Abstract
WW domain-containing oxidoreductase (WWOX) has been reported to be a tumor suppressor in multiple cancers, including prostate cancer. WWOX can induce apoptotic responses to inhibit tumor progression, and the other mechanisms of WWOX in tumor suppression have also been reported recently. In this study, we found significant down-regulation of WWOX in prostate cancer specimens and prostate cancer cell lines compared with the normal controls. In addition, an ectopically increased WWOX expression repressed tumor progression both in vitro and in vivo. Interestingly, overexpression of WWOX in 22Rv1 cells led to cell cycle arrest in the G1 phase but did not affect sub-G1 in flow cytometry. GFP-WWOX overexpressed 22Rv1 cells were shown to inhibit cell cycle progression into mitosis under nocodazole treatment in flow cytometry, immunoblotting and GFP fluorescence. Further, cyclin D1 but not apoptosis correlated genes were down-regulated by WWOX both in vitro and in vivo. Restoration of cyclin D1 in the WWOX-overexpressed 22Rv1 cells could abolish the WWOX-mediated tumor repression. In addition, WWOX impair c-Jun-mediated cyclin D1 promoter activity. These results suggest that WWOX inhibits prostate cancer progression through negatively regulating cyclin D1 in cell cycle lead to G1 arrest. In summary, our data reveal a novel mechanism of WWOX in tumor suppression.
Abbreviations
| WWOX | = | WW domain-containing oxidoreductase |
| GFP | = | Green fluorescent protein |
| CCND1 | = | cyclin D1 |
| ATCC | = | American Type Culture Collection |
| rEGF | = | Recombinant Epidermal Growth Factor |
| RT-PCR | = | Reverse transcription polymerase chain reaction |
Introduction
Prostate cancer is the most commonly diagnosed malignancy and the second leading cause of cancer death following lung cancer in the United States.Citation1 Over the past 25 years, the incidence of prostate cancer has substantially increased. The development of prostate cancer is thought to be caused by multiple events including loss of cell cycle control, escape from apoptosis, inactivation of DNA repair genes and genomic rearrangement.Citation2
The cyclin D1 proto-oncogene is an important regulator of G1 to S phase transition, and its overexpression is commonly found in cancers including prostate cancer.Citation3 Dysregulation of cyclin D1 has been reported to contribute to an increase not only in tumor cell growth but also in chromosome instability.Citation4 These alterations may further contribute to tumor progression and the acquisition of an aggressive phenotype. Recently, many molecules have been reported to be involved in the development and metastasis of prostate cancer including cyclin D1, retinoblastoma, p53 and WW domain-containing oxidoreductase (WWOX).Citation5 Several of these molecules tightly regulate the cell cycle, indicating that disorder of the cell cycle is an important event in prostate cancer pathogenesis.
The WWOX gene is located at chromosome 16q23.3–24.1 and has been identified to be the common chromosomal fragile site, FRA16D.Citation6 The WWOX protein contains 2 N-terminal WW domains, a nuclear localization sequence and a central short dehydrogenase reductase domain.Citation7 WWOX has been reported to be abundantly expressed in hormonally active tissues including prostate, suggesting that WWOX may play an important role in hormone-regulated cancers.Citation8 Previous studies have indicated that WWOX may be a candidate tumor suppressor gene in multiple cancers, and apoptosis has been proposed to be the possible mechanism of WWOX tumor suppression.Citation8,9 Ectopic WWOX expression has been reported to result in lung and prostate tumor cell apoptosis through a caspase-dependent mechanism.Citation9,10 In addition, WWOX has been shown to directly interact with p53 to enhance an apoptotic response.Citation11
Recent studies have indicated that WWOX-mediated tumor suppression may be through multiple mechanisms. The tumorigenic suppression of WWOX in breast cancer has been reported to be through the inhibition of tumor growth but not through an increase in apoptosis.Citation12 In addition, WWOX has been shown to decrease the activity of integrin and adhesion of tumor cells to fibronectin to suppress ovary cancer tumorigenicity.Citation13 These findings suggest that other mechanisms of WWOX as a tumor suppressor may exist.
Due to the reported association between WWOX and hormone pathways in breast cancer, the role of WWOX in prostate cancer, another cancer with strong dependence on sex hormones, deserves exploration, and its significance may further be related to castration resistance status. In this study, we explored the role of WWOX in prostate tumor and prostate cancer cells focusing on its regulation of the cell cycle. We found that WWOX induced cell cycle arrest in the G1 phase through the downregulation of cyclin D1 in prostate cancer.
Materials and Methods
Clinical specimens
Human prostate specimens were obtained from Kaohsiung Veterans General Hospital (Kaohsiung, Taiwan) and National Cheng Kung University Hospital (Tainan, Taiwan). All patients gave informed consent and the protocol was approved by the IRB Committee before a tissue sample was collected during their planned surgery. The samples were snap frozen and stored in liquid nitrogen until use.
