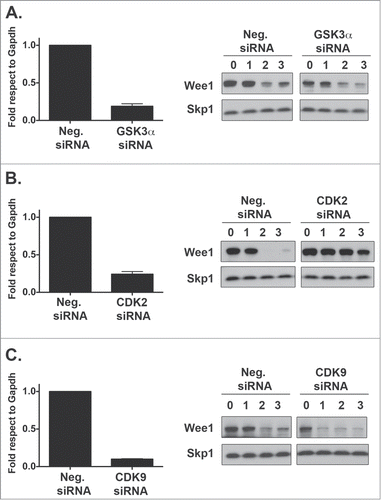
To further identify the possible kinases controlling Wee1 degradation, we depleted GSK3β, GSK3α, CDK9, and CDK2 in HeLa cells and measured Wee1 turnover via a cyclohexamide degradation assay (, ). GSK3β depletion decreased Wee1 turnover (). In addition, GSK3β was required for turnover of 2 other cell cycle regulators, cyclin B1 and p27kip1 (). Similar to GSK3β, CDK2 depletion also stabilized Wee1 () whereas GSK3α and CDK9 depletion did not have any effect on Wee1 turnover (). These studies indicate that GSK3β and CDK2 may control Wee1 degradation.
Figure 3. GSK3β Knockdown reduces Wee1 turnover. HeLa cells were transfected with GSK3β (A) siRNA and the extent of degradation determined after a cycloheximide degradation assay. Protein levels of Wee1 and cell cycle markers such as CyclinB1 and p27kip1 were assessed by Western blot and compared to the SKP1 loading control (B). mRNA knockdown was assessed by RT-PCR. This represents the average of 3 independent experiments plus the standard deviation of the mean. For protein levels, one representative Western blot is shown.
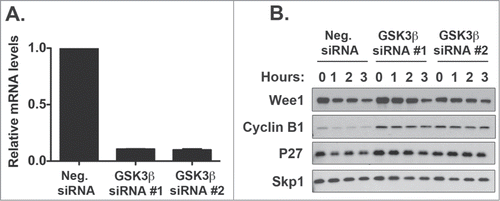
Figure 4. GSK3α or CDK9 knockdown does not affect Wee1 turnover. HeLa cells were transfected with GSK3α (A) CDK2 (B) and CDK9 (C) siRNAs and the extent of degradation determined after a cycloheximide degradation assay. Protein levels of Wee1 and cell cycle markers such as CyclinB1 and p27 were assessed by Western blot and compared to the SKP1 loading control. mRNA knockdown was assessed by RT-PCR. RT-PCR plot represents the average of 3 independent experiments plus the standard deviation of the mean.
To further confirm the role of GSK3 and CDK2 in regulating Wee1 protein levels, we tested several commercially available dual GSK3/CDK2 inhibitors. All compounds increased the steady-state levels of K328M-Wee1-Luciferase, suggesting that GSK3β and/or CDK2 are required for Wee1 turnover ().
Figure 5. GSK3 inhibitors stabilize K328M-Wee1-luciferase. HeLa cells were transfected with K328M-Wee1-luciferase or luciferase alone. 24 hours after transfection, cells were incubated with DMSO, ARA-014418 (GSK3α/β inhibitor), Bio-acetoxime (GSK3α/β inhibitor), and BIO (GSK3α/β inhibitor) for 24 hours. Brite-lite reagent was then added to transfected cells and the steady-state levels of K328M-Wee1-luciferase or luciferase was calculated. The ratio of K328M-Wee1-luciferase to luciferase alone was then plotted and normalized to DMSO (100%).
