Abstract
Tumor suppressor p53 plays a crucial antiviral role and targeting of p53 by viral proteins is a common mechanism involved in virus oncogenesis. The activity of p53 is tightly regulated at the post-translational levels through a myriad of modifications. Among them, modification of p53 by SUMO has been associated with the onset of cellular senescence. Kaposi´s sarcoma-associated herpesvirus (KSHV) expresses several proteins targeting p53, including the latent protein LANA2 that regulates polyubiquitylation and phosphorylation of p53. Here we show that LANA2 also inhibits the modification of p53 by SUMO2. Furthermore, we show that the reduction of p53-SUMO2 conjugation by LANA2, as well as the p53-LANA2 interaction, both require the SUMOylation of the viral protein and its interaction with SUMO or SUMOylated proteins in a non-covalent manner. Finally, we show that the control of p53-SUMO2 conjugation by LANA2 correlates with its ability to inhibit SUMO2- and type I interferon-induced senescence. These results highlight the importance of p53 SUMOylation in the control of virus infection and suggest that viral oncoproteins could contribute to viral infection and cell transformation by abrogating p53 SUMOylation.
Keywords:
Introduction
Tumor suppressor p53 is targeted by all oncogenic viruses to circumvent host growth surveillance and increase virus replication.Citation1 Generally, the regulation of p53 functions by viral oncoproteins involves mainly the alteration of its post-translational modifications ubiquitylation, acetylation or phosphorylation.Citation2
Kaposi's sarcoma-associated herpesvirus (KSHV) encodes several proteins that can compromise p53 activity, including the latency-associated nuclear antigen 2 (LANA2) (also called vIRF3). LANA2 inhibits both the transcriptional activation and apoptosis induced by p53 through the inhibition of p53 phosphorylation, by altering its oligomerization and DNA-binding affinity, and by increasing its polyubiquitylation.Citation3-8 Apart from p53, LANA2 also compromises the activity of other tumor suppressors such as pRb or PML.Citation9,10 Interestingly, the control of these 2 tumor suppressors by LANA2 involves the regulation of their SUMO conjugation.Citation9,10 Since p53 activity can be also regulated by SUMO we evaluated whether LANA2 could modulate p53 SUMOylation.
Here, we demonstrate that LANA2 interacts with p53 and downmodulates p53-SUMO2 conjugation. We also show that both, the inhibition of p53-SUMO2 conjugation and the interaction of LANA2 with p53 require an intact SUMO interacting domain (SIM) as well as SUMOylation domains in LANA2. Finally, we show that there is a positive correlation between the reduction of p53-SUMO2 conjugation by LANA2 and its ability to inhibit cell senescence induced in response to SUMO2 overexpression or type I interferon treatment. These results suggest that SUMOylation may be relevant for the functions of p53 in antiviral immunity.
Results and Discussion
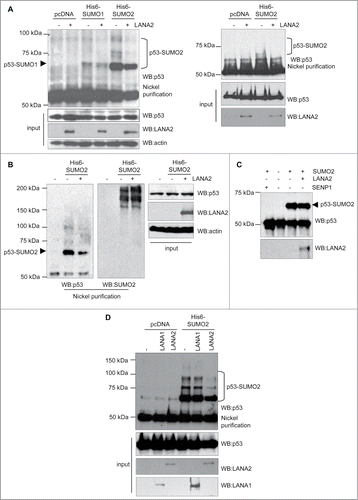
In order to analyze the effect of LANA2 expression on p53 SUMOylation, we co-transfected HEK-293 cells with Ubc9 and His6-SUMO1 or His6-SUMO2 plasmids, in the presence or absence of pcDNA-LANA2, and 48 h after transfection whole protein extracts and Histidine-tagged purified proteins were analyzed by Western-blot using anti-p53 antibody. As shown in A (left panel) p53-SUMO2 protein levels clearly decreased after expression of LANA2, whereas the p53-SUMO1 levels were not noticeably altered, suggesting that LANA2 inhibited the conjugation of SUMO2 to p53. Similar analysis of endogenous p53 on B cells (A, right panel) provided identical results. To evaluate whether LANA2 induces a general inhibition of SUMO2 conjugation, Histidine-tagged purified proteins obtained from HEK-293 cells transfected with the indicated plasmids were analyzed by Western-blot using anti-SUMO2 antibody. We observed again a clear decrease in the levels of p53-SUMO2 protein but we did not detect a reduction in the levels of total SUMO2-conjugated proteins in cells expressing LANA2 (). We then evaluated whether LANA2 can directly modulate p53-SUMO2 conjugation using an in vitro SUMOylation assay. As shown in , in vitro SUMOylation of p53 was not significantly affected by the presence of LANA2. However, when we added SENP1 (Biomol) to the reaction, we detected a complete inhibition of p53-SUMO2 conjugation, suggesting that LANA2 does not directly modulate p53-SUMO2 conjugation. In addition to LANA2, another latent KSHV protein, LANA1, also inhibits p53.Citation5 We decided to evaluate whether LANA1 could also inhibit the SUMOylation of p53. Western-blot analysis of HEK-293 cells transfected with His6-SUMO2 and pcDNA, LANA1 or LANA2, with anti-p53 antibody revealed that LANA2 reduced the levels of p53-SUMO2, confirming our results (). However, we did not observe a reduction in p53-SUMO2 conjugation when LANA1 was expressed (). All together, these results indicate that KSHV protein LANA2 inhibits the conjugation of SUMO2 to p53 in vivo.