Cell culture
PC-3, LNCaP and 22Rv1 cell lines (ATCC) were cultured in RPMI-1640, supplemented with 10% fetal bovine serum and antibiotics. DU145 (ATCC) cell line was cultured in Minimum Essential Eagle's medium supplemented with 10% fetal bovine serum and 2mM L-glutamine with antibiotics. PWR-1E and RWPE-1 (ATCC) human non-tumorigenic prostate epithelial cell lines were cultured in keratinocyte serum-free medium supplemented with 0.2 ng/ml rEGF and 30 mg/ml bovine pituitary extract. The cultures were maintained in a 5% CO2 humidified atmosphere at 37°C.
Cell cycle analysis
To examine the effect of WWOX on the cell cycle distribution of asynchronous populations of prostate cancer cells, replicative DNA synthesis and DNA content were analyzed using univariate flow cytometric analysis. Transfected 22Rv1 cells were harvested by trypsin-EDTA release and fixed in ice-cold 70% ethanol. At least 1 to 2 hours before flow cytometric analysis, the cells were resuspended in a 1-mL aliquot of modified Vindelov's DNA staining solution (10 μg/mL RNase A and 5 μg/mL propidium iodide in phosphate buffered saline). Flow cytometric analysis was performed with a flow cytometry system (FACS Calibur-S System, BD Bioscience). Cells in the G1, S, and G2-M phases of the cell cycle were determined with Modfit LT software.
Cell proliferation and clonogenic assay
The cells were transiently transfected with control plasmid, GFP plasmid, GFP-WWOX plasmid or cyclin D1 plasmid DNA or WWOX siRNA using Lipofectamine 2000 (Invitrogen). After transfection, the medium was removed, and the cells were washed with phosphate buffered saline twice and resuspended to count the cells at 0, 24, 48 and 72 hours. To determine the long-term effects, the cells were transfected with the indicated plasmids. After being rinsed with fresh medium, the cells were allowed to grow for 14 d to form colonies, which were then stained with crystal violet. A clonogenic assay was used to elucidate the possible differences in long-term effects of WWOX and cyclin D1 on human prostate cells.
Western blotting
Lysis buffer (4°C) was added to the cells for 10 minutes, and the cell lysates were then collected, added to sample buffer and heated to 99°C for 10 minutes. The protein samples were electrophoresed by 10% SDS PAGE to separate the proteins by molecular weight. The proteins were transferred from the gel to PVDF membranes using an immersion transfer device. After blocking with 5% milk in TBST, the membranes were incubated with the indicated primary antibodies for WWOX, actin, cyclin D1, p16, p21, p27 (Santa Cruz), caspase 3 and cleaved caspase 3 for 2 hours at room temperature. The membranes were washed with TBST 3 times for 10 minutes each time and then incubated with the appropriate secondary antibodies. X-ray films were used to detect horseradish peroxidase conjugated signaling.
Xenograft tumor growth
Nude mice were obtained from National Laboratory Animal Center. For the animal experimental, each mouse was subcutaneously inoculated with 1×105 manipulated 22Rv1 cells in the left and right flank, respectively. The tumor size was measured every 2 to 3.
Immunohistochemistry
Tumor arising from xenograft model were collected to examine indicated protein expression. The slides were dewaxed and rehydrated in PBS, and sections were immersed in 0.01M citrate buffer (pH 6.0) and then microwaved for 15 minutes for antigen retrieval. Endogenous peroxidase activity was blocked using 3% H2O2. The slides were then transferred into a humidified chamber, incubated with 10% horse or goat serum for 30 minutes, and then incubated with indicated primary antibody overnight at 4°C. After primary antibody incubation, the slides were immersed in peroxidase-labeled secondary antibody for 30 minutes at room temperature. To detect the antibody conjugated antigen reaction, the sections were incubated in 3-amino-9-ethylcarbazole substrate-chromogen for 30 minutes and counterstained with hematoxylin (K4005, EnVision System-HRP (AEC); Dako Cytomation).
Reporter assay
The PGL4-cyclin D1 promoter plasmid was given from Dr. Wang. For reporter assays, the cells were transiently transfected with the WWOX plasmid and the Renilla luciferase plasmid as a normalization control using Lipofectamine 2000 (Invitrogen). Reporter assays were performed 48 hours posttransfection using the Dual-Luciferase Reporter Assay system (Promega).
Statistical analysis
Data were expressed as means ± SD. Statistical comparisons of the results were made using paired t tests or 2-way ANOVA. All tests were 2-sided, and a p value of less than 0.05 was considered to be statistically significant. SPSS version 17 software was used to analyze all parameters (SPSS Inc.).