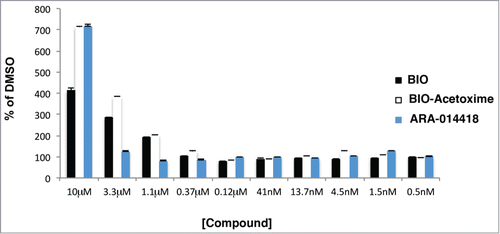
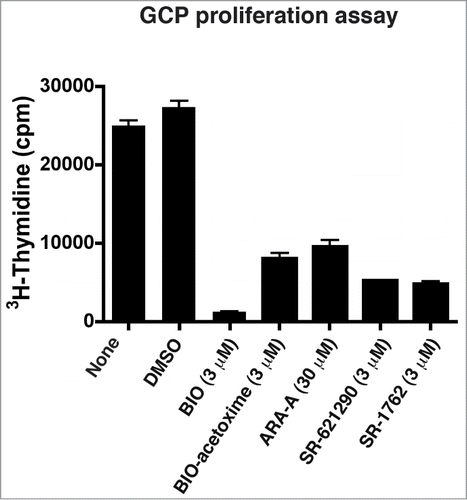
Figure 6. GSK3 inhibitors inhibit GCP proliferation. The compounds tested decrease GCP proliferation in the presence SHH (75 ng/ml). Purified GCPs were treated for 24 hours with the compounds and then3H-thyimidine was added to the media for an additional 24 hours. Plots representing the amounts of 3H-thyimidine incorporated by GCPs from one representative experiment performed in triplicate is shown. Plots represent the mean and the standard error of the mean.
GSK3β and inhibition reduces GCP proliferation
Our prior studies suggest that inhibiting Wee1 turnover limits cell cycle progression. We thus hypothesized that inhibitors, which stabilize Wee1 should reduce cell proliferation. We chose the cerebellar granule cell progenitor (GCP) system to test the effect of GSK3 inhibition on proliferation since we found that GCPs abundantly express GSK3 and Wee1 (Hatten and colleagues, unpublished observations). During postnatal cerebellar development, granule cell progenitors (GCPs) undergo a remarkable expansion generating 45 billion granule neurons in the human brain.Citation11 Therefore, they are an opportune model to study cell division during neurogenesis. Furthermore, GCP neurogenesis also provides a model for pediatric brain tumors as several subgroups of medulloblastoma are thought to arise from GCPs.Citation10,11
GCPs can be purified to near homogeneity (99% purity) and utilized in proliferation assays. After adding the mitogenic factor Sonic Hedgehog (SHH), all the tested GSK3 inhibitors (BIO, BIO-Acetoxime, ARA-A, SR-621291, SR-1762) reduced 3H-Thymidine incorporation with respect to DMSO and to non-treated cells, suggesting that GSK3β is required for GCP proliferation ().
Discussion
We report here the development of potent compounds that selectively increase Wee1 protein levels. As these molecules inhibit GSK3 activity, we suggest that GSK3 controls Wee1 turnover. Indeed, depletion of GSK3β inhibited Wee1 turnover and commercially available GSK3 inhibitors stabilized Wee1. All GSK3 inhibitors tested decreased proliferation of granule cell progenitors, which are thought to give rise to some forms of medulloblastoma. Collectively, these studies suggest that GSK3 inhibition may be effective in reducing proliferation of some brain tumors.