Figure 1. LANA2 reduces SUMO2 conjugation to p53 in vivo. (A) SUMO2 conjugated p53 decreases in both, HEK-293 cells (left panel) and MHH–PREB-1 cells (right panel) after expression of LANA2. (B) LANA2 does not have a general effect on SUMO2 conjugation process. (C) LANA2 does not significantly affect p53-SUMO2 conjugation in vitro. (D) LANA2 but not LANA1 inhibits p53-SUMO2 modification in HEK-293 cells.
Both, SIM and SUMOylation domains in LANA2 are required for its control of PML nuclear bodies.Citation11 Therefore, we evaluated the conjugation of p53 to SUMO2 in HEK-293 cells co-transfected with pcDNA, LANA2-WT, a LANA2 mutant in the SIM domain (LANA2ΔSIM), a SUMOylation mutant (LANA2ΔSUMO), or the double SIM and SUMOylation mutant of LANA2 (LANA2TOTAL), together with an empty vector or Ubc9 and His6-SUMO2. Expression of LANA2-WT induced a reduction in the levels of p53-SUMO2 that was less evident after expression of LANA2ΔSIM or LANA2ΔSUMO, and almost undetectable after expression of LANA2TOTAL (). We then analyzed whether the reduction in p53-SUMO2 conjugation by LANA2 requires the interaction between both proteins. Anti-p53 immunoprecipitates obtained from HEK-293 cells transfected with pcDNA, LANA2-WT, or the LANA2 mutants were probed with anti-LANA2 antibody. As shown in , p53 co-immunoprecipitated with LANA2-WT. However, we did not detect an interaction between p53 and the LANA2 mutants. All together, these results demonstrate that LANA2 requires intact SIM and SUMOylation domains to interact with p53 and to effectively inhibit p53-SUMO2 conjugation.
Figure 2. LANA2 requires intact SIM and SUMOylation domains to inhibit p53-SUMO2 conjugation, to interact with p53, and to inhibit senescence induced by type I interferon treatment or SUMO2 overexpression. (A) LANA2 requires intact SIM and SUMOylation domains to efficiently reduce p53-SUMO2 conjugation. (B) Interaction between p53 and LANA2 requires intact SIM and SUMOylation domains in LANA2. (C) Inhibition of SUMO2-induced senescence by LANA2-WT but not by LANA2 mutants in the SIM or SUMOylation domains. Senescence was determined by using a senescence β-galactosidase staining kit. The results are presented as mean of 3 independent experiments +/− SD and analyzed by Student's t-test (*, P < 0.05 versus cells co-transfected with SUMO2 and pcDNA). (D) Inhibition of senescence induced by interferon treatment by LANA2-WT but not by the mutants of LANA2 in the SIM or SUMOylation domains. H1299-p53 cells transfected with LANA2-WT, LANA2ΔSIM, LANA2ΔSUMO or LANA2TOTAL were analyzed by Western blotting (upper-left panel) and treated or not with 500 U/ml β-interferon for 4 d after which cell senescence was evaluated by β-galactosidase staining. Representative pictures of SA-β-Gal staining (upper-right panel) and percentage of SA-β-Gal-positive cells (lower panel) in response to interferon treatment. The percentage of positive staining was determined by dividing the number of β-Gal-positive cells into the total number within 10 random fields. The results are presented as mean of 3 independent experiments +/− SD and analyzed by Student's t-test (***, P < 0.0005 vs. pcDNA transfected cells).