Results
WWOX expression was decreased in the prostate tumors and cancer cell lines
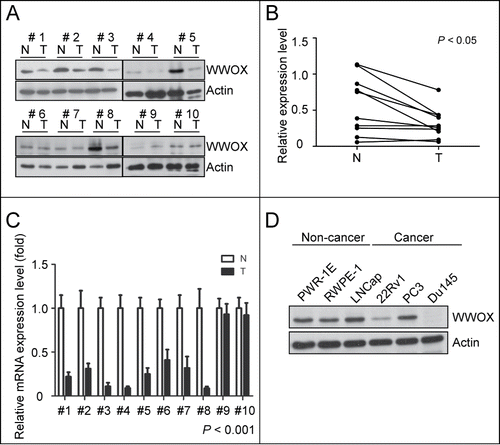
To investigate the role of WWOX in prostate cancer, we examined the expression of WWOX in 10 representative N/T pair specimens of prostate tissue. In 7 of 10 patients, the WWOX protein expressions in the tumors were lower than in the normal tissues of the prostate specimens (). Quantitative analysis of the WWOX protein expression showed a significant reduction in the tumors by the paired t test (). Similarly, the mRNA level of WWOX in 8 of 10 patients was reduced in the tumors (). These findings revealed that the WWOX expression was downregulated by repressing mRNA in the prostate cancer tumors. In addition, the WWOX protein expression was decreased in the prostate cancer cell lines (22Rv1 and DU145) compared to the non-cancer prostate cell lines (PWR-1E and RWPE-1) (). These findings imply that WWOX plays a role in suppressing tumors in prostate cancer.
Figure 1. WWOX expression is reduced in prostate tumors and cancer cell lines. Western blot (A) and qPCR (C) analysis for 10 representative NT pairs specimens of prostate tissue. (A) 7 of 10 WWOX protein expression in tumor are lower than normal tissue by Western blot using anti-WWOX and anti-actin served as internal control. (B) Western blot results of (A) were quantified by densitometer and analyzed by paired t test (p < 0.05) (C) 8 of 10 WWOX mRNA are decreased in tumor tissue by qPCR using GAPDH served as internal control. Statistical significance was estimated using the paired t test analysis (p < 0.001) (D) The WWOX protein expression level in different prostate cancer and noncancer cell lines were examined by Western blot. WWOX expression is lower in prostate cancer-derived cells (22Rv1 and DU145) than noncancer prostate cells (PWR-1E and RWPE-1).
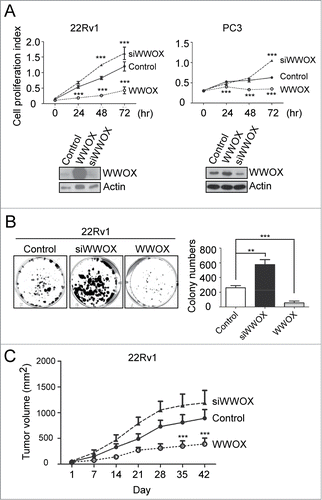
WWOX repressed prostate tumor progression in vivo and in vitro
To further validate the biological function of WWOX in prostate cancer, both overexpression and knockdown of WWOX were investigated in 22Rv1 and PC3 cells. The ectopic expression of WWOX dramatically inhibited cell proliferation in both 22Rv1 and PC3 cells. In contrast, knockdown of WWOX enhanced cell growth (). In addition, WWOX-silenced 22Rv1 and PC3 attenuate cell proliferation. A colony-forming assay was used to investigate the long-term effect of WWOX. Likewise, the data showed that the overexpression of WWOX in 22Rv1 cells led to fewer and smaller colonies, and that silencing WWOX in 22Rv1 cells significantly increased the number of colonies compared with the vector control (). To investigate WWOX in vivo, WWOX-overexpressing or -silenced 22Rv1 cells were injected subcutaneously in a number of nude mice. Smaller tumors were observed in the nude mice implanted with cancer cells overexpressing WWOX compared with those implanted with cancer cells with the control plasmid (). At the end point, the average volume of the tumors expressing WWOX and of the control plasmid were 390 ± 115 and 890 ± 170 mm3, respectively (p < 0.001, two-way ANOVA). In addition, the average volume of the WWOX-silenced tumors was 1190 ± 240 mm3, which was slightly bigger than the control group (890 ± 170 mm3). Taken together, WWOX suppressed cell proliferation in vitro and tumor growth in vivo, suggesting that WWOX acts as a tumor suppressor in prostate cancer.
Figure 2. WWOX inhibits prostate cancer progression both in vitro and in vivo. (A) Overexpress or knockdown WWOX in 22Rv1 and PC3 cell lines. After transfection, cell were collected and counted at 0, 24, 48 and 72 hours. Cell proliferation index represents proliferated cell number compared to 0 hour. Representative WWOX expression level are validated by Western blot. Overexpression of WWOX in 22Rv1 cell inhibit cell proliferation. In contrast, knockdown WWOX enhances cell growth. (B) Colony-forming assay of control, WWOX- silenced and WWOX-overexpressed 22RV1 cell. Quantification of colony numbers are shown on right panel. (C) Control, WWOX- silenced and WWOX-overexpressed 22RV1 cell of xenograft in nude mice. Tumor volume were measured every week. Overexpression of WWOX inhibit tumor progression in vivo. Statistical significance was estimated using Two-way ANOVA analysis. Data are representative of 3 to 5 independent performed experiments; mean ± SD; **, p < 0.005; ***, p < 0.001.