Both the screening hit SR-621960 and the synthetic analogs SR-1772, SR-1774, SR-1775, and SR-1762 induced Wee1 stabilization. The kinase profiling assay of these compounds indicate GSK3β, GSK3α, CDK2 and CDK9 as the main targets. GSK3β and CDK2 knockdown reduced endogenous Wee1 protein degradation whereas GSK3α or CDK9 depletion had no effect on Wee1 turnover. CDK regulation of Wee1 turnover is in line with previous results since CDK1/cyclin B1 has been reported to phosphorylate Wee1 and to promote its degradation during mitosisCitation17 and we identified CDK2/cyclin A1 as a possible regulator of Wee1 turnover.Citation18,19 Thus, it is feasible that CDKs also regulate Wee1 turnover during interphase and that the reason the SR-621291 analogs stabilize Wee1 is due to inhibition of CDK activity. It is equally plausible that SR-621291 analogs stabilize K328M-Wee1 luciferase due to GSK3β inhibition. This would be consistent with known GSK3β inhibitors such as lithium chloride and SB415286 decreasing cell proliferation and inducing G2/M arrest.Citation20-22 Further, GSK3β activity has been postulated to control cyclin-B1/CDK1 activity, in part, through phosphorylation of hBora, which is involved in phosphorylation and activation of Plk1.Citation23
In the present study, we report that GSK3β may regulate mitotic entry through Wee1 phosphorylation, to induce Wee1 degradation.Citation17,18,24,25 Further studies are required to determine whether GSK3β-mediated degradation of Wee1 is direct. Our prior studies demonstrated that GSK3β phosphorylates Wee1 in vitro on sites required for turnover.Citation13 GSK3β phosphorylation promotes the binding of E3 ubiquitin ligases such as Fbw7 and β-TrCP, allowing subsequent ubiquitination and proteolysis of the substrates.Citation25 Since SCF-β-TrCP is known to ubiquitinate Wee1 to target it for degradation, it is conceivable that GSK3β promotes this event. However, we find that GSK3β depletion stabilizes p27kip1 and cyclin B1 suggesting that it may be a general regulator of protein turnover, which may indirectly control Wee1 turnover. Indeed, GSK3β has been shown to control turnover of many cellular substrates.Citation26 Further, GSK3β has been found to phosphorylate many proteins and play important roles in a variety of cellular processes such as cell proliferation, differentiation, cell cycle, and apoptosis.Citation27,28 Thus, it is possible that GSK3β inhibition or depletion arrests cells in a particular cell cycle phase where Wee1 levels are high. Future studies are required to better define whether Wee1 stabilization after GSK3β inhibition or depletion is a consequence of affecting the cell cycle.
Our studies suggest that GSK3β inhibition reduces cell proliferation in part due to Wee1 stabilization. Importantly, GSK3β inhibitors decreased proliferation of granule cell progenitors. GCPs are of special interest both to the development of the cerebellar circuitry and to medulloblastoma. GCPs are one of 2 principal classes of neurons in the developing cerebellum. GSK3 antagonizes the canonical WNT pathway playing a central role in neural development and adult neurogenesis. Without WNT signals, cytoplasmic β-catenin is maintained at a low level regulated by 4 different proteins: axin, adenomatous polyposis coli (APC), casein kinase 1 α (CK1α) and GSK3. Upon binding of Wnt to the receptor complex, GSK3 is phosphorylated and inhibited, allowing increased levels of β-catenin.Citation29-31 It is commonly accepted that GSK3 inhibition and constitutive WNT activation increases neurogenesis in the subventricular zone and the hippocampus.Citation32-34 By contrast, activation of the WNT/ β-catenin signaling pathway results in proliferation inhibition and premature differentiation of GCPs, which is in line with our current studies.Citation35,36,37 Potentially, GSK3β inhibition may decrease GCP proliferation via increasing Wee1 levels and activating WNT/ β-catenin signaling.
Both GSK3β and CDK2 kinases have emerged as potential molecular therapeutic targets in cancer given their well-characterized roles in the regulation of gene expression and oncogenic signaling in multiple cancers including medulloblastoma.Citation38-40 Whereas increased CDK2 activity is linked to tumorigenesis, both activation and inhibition of GSK3β has been linked to cancer proliferation, migration and invasion.Citation41,42 Furthermore, GSK3β inhibition has either increased or decreased proliferation depending on the setting.Citation43-45 Therefore, the potential therapeutic benefit of inhibiting GSK3β in medulloblastoma should be carefully determined depending on the tumor subtype.
Materials and Methods
Luciferase assay
HeLa cells expressing K328MWee1-luciferase, N-cyclin B-luciferase, or luciferase alone were treated with the indicated compounds for 24-hours after which britellite was added. We have previously described similar assays.Citation16
In vitro kinase assays
In vitro kinase assay to detect GSK3β, GSK3α, CDK2 and CDK9 as well as complete kinase profile of 296 kinases was performed by Reaction Biology Corporation.
