Exposure to radiation and its damaging effects are a threat to all creatures which multiple repair systems, particularly DNA repair systems, are well orchestrated to counteract. Methods for response beyond the highly characterized genetic repair systems continue to be elucidated. We have recently demonstrated that purinergic nucleotide receptor signaling may play a significant role in coping with genotoxic stress.Citation1 Purinergic P2Y14 receptor deficient mice, like some other purinergic receptor null mice, do not display distinctive phenotypes under normal homeostatic conditions. However, particular types of stress unveil the role of purinergic receptors including that of P2Y14. In the case of P2Y14, the mice are sensitive to radiation-induced senescence at both the cellular and whole-organism level 1.
Figure 1. Hypothetical schema of the role of P2Y14 signaling axis in the stress-induced stem cell senescence.
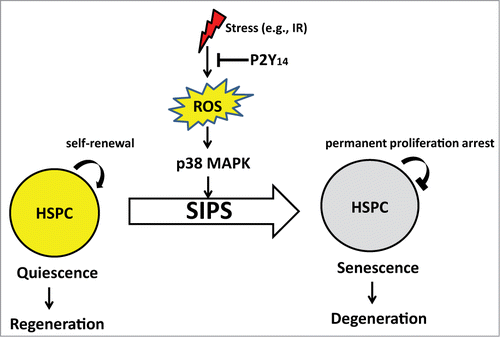
The P2Y14 receptor has been previously defined to alter immune cell responsiveness and to serve as a receptor activated by UDP-sugars.Citation2,3 UDP-sugars are generally intracellular intermediates and the receptor is therefore, likely to signal events associated with cell stress and cell disruption. A prompt but timely response to cell stress is critical to prevent or lessen stress-induced cellular damage. In this context, the accumulation of nucleotides does not require time and energy-consuming protein synthesis and is thereby appropriate for ensuring an immediate response to a “danger signal.”
Compromised stress responses are causatively associated with multiple diseases including age-related disorders. P2Y14 deficiency leads to an increased sensitivity of haematopoietic stem progenitor cells (HSPC) to stress-induced premature senescence (SIPS), and cell death, resulting in defective self-renewal and impaired reconstitution activity following serial transplantation.Citation1
Recently, cytokines, chemokines and proteases that are involved mostly in inflammatory responses were revealed as non–cell-autonomous modulators of senescence (referred as the senescence-associated secretory phenotype (SASP)).Citation4 Ligands of purinergic receptors are secreted into the extracellular environment in response to inflammation and other stresses, and have been reported to modulate the expression of central components of SASP factors.Citation5 It is therefore conceivable that P2Y14 receptor signaling may be functionally interlinked to the modulation of SASP factors, thereby regulating stress-induced stem cell senescence.
Of special interest is the fact that HSPCs derived from aged P2Y14-deficient animals (e.g., 21–25 month old) also exhibit increased cellular senescence even in the absence of exogenous stress.Citation1 This indicates the potential impact of P2Y14 not only on SIPS but also on the natural aging process. While senescence is already in progress at the cellular level in aged P2Y14 deficient animals, these mice display no observable features of accelerated aging at the level of the whole organism. Cellular senescence appears to be tightly linked to tissue and organism aging, but whether it is a driving mechanism for tissue and organism aging remains an open question. While aging is relatively apparent at the organism level by the presence gray hair and wrinkles, and at the tissue level by the deterioration of reproductive (ex: loss of reproductive capacity), cognitive (ex: loss of memory) and sensory (ex: loss of hearing or eyesight) functions, aging at the cellular and molecular level is difficult to define, since only a handful of senescence makers were available until recently. Considering that more than 800 genes are known to associate with Arabidopis senescence,Citation6 further identification and functional characterization of senescence-associated genes in mammals is critical. It would also be necessary to investigate more about the fundamental similarities and differences between molecular pathways of replicative stress, and oncogene-induced senescence and whether and how any of these contributes to organismal aging.
Of note, P2Y14 expression is predominant in immature stem progenitors both in humans and mice, and defines a subtype of deeply quiescent (G0) HSPCs in human.Citation7 Thus, it is reasonable to explore whether disruption of P2Y14 signaling in stem progenitor cells, where P2Y14 is predominantly expressed, limits the capacity of stem cells to reside in a quiescent state in response to stress enabling them to move toward senescence (Fig. 1). Agonists and antagonists for P2Y14 were identified. The remaining question is therefore whether SIPS is modifiable by a P2Y14 receptor based strategy?
It may not be possible to stop or reverse the ticking of the “biological clock” (intrinsic aging) and stress seems to be unavoidable. However, stress-induced (extrinsic) aging is possibly modifiable with more in-depth knowledge of the molecular pathways governing stem cell transitions from quiescence (transient) to senescence (permanent).
References
- Cho J, et al. J Clin Invest 2014; 124:3159-71; PMID:24937426; http://dx.doi.org/10.1172/JCI61636
- Barrett MO, et al. Mol Pharmacol 2013; 84:41-9; PMID:23592514; http://dx.doi.org/10.1124/mol.113.085654
- Scrivens M, Dickenson JM. Brit J Pharm 2005; 146:435-44; PMID:15997228; http://dx.doi.org/10.1038/sj.bjp.0706322
- Campisi J, et al. Semin Cancer Biol 2011; 21:354-9; PMID:21925603
- Idzko M, et al. Brit J Pharmacol 2003; 138:1244-50; PMID:12711624; http://dx.doi.org/10.1038/sj.bjp.0705145
- Buchanan-Wollaston V, et al. Plant J 2005; 42:567-85; PMID:15860015; http://dx.doi.org/10.1111/j.1365-313X.2005.02399.x
- Lee BC, et al. Genes Dev 2003; 17:1592-604; PMID:12842911; http://dx.doi.org/10.1101/gad.1071503