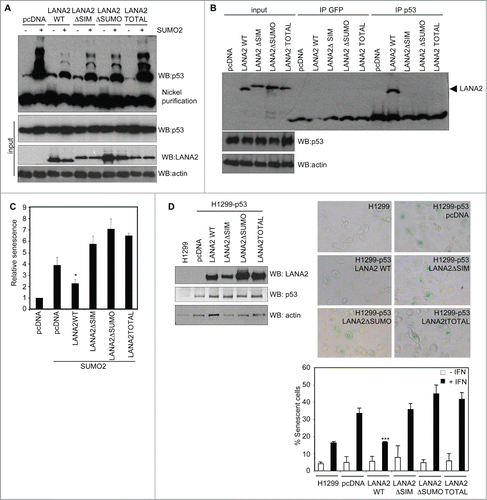
Overexpression of SUMO2 induces a premature senescence phenotype in correlation with the induction of SUMOylation of both, p53 and Rb.Citation12 Therefore, we analyzed the senescence of HEK-293 cells transfected by using a senescence β-galactosidase staining kit (Cell Signaling). As shown in , the induction of senescence observed after overexpression of SUMO2 was significantly reduced after co-transfection of LANA2-WT. In contrast, we did not detect a decrease in β-galactosidase staining after expression of any of the LANA2 mutants, indicating that LANA2 inhibits SUMO2-induced senescence by a mechanism that requires intact SIM and SUMOylation domains in the viral protein.
In addition, SUMOylation of p53 has a role in the induction of senescence in response to type I interferon treatment.Citation13 For this reason we evaluated senescence in H1299-p53 cellsCitation13 transfected with LANA2-WT or the LANA2 mutants after treatment with 500 U/ml of β-interferon. As shown in , the percentage of β-galactosidase positive cells observed after interferon treatment was significantly reduced when LANA2-WT was expressed, but it was not affected by the expression of the LANA2 mutants. All together, our results indicate that there is a direct correlation between inhibition of p53-SUMO2 conjugation by LANA2 and its ability to inhibit senescence induced by SUMO2 overexpression or interferon treatment.
It has been shown that, at least in some conditions, SUMO enhances p53 activity.Citation12,14,15 Furthermore, SUMOylation of p53 is induced in response to DNA damage, interferon treatment, or viral infection, and SUMOylation has an important role in the induction of senescence by p53,Citation12,13,16,17 suggesting that this modification may represent a physiological response to activate p53 after virus infection. Thus, it is possible to speculate that inhibition of p53 SUMOylation may be a mechanism used by viral proteins to control p53 activity. Along these lines, another viral oncoprotein, human papillomavirus E6, inhibits the SUMO ligase activity of PIASγ, inhibiting p53-SUMO2 conjugation, and counteracting the onset of cell senescence associated with induction of SUMOylation.
KSHV encodes several proteins with potential oncogenic capacity. One of them is LANA2, a protein that has been reported to be absolutely required for proliferation and survival of PEL cells.Citation18 Our results suggest that modulation of p53-SUMO2 conjugation by LANA2 might be an additional mechanism contributing to the establishment of a latent infection, proliferation and transformation of the infected cells.
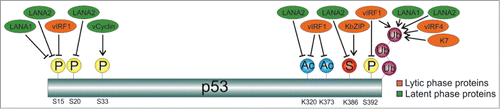
In summary, this report identifies a new mechanism of p53 post-translational regulation exerted by KSHV to control its activities (). Finally, this is also a new example of the important role played by SUMO in the regulation of viral proteins. It is then tempting to consider the possibility of targeting the interaction of viral proteins with SUMO as a promising potential therapeutic intervention against viral pathogenesis that merits further investigation.