siRNA transfection
HeLa cells were transfected with siRNAs targeting GSK3β, GSK3α, CDK2 and CDK9 and processed for degradation assay as previously described.Citation9 The following siRNAs were used in this study: Negative siRNA (Neg. siRNA siRNA, Invitrogen, Cat # 4390843), GSK3β siRNA #1 (Invitrogen, Cat # s6241), GSK3β siRNA #2 (Invitrogen, Cat # s6242), GSK3α (Invitrogen, Cat # s6237), CDK2 (Invitrogen, Cat # s206), CDK9 (Invitrogen, Cat # s2834). Wee1, Cyclin B1, and p27kip1 Western blots were processed as previously described.Citation9
Cycloheximide degradation assay
100 μg/ml Cycloheximide or DMSO were added to HeLa cells 2 days after they were transfected with siRNAs. Cells were harvested at specific time points and extracts were prepared as described below followed by SDS-PAGE and Western blotting.
Cell extract preparation, antibodies
Cells were homogenized and extracts were prepared using lysis buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1× Protease Inhibitor Cocktail, 1 μM Microcystin LR). Cells were lysed by the freeze-thaw method (liquid nitrogen/37°C water bath). The soluble fraction was recovered by centrifugation at 14,000 RPM for 20 min at 4°C. Protein concentration was measured with the BCA Protein Assay kit (Pierce) and 30 μg of protein from each sample was resolved by SDS-PAGE. The resolved bands were transferred onto a nitrocellulose membrane and then probed with the indicated antibodies.
Primary Antibodies: anti-Skp1 p19 antibody (H-163) (Santa Cruz Biotechnology, Cat # sc-7163), anti-Wee1 antibody (Cell Signaling, Cat # 4936S), anti-cyclin B1 (Abcam, Cat # ab72), anti-p27 antibody (Santa Cruz Biotechnology, Cat # sc-776). Secondary antibodies: Anti-mouse IgG-HRP antibody (GE Healthcare, Cat # NXA931), anti-rabbit IgG-HRP antibody (GE Healthcare, Cat # NA9340V).
GCP Isolation and compound treatment
GCPs were purified from cerebellar cortex from P6 CD-1 (Jackson Laboratory) mice using Percoll gradient sedimentation to yield an enriched GCP fraction and preplating on a Petri dish to remove contaminating glial cells. Purified GCPs were resuspended in medium (DMEM/F12, 1.5% Glucose, 20 mM Glutamine, 10% Horse serum, 5% FBS) and 3 × 1016 cells/well were plated in 6-well dishes or 3 × 105 cells/well in 96-well plates. For compound treatment, 30 μM of Ara-A (Torcris, Cat 3966) or 3 μM of BIO (Tocris, Cat 3194), BIO-Acetoxime (Torcris, Cat 3874), SR-6212901 or SR-1762, or DMSO was added to the culture for 24 h. Human recombinant Sonic Hedgehog (Applied Stem Cell) was added at 75 ng/ml. Subsequently, GCPs were used for3H-Thymidine incorporation assay.
3H-Thymidine assay
After plating and treating the cells with the compounds for 24 h, 1 μCi of [methyl-3H]-Thymidine (Amersham) was added to each well and the cells were harvested 22 hours later and analyzed using TOP-Count (Perkin Elmer).
RNA isolation and qRT-PCR
Cells were lysed in 1 ml of Trizol (Invitrogen) and the RNA was further purified with the RNeasy Mini Kit (Qiagen). RNA was then reverse-transcribed with the High Capacity cDNA Reverse Transcripiton kit (Applied Biosystems). qRT-PCR was performed using a Sybergreen Gene Expression Master Mix (Applied Biosystems) in a CFX384 Touch™ Real-Time PCR Detection System (Bio-Rad). Fold change in gene expression was estimated using the computed tomography (CT) comparative method (2– DDCT) normalizing to GAPDH CT values and relative to control samples.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary_File__GSK3_inhibitors.pdf
Download PDF (100 KB)Acknowledgments
We thank Dr. Mary E. Hatten and Yin Fang for assistance with the GCP proliferation assay. We thank all members of the Center for Therapeutic Innovation and the Miami Project for Paralysis for helpful discussions.
Funding
This work was partly funded by R01NS067289 (to NGA) and U54MH074404 (to WRR; H. Rosen, PI).