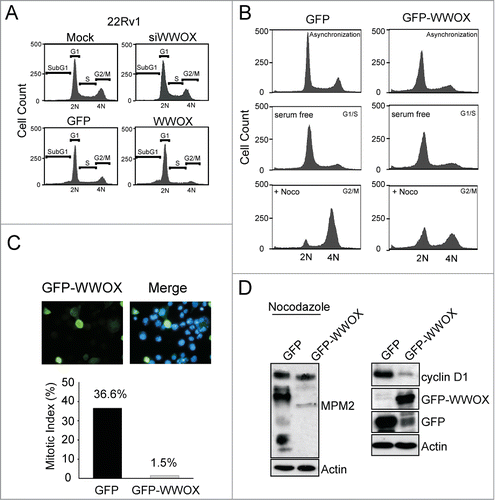
WWOX-mediated tumor suppression is through G1 arrest
The cell cycle status of WWOX-overexpressing or WWOX-silenced 22Rv1 cells was analyzed with flow cytometry. Interestingly, WWOX-overexpressing 22Rv1 cells arrested the cell cycle in the G1 phase but not in the sub-G1 phase 24 hours after transfection (). However, WWOX silencing did not affect the cell cycle. The percentages of transfected cells in the different cell cycle phases are shown in . More WWOX-overexpressing cells (78.4%) stayed in the G1 phase compared with GFP-overexpressing cells (57.2%), and there were fewer WWOX-overexpressing cells in the S phase (reduced by approximately 2.5-fold) and in the G2/M phase (reduced by 3-fold) compared with the GFP control group. There were no significant changes in the number of cells in the sub-G1 phase, defined as apoptotic cells, in either group. These findings suggest that WWOX provokes cell cycle arrest in the G1 phase. To further validate that WWOX induces cell cycle arrest in the G1 phase, GFP-expressing and WWOX-overexpressing 22Rv1 cells were starved to allow for synchronization in the G1 phase followed by nocodazole treatment for 14 hours to trigger the cells into the G2/M phase. Analysis of cell cycle distribution by flow cytometry was shown in cell count versus DNA content profiles of the cells (). The GFP and GFP-WWOX plasmids were transfected into 22Rv1 and mitotic cells with GFP fluorescence and then counted. The mitotic index was calculated as the percentage of mitotic cells, and was found to be lower in WWOX-overexpressing 22Rv1 cells compared with the GFP controls (36.6% vs. 1.5%) ().The mitosis-specific phosphoprotein expressions, as labeled by MPM2 antibodies, were reduced in the WWOX-overexpressing cells under nocodazole treatment (). This suggests that WWOX overexpression inhibits cells from entering into mitosis, even with nocodazole treatment. These findings support that WWOX-mediated cell growth inhibition is through G1 arrest in prostate cancer.
Table 1. Quantification of cell cycle phases in WWOX-overexpressed and WWOX-silenced 22Rv1 cells
Figure 3. WWOX-mediated cell growth inhibition is through G1 arrest. (A) The cell cycle phases are analyzed by flow cytometer in 22Rv1 cell (Mock, GFP, WWOX-overexpressed and WWOX-silenced). The number of WWOX-overexpressed cell increase in G1 and reduce in G2/M phase compare to GFP-expressed cell. (B) GFP-expressed and WWOX-overexpressed 22Rv1 cells were starved to synchronize in G1 phase followed by nocodazole treatment for 14 hours to trigger cell into mitosis. Cells were harvested to determine cell cycle distributions by flow cytometry as shown in cell count vs DNA content profiles of the cells. (C) The GFP and GFP-WWOX plasmid were transfected into 22Rv1 cell and mitotic cells with GFP fluorescence were counted. The mitotic index indicate the percentage of mitotic cells. (D) Cyclin D1 and MPM2 protein expression were detected in GFP-expressed and WWOX-overexpressed 22Rv1 cells with or without nocodazole treatment.