Figure 3. Regulation of p53-posttranslational modifications by KSHV proteins. While latent protein vCyclin induces phosphorylation of p53 at S33,Citation22 both latent proteins LANA1 and LANA2 inhibit phosphorylation of p53 at S15Citation3,23 and promote p53 ubiquitylation.Citation3,24 In addition, LANA2 also inhibits phosphorylation of p53 at S20,Citation3 acetylation of p53 at K320Citation3 and SUMO-2 modification of p53 (this report). The lytic protein vIRF1 inhibits phosphorylation of p53 at S15 and S392,Citation25 acetylation at K320 and K373 Citation25 and promotes p53 ubiquitylationCitation26; vIRF4 and K7 also induce p53 ubiquitylation,Citation7,27 and KbZIP induces SUMO2 conjugation of p53.Citation28
Materials and Methods
Cell lines and transfections
HEK-293, and H1299 cells were maintained in DMEM supplemented with 10% heat inactivated-fetal calf serum (Gibco), 5 mmol/L L-glutamine (Invitrogen) and 1% penicillin-streptomycin solution (Sigma, 10,000 U/mL penicillin and 10 mg/mL streptomycin). MHH-PREB-1 cells were maintained in RPMI 1640 supplemented with 10% heat inactivated-fetal calf serum, 5 mmol/L L-glutamine and 1% penicillin-streptomycin solution. Transfection of HEK-293 was done using X-treme (Roche), and H1299 cells were transfected with lipofectamine 2000, following the manufacturer's instructions. For electroporation, MHH-PREB-1 cells (107 cells) were washed in RPMI 1640 without fetal calf serum, resuspended in 250 μl of the same medium, and placed with 20 μg of plasmid DNA in 0.4-cm gap electroporation cuvettes. Cells were transfected using an electroporator (Bio-Rad Laboratories) at 250 V and 960 μF.
Plasmids and reagents
pcDNA-p53, pcDNA-His6-SUMO1, pcDNA-His6-SUMO2, pcDNA-SV5-Ubc9, pcDNA-LANA2, pcDNA-LANA2ΔSIM, and pcDNA-LANA2ΔSUMO have been previously described.Citation8,10,11,19,20 Interferon was purchased from GenScript.
Immunoprecipitation assay
Cells were lysed in TNN buffer (100 mM Tris-HCl, pH 8, 250 mM NaCl, 0.5% NP-40) at 4°C, centrifuged at 15,800 x g for 5 min and immunoprecipitated overnight at 4°C after addition of 1 μl of the specified antibody and 50 μl of 50% protein A-Sepharose CL-4B beads (Amersham Biosciences). Beads were then washed 4 times with TNN buffer and resuspended in 30 μl of SDS-PAGE loading buffer.
Western-blot analysis and antibodies
For Western-blot analysis, cells were washed in PBS, scraped in SDS-gel loading buffer and boiled for 5 min. Proteins of total extracts were separated by SDS-PAGE and transferred to nitrocellulose membrane. The following antibodies were used: anti-p53 (DO-1) (sc-126, Santa Cruz), anti-LANA2 (Novus Biologicals), anti-actin (MP Biomedicals), anti-SUMO2 (Zymed laboratories), and anti-LANA (Advanced Biotechnologies Inc.) antibodies.
In vitro SUMOylation assay
In vitro SUMO conjugation assays were performed on [35S]methionine-labeled in vitro-transcribed/translated proteins as described previously.Citation21
Purification of His-tagged conjugates
Purification of His-tagged conjugates using Ni2+-NTA-agarose beads was performed as described.Citation10
Senescence determination
For SA-β-Gal activity cells treated as indicated were stained with the senescence β-galactosidase kit (Cell Signaling) following the manufacturer´s instructions. Percentage positive staining was determined by dividing the number of β-Gal-positive cells into the total number within 10 random fields from duplicate dishes.
Statistical analysis
For statistical analysis between control and different groups the Student's t test was applied. The significance level chosen for the statistical analysis was P<0 .05
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Funding at the laboratory of CR is provided by BFU-2011–27064. CFC-H is supported by La Caixa fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. AF is a FPU predoctoral fellow from MECD. MC is a “Miguel Servet” investigator (ISCIII). Work in the laboratory of MC is funded by ISCIII (CP/11/00273).