References
- Do K, Doroshow JH, Kummar S. Wee1 kinase as a target for cancer therapy. Cell Cycle 2013; 12:3159-64; PMID: 24013427; http://dx.doi.org/10.4161/cc.26062
- Vriend LE, De Witt Hamer PC, Van Noorden CJ, Wurdinger T. WEE1 inhibition and genomic instability in cancer. Biochimica Biophys Acta 2013; 1836:227-35; PMID:23727417
- Stathis A, Oza A. Targeting Wee1-like protein kinase to treat cancer. Drug News Perspect 2010; 23:425-9; PMID:20862394
- Leijen S, Beijnen JH, Schellens JH. Abrogation of the G2 checkpoint by inhibition of Wee-1 kinase results in sensitization of p53-deficient tumor cells to DNA-damaging agents. Current Clin Pharmacol 2010; 5:186-91; PMID:20406171; http://dx.doi.org/10.2174/157488410791498824
- Murrow LM, Garimella SV, Jones TL, Caplen NJ, Lipkowitz S. Identification of WEE1 as a potential molecular target in cancer cells by RNAi screening of the human tyrosine kinome. Breast Cancer Res Treat 2010; 122:347-57; PMID:19821025; http://dx.doi.org/10.1007/s10549-009-0571-2
- Mir SE, De Witt Hamer PC, Krawczyk PM, Balaj L, Claes A, Niers JM, Van Tilborg AA, Zwinderman AH, Geerts D, Kaspers GJ, et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell 2010; 18:244-57; PMID:20832752; http://dx.doi.org/10.1016/j.ccr.2010.08.011
- Mahajan K, Mahajan NP. WEE1 tyrosine kinase, a novel epigenetic modifier. Trends Genet 2013; 29:394-402; PMID:23537585; http://dx.doi.org/10.1016/j.tig.2013.02.003
- Mahajan K, Fang B, Koomen JM, Mahajan NP. H2B Tyr37 phosphorylation suppresses expression of replication-dependent core histone genes. Nat Struct Mol Biol 2012; 19:930-7; PMID:22885324; http://dx.doi.org/10.1038/nsmb.2356
- Madoux F, Simanski S, Chase P, Mishra JK, Roush WR, Ayad NG, Hodder P. An ultra-high throughput cell-based screen for wee1 degradation inhibitors. J Biomol Screen 2010; 15:907-17; PMID:20660794; http://dx.doi.org/10.1177/1087057110375848
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 1999; 22:103-14; PMID:10027293; http://dx.doi.org/10.1016/S0896-6273(00)80682-0
- Roussel MF, Hatten ME. Cerebellum development and medulloblastoma. Curr Top Dev Biol 2011; 94:235-82; PMID:21295689; http://dx.doi.org/10.1016/B978-0-12-380916-2.00008-5
- Simanski S, Madoux F, Rahaim RJ, Chase P, Schurer S, Cameron M, Mercer BA, Hodder P, Roush WR, Ayad NG. Identification of small molecule inhibitors of Wee1 degradation and mitotic entry. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD) 2010; PMID:23762957
- Penas C, Ramachandran V, Simanski S, Lee C, Madoux F, Rahaim RJ, Chauhan R, Barnaby O, Schurer S, Hodder P, et al. Casein kinase 1delta-dependent Wee1 protein degradation. J Biolog Chem 2014; 289:18893-903; PMID:24817118; http://dx.doi.org/10.1074/jbc.M114.547661
- Bibian M, Rahaim RJ, Choi JY, Noguchi Y, Schurer S, Chen W, Nakanishi S, Licht K, Rosenberg LH, Li L, et al. Development of highly selective casein kinase 1delta/1epsilon (CK1delta/epsilon) inhibitors with potent antiproliferative properties. Bioorg Med Chem Lett 2013; 23:4374-80; PMID:23787102; http://dx.doi.org/10.1016/j.bmcl.2013.05.075
- Reed SI. Ratchets and clocks: the cell cycle, ubiquitylation and protein turnover. Nat Rev Mol Cell Biol 2003; 4:855-64; PMID:14625536; http://dx.doi.org/10.1038/nrm1246