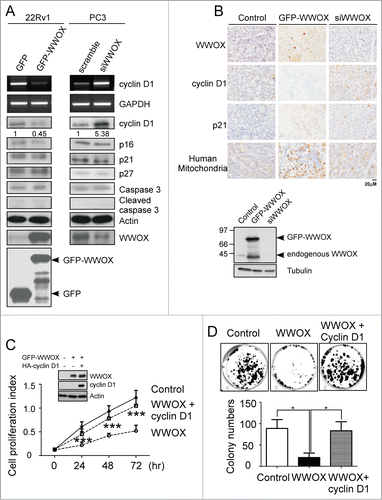
Cyclin D1 was required for WWOX-mediated G1 arrest
To investigate whether the effect of WWOX was through G1 arrest or apoptosis, the protein markers were examined using Western blotting and RT-PCR. The results showed that the cyclin D1 protein expression was reduced in WWOX-overexpressing 22Rv1 cells and increased in WWOX-silenced PC3 cells compare to the control cells (). However, there were no changes in the expressions of p16, p21, p27 or cleaved caspase 3, suggesting that apoptosis was not involved in the WWOX-mediated prostate cancer repression. In addition, we examined whether WWOX regulates cyclin D1expression through transcriptional regulation. The ectopic expression of WWOX resulted in a reduction of cyclin D1 mRNA level in the 22Rv1 cells, whereas cyclin D1 mRNA was increased in the WWOX-silenced PC3 cells. These data imply that WWOX negatively regulates cyclin D1 both in mRNA and protein. To further confirm the effect of WWOX in vivo. The tumor arising from GFP, GFP-WWOX and siWWOX were examined by immunohistochemistry (). The data indicate similar results that WWOX could reduce cyclin D1 protein expression but not affect p21 protein expression in vivo. Besides, the human 22Rv1 cells xenograft tumor were verified by human mitochondria marker immunostaining. As shown in , the overexpression of WWOX resulted in a decrease of cyclin D1 in both mRNA and protein. To further explore the role of cyclin D1, it was co-expressed with WWOX. Restoration of cyclin D1 abolished WWOX-mediated cell proliferation inhibition compared to WWOX-overexpressing 22Rv1 cells (). Similar results were found in the colony-formation assay (), where WWOX overexpression decreased the colonization ability of 22Rv1 cells. However, restoration of cyclin D1 could almost completely recover the WWOX-mediated suppression of colonization. These finding suggest that cyclin D1 plays a key role in WWOX-mediated cell proliferation suppression, and that the re-expression of cyclin D1 can abolish the inhibitory effect of WWOX on cell proliferation. Taken together, our results support that WWOX induces cell cycle arrest in the G1 phase through cyclin D1 to suppress the progression of prostate cancer.
Figure 4. (See previous page). WWOX-mediated G1 arrest is through cyclin D1. (A) The ectopic expressed WWOX in 22Rv1 cell decrease cyclin D1 but not apoptotic-associated protein expression. In contrast, WWOX-silenced PC3 cell increase cyclin D1 expression. The protein expressions were measured by the antibodies shown at right. Cyclin D1 mRNA expression was measured under the same condition using RT-PCR. (B) Manipulated-22Rv1 derived tumor were collected to examine protein expression by immunohistochemistry. The protein expressions were measured by the antibodies shown at left. Representative WWOX expression level in injected-22Rv1 are validated by Western blot. Overexpress WWOX in 22Rv1 cell inhibit cell proliferation compare to control. While co-transfection of WWOX and cyclin D1 plasmids suppress WWOX-mediated cell growth. (C) After transfection, cell were collected and counted at 0, 24, 48 and 72 hours. Cell proliferation index represent proliferated cell number compare to 0 hour. Representative WWOX and cyclin D1 expression level are validated by Western blot. (D) Colony-forming assay of 22RV1 cell (control, WWOX and WWOX+cyclin D1). Quantification of colony numbers are shown on bottom panel. Data are representative of 3 independent performed experiments; mean ± SD; **, p < 0.005; ***, p < 0.001.
WWOX-mediated cyclin D1 decrease is through c-Jun
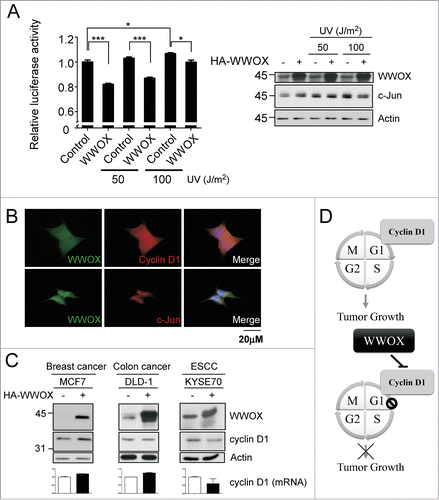
WWOX interacts with and suppresses several transcription factors, such as c-Jun, through sequesters them in the cytoplasmic compartment.Citation14 To further clarify whether WWOX-mediated cyclin D1 reduction is through c-Jun. The cyclin D1 promoter activity were examined using luciferase reporter assay with or without WWOX overexpression. UV treatment is to induce c-Jun overexpression and activation.Citation15 The data indicate that UV-induced c-Jun increase cyclin D1 promoter activity (). However, WWOX impair c-Jun-mediated cyclin D1 promoter activity. Representative WWOX and c-Jun expression level are validated by immunobloting. Taken together, WWOX-mediated cyclin D1 decrease maybe in part through c-Jun. In addition, subcellular localization of endogenous WWOX, cyclin D1 and c-Jun were examined by immunofluorescence. The data indicate that WWOX, cyclin D1 and c-Jun are detected in cytoplasm and WWOX can be co-localized with either cyclin D1 or c-Jun (). To verify whether the mechanism of WWOX-mediated cyclin D1 reduction was contributed to variant cancers. WWOX was overexpressed in breast, colon and esophageal cancer cell lines and cyclin D1 mRNA and protein expression were examined. The finding indicates that WWOX overexpression decrease cyclin D1 in both mRNA and protein in esophageal cancer cell line, but WWOX does not regulate cyclin D1 in breast and colon cancer cell lines (). These results show that WWOX-mediated cyclin D1 repression may be demonstrated in prostate cancer and ESCC cell lines but not in all cancer cell lines.