References
- Collot-Teixeira S, Bass J, Denis F, Ranger-Rogez S. Human tumor suppressor p53 and DNA viruses. Rev Med Virol 2004; 14:301-19; PMID:15334538; http://dx.doi.org/10.1002/rmv.431
- Jayaraman L, Prives C. Covalent and noncovalent modifiers of the p53 protein. Cell Mol Life Sci 1999; 55:76-87; PMID:10065153; http://dx.doi.org/10.1007/s000180050271
- Baresova P, Musilova J, Pitha PM, Lubyova B. p53 Tumor Suppressor Protein Stability and Transcriptional Activity Are Targeted by Kaposi's Sarcoma-Associated Herpesvirus-Encoded Viral Interferon Regulatory Factor 3. Mol Cell Biol 2014; 34:386-99; PMID:24248600; http://dx.doi.org/10.1128/MCB.01011-13
- Chudasama P, Konrad A, Jochmann R, Lausen B, Holz P, Naschberger E, et al. Structural proteins of Kaposi's sarcoma-associated herpesvirus antagonize p53-mediated apoptosis. Oncogene 2014; PMID:24469037
- Friborg J, Jr., Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 1999; 402:889-94; PMID:10622254
- Gwack Y, Hwang S, Byun H, Lim C, Kim JW, Choi EJ, et al. Kaposi's sarcoma-associated herpesvirus open reading frame 50 represses p53-induced transcriptional activity and apoptosis. J Virol 2001; 75:6245-8; PMID:11390631; http://dx.doi.org/10.1128/JVI.75.13.6245-6248.2001
- Lee HR, Toth Z, Shin YC, Lee JS, Chang H, Gu W, et al. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor 4 targets MDM2 to deregulate the p53 tumor suppressor pathway. J Virol 2009; 83:6739-47; PMID:19369353; http://dx.doi.org/10.1128/JVI.02353-08
- Rivas C, Thlick AE, Parravicini C, Moore PS, Chang Y. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J Virol 2001; 75:429-38; PMID:11119611; http://dx.doi.org/10.1128/JVI.75.1.429-438.2001
- Marcos-Villar L, Gallego P, Munoz-Fontela C, de la Cruz-Herrera CF, Campagna M, Gonzalez D, Lopitz-Otsoa F, Rodríguez MS, Rivas C. Kaposi's sarcoma-associated herpesvirus lana2 protein interacts with the pocket proteins and inhibits their sumoylation. Oncogene 2014; 33:495-503; PMID:23318443; http://dx.doi.org/10.1038/onc.2012.603
- Marcos-Villar L, Lopitz-Otsoa F, Gallego P, Munoz-Fontela C, Gonzalez-Santamaria J, Campagna M, Shou-Jiang G, Rodriguez MS, Rivas C. Kaposi's sarcoma-associated herpesvirus protein LANA2 disrupts PML oncogenic domains and inhibits PML-mediated transcriptional repression of the survivin gene. J Virol 2009; 83:8849-58; PMID:19553342; http://dx.doi.org/10.1128/JVI.00339-09
- Marcos-Villar L, Campagna M, Lopitz-Otsoa F, Gallego P, Gonzalez-Santamaria J, Gonzalez D, Rodriguez MS, Rivas C. Covalent modification by SUMO is required for efficient disruption of PML oncogenic domains by Kaposi's sarcoma-associated herpesvirus latent protein LANA2. J Gen Virol 2011; 92:188-94; PMID:20881090; http://dx.doi.org/10.1099/vir.0.024984-0
- Li T, Santockyte R, Shen RF, Tekle E, Wang G, Yang DC, Chock PB. Expression of SUMO-2/3 induced senescence through p53- and pRB-mediated pathways. J Biol Chem 2006; 281:36221-7; PMID:17012228; http://dx.doi.org/10.1074/jbc.M608236200
- Marcos-Villar L, Perez-Giron JV, Vilas JM, Soto A, de la Cruz-Hererra CF, Lang V, Collado M, Vidal A, Rodríguez MS, Muñoz-Fontela C, et al. SUMOylation of p53 mediates interferon activities. Cell Cycle 2013; 12:2809-16; PMID:23966171; http://dx.doi.org/10.4161/cc.25868
- Gostissa M, Hengstermann A, Fogal V, Sandy P, Schwarz SE, Scheffner M, Del Sal G. Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. Embo J 1999; 18:6462-71; PMID:10562558; http://dx.doi.org/10.1093/emboj/18.22.6462