- Watanabe N, Broome M, Hunter T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J 1995; 14:1878-91; PMID:7743995
- Watanabe N, Arai H, Iwasaki J, Shiina M, Ogata K, Hunter T, Osada H. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc Natl Acad Sci U S A 2005; 102:11663-8; PMID:16085715; http://dx.doi.org/10.1073/pnas.0500410102
- Owens L, Simanski S, Squire C, Smith A, Cartzendafner J, Cavett V, Caldwell Busby J, Sato T, Ayad NG. Activation domain-dependent degradation of somatic Wee1 kinase. J Biol Chem 2010; 285:6761-9; PMID:20038582; http://dx.doi.org/10.1074/jbc.M109.093237
- Enders GH. Gauchos and ochos: a Wee1-Cdk tango regulating mitotic entry. Cell Div 2010; 5:12; PMID:20465818; http://dx.doi.org/10.1186/1747-1028-5-12
- Tighe A, Ray-Sinha A, Staples OD, Taylor SS. GSK-3 inhibitors induce chromosome instability. BMC Cell Biol 2007; 8:34; PMID:17697341; http://dx.doi.org/10.1186/1471-2121-8-34
- Korur S, Huber RM, Sivasankaran B, Petrich M, Morin P, Jr., Hemmings BA, Merlo A, Lino MM. GSK3beta regulates differentiation and growth arrest in glioblastoma. PloS One 2009; 4:e7443; PMID:19823589; http://dx.doi.org/10.1371/journal.pone.0007443
- Pizarro JG, Folch J, Esparza JL, Jordan J, Pallas M, Camins A. A molecular study of pathways involved in the inhibition of cell proliferation in neuroblastoma B65 cells by the GSK-3 inhibitors lithium and SB-415286. J Cell Mol Med 2009; 13:3906-17; PMID:18624766; http://dx.doi.org/10.1111/j.1582-4934.2008.00389.x
- Lee YC, Liao PC, Liou YC, Hsiao M, Huang CY, Lu PJ. Glycogen synthase kinase 3 beta activity is required for hBora/Aurora A-mediated mitotic entry. Cell Cycle 2013; 12:953-60; PMID:23442801; http://dx.doi.org/10.4161/cc.23945
- Smith A, Simanski S, Fallahi M, Ayad NG. Redundant ubiquitin ligase activities regulate wee1 degradation and mitotic entry. Cell Cycle 2007; 6:2795-9; PMID:18032919; http://dx.doi.org/10.4161/cc.6.22.4919
- Watanabe N, Arai H, Nishihara Y, Taniguchi M, Watanabe N, Hunter T, Osada H. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc Nat Acad Sci U S A 2004; 101:4419-24; PMID:15070733; http://dx.doi.org/10.1073/pnas.0307700101
- Linding R, Jensen LJ, Ostheimer GJ, van Vugt MA, Jorgensen C, Miron IM, Diella F, Colwill K, Taylor L, Elder K, et al. Systematic discovery of in vivo phosphorylation networks. Cell 2007; 129:1415-26; PMID:17570479; http://dx.doi.org/10.1016/j.cell.2007.05.052
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J 2001; 359:1-16; PMID:11563964; http://dx.doi.org/10.1042/0264-6021:3590001
- Takahashi-Yanaga F. Activator or inhibitor? GSK-3 as a new drug target. Biochem Pharmacol 2013; 86:191-9; PMID:23643839; http://dx.doi.org/10.1016/j.bcp.2013.04.022
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006; 127:469-80; PMID:17081971; http://dx.doi.org/10.1016/j.cell.2006.10.018
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009; 17:9-26; PMID:19619488; http://dx.doi.org/10.1016/j.devcel.2009.06.016
- Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, Hanash S, Misek DE, Katabuchi H, Williams BO, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell 2007; 11:321-33; PMID:17418409; http://dx.doi.org/10.1016/j.ccr.2007.02.016
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells 2007; 25:2827-36; PMID:17673525; http://dx.doi.org/10.1634/stemcells.2007-0177