Figure 5. WWOX-mediated cyclin D1 decrease is through c-Jun. (A) The cyclin D1 promoter activity were determined using luciferase assay with or without WWOX overexpression under UV treatment. After UV exposure 1 hour, harvest cell to do reporter assay. Representative WWOX and c-Jun expression level are validated by Western blot. mean ± SD; *, P < 0.05; ***, P < 0.001 (B) Endogenous WWOX, cyclin D1 and c-Jun subcellular localization were examined by immunofluorescence. DAPI is used to indicate nucleus. (C) The mechanism of WWOX-mediated cyclin D1 decrease were examined in variant cancer types. The protein expressions were measured by the antibodies shown at left. The cyclin D1 mRNA expression were determined by qPCR. (D) The model to illustrate the WWOX-mediated tumor growth inhibition.
Discussion
We found that WWOX acts as a tumor suppressor in prostate cancer through regulating the cell cycle. At first, both protein and mRNA expressions of WWOX were decreased in human prostate cancer tissues. WWOX mRNA levels are frequently downregulated in variant tumors. There are at least 3 potential upstream mediator(s) that regulate WWOX transcription including our unpublished data descript below. First, WWOX is located at 16q23.3–24.1, that was reported is one of the common fragile sites, FRA16D.Citation6 The 16q23.3–24.1 is a frequent homozygous deletion region in various carcinoma. Second, hypermethylation of the WWOX promoter has been reported in both cancer cell lines and primary tumor.Citation16 These 2 recently known mechanisms may contribute to WWOX mRNA reduction in tumor tissues. Third, several potential miRNAs, miR-200b, 200c, 429 and 330, are predicted to interact with 3′UTR region of WWOX. That indicate these miRNAs may downregulate WWOX transcription or translation, but our data demonstrated that WWOX did not affect by these candidate miRNAs (data not show). Whether WWOX mRNA levels were regulated by transcriptional factor is worth to study in the future. We showed the suppression of tumorigenicity following overexpression of WWOX in 22Rv1 prostate cancer cells both in vitro and in vivo. In addition, further evidence supports the role of WWOX as a tumor suppressor as opposite results were found in WWOX-knockdown PC-3 prostate cancer cells. Qin and coworkers reported similar findings supporting WWOX as a tumor suppressor in prostate cancer.Citation17
WWOX has been reported to suppress tumorigenicity through apoptosis according in prostate cancer and breast cancer.Citation10,17 These studies found an increase in the sub-G1 population, cleaved poly (ADP-ribose) polymerase-1 and activation of caspase 3 and 9 with the overexpression of WWOX in cells. Interestingly, our results showed that the overexpression of WWOX in 22Rv1 cells did not lead to cell apoptosis, but rather resulted in cell cycle arrest in the G1 phase. This is consistent with the findings of Hu and colleagues in hepatic carcinoma.Citation18 The disparity between studies may be caused by different study time periods and cell lines, however all of the studies ultimately resulted in suppressed tumorigenicity. Moreover, we found that the overexpression of WWOX resulted in decreased expressions of cyclin D1 both in mRNA and protein, but did not change levels of apoptosis-related proteins such as caspase 3. In addition, the ectopic expression of WWOX prevented entry to mitosis, and restoration of cyclin D1 in 22Rv1 cells abolished the effect of WWOX-mediated cell cycle arrest in the G1 phase. Taken together, our results consistently support that the loss of WWOX in prostate cancer results in an increase of cyclin D1 and promotes cell cycle progression. Restoration of WWOX mediated cancer cell cycle arrest in the G1 phase through repression of cyclin D1 to inhibit tumor progression (). Several studies have reported that restoring the expression of WWOX in multiple cancers such as breast cancer and lung cancer leads to tumor repression through an increase in apoptosis,Citation10,17 however little is known about the regulation of the cell cycle by WWOX. WWOX is known to interact with several transcription factors such as p53, AP-2γ, c-Jun and β-catenin to regulate cellular signaling and tumor development.Citation11,14,19,20 