- Muller S, Berger M, Lehembre F, Seeler JS, Haupt Y, Dejean A. c-Jun and p53 activity is modulated by SUMO-1 modification. J Biol Chem 2000; 275:13321-9; PMID:10788439; http://dx.doi.org/10.1074/jbc.275.18.13321
- Bischof O, Schwamborn K, Martin N, Werner A, Sustmann C, Grosschedl R, Dejean A. The E3 SUMO ligase PIASy is a regulator of cellular senescence and apoptosis. Mol Cell 2006; 22:783-94; PMID:16793547; http://dx.doi.org/10.1016/j.molcel.2006.05.016
- Yates KE, Korbel GA, Shtutman M, Roninson IB, DiMaio D. Repression of the SUMO-specific protease Senp1 induces p53-dependent premature senescence in normal human fibroblasts. Aging Cell 2008; 7:609-21; PMID:18616636; http://dx.doi.org/10.1111/j.1474-9726.2008.00411.x
- Wies E, Mori Y, Hahn A, Kremmer E, Sturzl M, Fleckenstein B, Neipel F. The viral interferon-regulatory factor-3 is required for the survival of KSHV-infected primary effusion lymphoma cells. Blood 2008; 111:320-7; PMID:17890449; http://dx.doi.org/10.1182/blood-2007-05-092288
- Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell 1998; 2:233-9; PMID:9734360; http://dx.doi.org/10.1016/S1097-2765(00)80133-1
- Vertegaal AC, Andersen JS, Ogg SC, Hay RT, Mann M, Lamond AI. Distinct and overlapping sets of SUMO-1 and SUMO-2 target proteins revealed by quantitative proteomics. Mol Cell Proteomics 2006; 5:2298-310; PMID:17000644; http://dx.doi.org/10.1074/mcp.M600212-MCP200
- Campagna M, Herranz D, Garcia MA, Marcos-Villar L, Gonzalez-Santamaria J, Gallego P, Gutierrez S, Collado M, Serrano M, Esteban M, Rivas C. SIRT1 stabilizes PML promoting its sumoylation. Cell Death Differ 2011; 18:72-9; PMID:20577263; http://dx.doi.org/10.1038/cdd.2010.77
- Chang PC, Li M. Kaposi's sarcoma-associated herpesvirus K-cyclin interacts with Cdk9 and stimulates Cdk9-mediated phosphorylation of p53 tumor suppressor. J Virol 2008; 82:278-90; PMID:17942552; http://dx.doi.org/10.1128/JVI.01552-07
- Curreli F, Friedman-Kien AE, Flore O. Glycyrrhizic acid alters Kaposi sarcoma-associated herpesvirus latency, triggering p53-mediated apoptosis in transformed B lymphocytes. J Clin Invest 2005; 115:642-52; PMID:15765147; http://dx.doi.org/10.1172/JCI200523334
- Cai QL, Knight JS, Verma SC, Zald P, Robertson ES. EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog 2006; 2:e116.
- Nakamura H, Li M, Zarycki J, Jung JU. Inhibition of p53 tumor suppressor by viral interferon regulatory factor. J Virol 2001; 75:7572-82; PMID:11462029; http://dx.doi.org/10.1128/JVI.75.16.7572-7582.2001
- Shin YC, Nakamura H, Liang X, Feng P, Chang H, Kowalik TF, Jung JU. Inhibition of the ATM/p53 signal transduction pathway by Kaposi's sarcoma-associated herpesvirus interferon regulatory factor 1. J Virol 2006; 80:2257-66; PMID:16474133; http://dx.doi.org/10.1128/JVI.80.5.2257-2266.2006
- Feng P, Scott CW, Cho NH, Nakamura H, Chung YH, Monteiro MJ, Jung JU. Kaposi's sarcoma-associated herpesvirus K7 protein targets a ubiquitin-like/ubiquitin-associated domain-containing protein to promote protein degradation. Mol Cell Biol 2004; 24:3938-48; PMID:15082787; http://dx.doi.org/10.1128/MCB.24.9.3938-3948.2004
- Chang PC, Izumiya Y, Wu CY, Fitzgerald LD, Campbell M, Ellison TJ, Lam KS, Luciw PA, Kung HJ. Kaposi's sarcoma-associated herpesvirus (KSHV) encodes a SUMO E3 ligase that is SIM-dependent and SUMO-2/3-specific. J Biol Chem 2010; 285:5266-73; PMID:20034935; http://dx.doi.org/10.1074/jbc.M109.088088