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 2009; 136:1017-31; PMID:19303846; http://dx.doi.org/10.1016/j.cell.2008.12.044
- Wrobel CN, Mutch CA, Swaminathan S, Taketo MM, Chenn A. Persistent expression of stabilized -catenin delays maturation of radial glial cells into intermediate progenitors. Dev Biol 2007; 309:285-97; PMID:17706960; http://dx.doi.org/10.1016/j.ydbio.2007.07.013
- Lorenz A, Deutschmann M, Ahlfeld J, Prix C, Koch A, Smits R, Fodde R, Kretzschmar HA, Schuller U. Severe alterations of cerebellar cortical development after constitutive activation of Wnt signaling in granule neuron precursors. Mol Cell Biol 2011; 31:3326-38; PMID:21690300; http://dx.doi.org/10.1128/MCB.05718-11
- Poschl J, Grammel D, Dorostkar MM, Kretzschmar HA, Schuller U. Constitutive activation of beta-catenin in neural progenitors results in disrupted proliferation and migration of neurons within the central nervous system. Dev Biol 2013; 374:319-32; PMID:23237957; http://dx.doi.org/10.1016/j.ydbio.2012.12.001
- Wen J, Yang HB, Zhou B, Lou HF, Duan S. beta-Catenin is critical for cerebellar foliation and lamination. PloS One 2013; 8:e64451; PMID:23691221; http://dx.doi.org/10.1371/journal.pone.0064451
- Hydbring P, Larsson LG. Cdk2: a key regulator of the senescence control function of Myc. Aging 2010; 2:244-50; PMID:20445224
- Knoepfler PS, Kenney AM. Neural precursor cycling at sonic speed: N-Myc pedals, GSK-3 brakes. Cell Cycle 2006; 5:47-52; PMID:16322694; http://dx.doi.org/10.4161/cc.5.1.2292
- Ling H, Samarasinghe S, Kulasiri D. Computational experiments reveal the efficacy of targeting CDK2 and CKIs for significantly lowering cellular senescence bar for potential cancer treatment. Biosystems 2013; 111:71-82; PMID:23254306; http://dx.doi.org/10.1016/j.biosystems.2012.12.001
- Atkins RJ, Stylli SS, Luwor RB, Kaye AH, Hovens CM. Glycogen synthase kinase-3beta (GSK-3beta) and its dysregulation in glioblastoma multiforme. J Clin Neurosci 2013; 20:1185-92; PMID:23768967; http://dx.doi.org/10.1016/j.jocn.2013.02.003
- Qu Z, Sun D, Young W. Lithium promotes neural precursor cell proliferation: evidence for the involvement of the non-canonical GSK-3beta-NF-AT signaling. Cell Biosc 2011; 1:18; PMID:21711903; http://dx.doi.org/10.1186/2045-3701-1-18
- Vidal F, de Araujo WM, Cruz AL, Tanaka MN, Viola JP, Morgado-Diaz JA. Lithium reduces tumorigenic potential in response to EGF signaling in human colorectal cancer cells. Int J Oncol 2011; 38:1365-73; PMID:21369697
- Sun A, Shanmugam I, Song J, Terranova PF, Thrasher JB, Li B. Lithium suppresses cell proliferation by interrupting E2F-DNA interaction and subsequently reducing S-phase gene expression in prostate cancer. Prostate 2007; 67:976-88; PMID:17440966; http://dx.doi.org/10.1002/pros.20586
- Nowicki MO, Dmitrieva N, Stein AM, Cutter JL, Godlewski J, Saeki Y, Nita M, Berens ME, Sander LM, Newton HB, et al. Lithium inhibits invasion of glioma cells; possible involvement of glycogen synthase kinase-3. Neuro-Oncol 2008; 10:690-9; PMID:18715951; http://dx.doi.org/10.1215/15228517-2008-041
- Madoux F, Mishra J, Mercer BA, Ayad N, Roush W, Hodder P, Rosen HR. Small molecule inhibitors of Wee1 degradation and mitotic entry. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD), 2010; PMID:21735595