C-Jun and Wnt/β-catenin are 2 critical pathway reported that to regulate cyclin D1 expression. Our data suggest that c-Jun could contribute to cyclin D1 promoter activation and WWOX can impair c-Jun mediated-cyclin D1 promoter activation in 22Rv1 cell (). The finding indicates that WWOX could decrease cyclin D1 mRNA expression through c-Jun pathway. On the other hand, previous data reported that low level of nucleus β-catenin increase the risk of relapse in prostate cancer.Citation21 However, it is inconsistent with the role of WWOX in prostate cancer. Taken together, WWOX-mediated cell cycle arrest in G1 may at least in part contributed by c-Jun pathway. Cell cycle disorder is a common phenomenon in cancer, and can lead to proliferation and also accumulation of mutations in cancer cells.Citation22 Cyclin D1 is an essential regulator of the G1toS transition in the cell cycle. Reduction of cyclin D1 may contribute cell cycle arrest in G1 phase lead to apoptosis in prostate cancer.Citation23 Previous studies have demonstrated that cyclin D1 is a proto-oncogene, and that its overexpression is common in multiple cancers including prostate cancer.Citation24 Depletion of cyclin D1 has been reported to induce cell cycle arrest and senescence in prostate cancer, furthermore, silence of cyclin D1 also contributed to inhibit cancer cell proliferation and attenuate the invasive capacity in glioblastoma.Citation25,26
The androgen receptor (AR) is known to play a critical role in prostate cancer progression. Therefore, depletion of AR function (either through androgen ablation or AR antagonist) is the first line of therapeutic treatment for prostate cancer. Androgen ablation triggers cell cycle arrest or cell death in prostate cancer.Citation27 However, tumor recurrence is common within a median of 2–3 years because of inappropriate restoration of androgen signaling. Castration-resistant prostate cancer treatment targeting AR such as abiraterone and enzalutamide has been reported to offer significant unexpected clinical benefits. Therefore even in castration-resistance prostate cancer, AR is considered to be the most important target. Androgen stimulates the accumulation of cyclin D1.Citation27 Previous studies have hypothesized that cyclin D1 acts as a negative feedback regulator to suppress androgen-dependent gene expression, however the current study emphasizes the opposite role of cyclin D1 in prostate cancer. The cyclin D1-positive phenotype has been reported to be increased in primary carcinoma and metastatic lymph node tissues compared to non-neoplastic tissues.Citation24 Therefore, cyclinD1 may be important for cell cycle entry after activation of androgen signaling. According to our results, WWOX was able to suppress cyclin D1 expression resulting in cell cycle arrest in the G1 phase to suppress prostate cancer progression. This finding suggests a novel therapeutic target for the treatment of prostate cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians 2014; 64:9-29; PMID:24399786
- Powell IJ, Bollig-Fischer A. Minireview: the molecular and genomic basis for prostate cancer health disparities. Mol Endocrinol 2013; 27:879-91; PMID:23608645; http://dx.doi.org/10.1210/me.2013-1039
- Alao JP, Gamble SC, Stavropoulou AV, Pomeranz KM, Lam EW, Coombes RC, Vigushin DM. The cyclin D1 proto-oncogene is sequestered in the cytoplasm of mammalian cancer cell lines. Mol Cancer 2006; 5:7; PMID:16503970; http://dx.doi.org/10.1186/1476-4598-5-7
- Casimiro MC, Pestell RG. Cyclin d1 induces chromosomal instability. Oncotarget 2012; 3:224-5; PMID:22538871
- Pluciennik E, Nowakowska M, Wujcicka WI, Sitkiewicz A, Kazanowska B, Zielinska E, Bednarek AK. Genetic alterations of WWOX in Wilms' tumor are involved in its carcinogenesis. Oncol Rep 2012; 28:1417-22; PMID:22842668; http://dx.doi.org/10.3892/or.2012.1940
- Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res 2000; 60:2140-5; PMID:10786676
- Chang NS, Doherty J, Ensign A, Lewis J, Heath J, Schultz L, Chen ST, Oppermann U. Molecular mechanisms underlying WOX1 activation during apoptotic and stress responses. Biochem Pharmacol 2003; 66:1347-54; PMID:14555208; http://dx.doi.org/10.1016/S0006-2952(03)00484-2
- Lewandowska U, Zelazowski M, Seta K, Byczewska M, Pluciennik E, Bednarek AK. WWOX, the tumour suppressor gene affected in multiple cancers. J Physiol Pharmacol 2009; 60 Suppl 1:47-56; PMID:19609013
- Qin HR, Iliopoulos D, Semba S, Fabbri M, Druck T, Volinia S, Croce CM, Morrison CD, Klein RD, Huebner K. A role for the WWOX gene in prostate cancer. Cancer Res 2006; 66:6477-81; PMID:16818616; http://dx.doi.org/10.1158/0008-5472.CAN-06-0956
- Fabbri M, Iliopoulos D, Trapasso F, Aqeilan RI, Cimmino A, Zanesi N, Yendamuri S, Han SY, Amadori D, Huebner K, et al. WWOX gene restoration prevents lung cancer growth in vitro and in vivo. Proc Natl Acad Sci U S A 2005; 102:15611-6; PMID:16223882; http://dx.doi.org/10.1073/pnas.0505485102
- Chang NS, Pratt N, Heath J, Schultz L, Sleve D, Carey GB, Zevotek N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J Biol Chem 2001; 276:3361-70; PMID:11058590; http://dx.doi.org/10.1074/jbc.M007140200
- Bednarek AK, Keck-Waggoner CL, Daniel RL, Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ, Aldaz CM. WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res 2001; 61:8068-73; PMID:11719429
- Gourley C, Paige AJ, Taylor KJ, Ward C, Kuske B, Zhang J, Sun M, Janczar S, Harrison DJ, Muir M, et al. WWOX gene expression abolishes ovarian cancer tumorigenicity in vivo and decreases attachment to fibronectin via integrin alpha3. Cancer Res 2009; 69:4835-42; PMID:19458077; http://dx.doi.org/10.1158/0008-5472.CAN-08-2974
- Gaudio E, Palamarchuk A, Palumbo T, Trapasso F, Pekarsky Y, Croce CM, Aqeilan RI. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res 2006; 66:11585-9; PMID:17178850; http://dx.doi.org/10.1158/0008-5472.CAN-06-3376
- Fritz G, Kaina B. Activation of c-Jun N-terminal kinase 1 by UV irradiation is inhibited by wortmannin without affecting c-iun expression. Mol Cell Biol 1999; 19:1768-74; PMID:10022864
- Ishii H, Vecchione A, Furukawa Y, Sutheesophon K, Han SY, Druck T, Kuroki T, Trapasso F, Nishimura M, Saito Y, et al. Expression of FRA16D/WWOX and FRA3B/FHIT genes in hematopoietic malignancies. Mol Cancer Res 2003; 1:940-7; PMID:14638866
- Qin HR, Iliopoulos D, Nakamura T, Costinean S, Volinia S, Druck T, Sun J, Okumura H, Huebner K. Wwox suppresses prostate cancer cell growth through modulation of ErbB2-mediated androgen receptor signaling. Mol Cancer Res 2007; 5:957-65; PMID:17704139; http://dx.doi.org/10.1158/1541-7786.MCR-07-0211
- Hu BS, Tan JW, Zhu GH, Wang DF, Zhou X, Sun ZQ. WWOX induces apoptosis and inhibits proliferation of human hepatoma cell line SMMC-7721. World J Gastroenterol 2012; 18:3020-6; PMID:22736928; http://dx.doi.org/10.3748/wjg.v18.i23.3020
- Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM. Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res 2004; 64:8256-61; PMID:15548692; http://dx.doi.org/10.1158/0008-5472.CAN-04-2055
- Bouteille N, Driouch K, Hage PE, Sin S, Formstecher E, Camonis J, Lidereau R, Lallemand F. Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. Oncogene 2009; 28:2569-80; PMID:19465938; http://dx.doi.org/10.1038/onc.2009.120
- Kypta RM, Waxman J. Wnt/beta-catenin signalling in prostate cancer. Nat Rev Urol 2012; 9:418-28; PMID:22710668; http://dx.doi.org/10.1038/nrurol.2012.116
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 2009; 9:153-66; PMID:19238148; http://dx.doi.org/10.1038/nrc2602
- Rico-Bautista E, Zhu W, Kitada S, Ganapathy S, Lau E, Krajewski S, Ramirez J, Bush JA, Yuan Z, Wolf DA. Small molecule-induced mitochondrial disruption directs prostate cancer inhibition via UPR signaling. Oncotarget 2013; 4:1212-29; PMID:23902736
- Drobnjak M, Osman I, Scher HI, Fazzari M, Cordon-Cardo C. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin Cancer Res 2000; 6:1891-5; PMID:10815912
- Noonan EJ, Place RF, Basak S, Pookot D, Li LC. miR-449a causes Rb-dependent cell cycle arrest and senescence in prostate cancer cells. Oncotarget 2010; 1:349-58; PMID:20948989
- Zhang X, Zhao M, Huang AY, Fei Z, Zhang W, Wang XL. The effect of cyclin D expression on cell proliferation in human gliomas. J Clin Neurosci 2005; 12:166-8; PMID:15749420; http://dx.doi.org/10.1016/j.jocn.2004.03.036
- Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal 2008; 6:e001; PMID:18301781; http://dx.doi.org/10.1621/nrs.06001
