How metazoan cell-killing genes, such as caspases (cysteine-dependent aspartate-directed proteases), evolved from their ancestors in unicellular organisms has been a hot topic for more than a decade. Due to the lack of clear evolutionary benefits for having such genes in single-cell eukaryotes, the route on which these genes were selected during evolution is still a mystery. Recent work, based on an unbiased genetic screen, provides more evidences for caspases/metacaspases originally being members of the protein quality control network, and later evolved as programmed cell death (PCD, apoptosis) executioners in the cell.Citation1
Caspases are a conserved family of cysteine proteases that are essential for the regulation of PCD in worms, flies, and mammals. For higher eukaryotes like humans, such function can be either beneficial, e.g., preventing over-proliferation of cells harboring aggressive mutations (cancer), or life threatening, e.g., causing progressive loss of structure or function of neurons (neurodegenerative disease). Two groups of caspase-like proteins have been identified,Citation2 both show significant similarity to the primary sequence and secondary structures of metazoan caspases. One group is designated as paracaspases found in animals and slime mold and the other group identified in plants, fungi, and protozoa, termed as metacaspases. Yeast (Saccharomyces cerevisiae) has an ancient caspase Mca1 (MetaCAspase 1), which is a close caspase relative that appears to behave as a cell death executioner under certain stresses.Citation2,3 Intriguingly, studies have also indicated that under normal culture condition Mca1 has non-death roles in altering cell cycle dynamics and clearance of protein aggregates ().Citation4,5
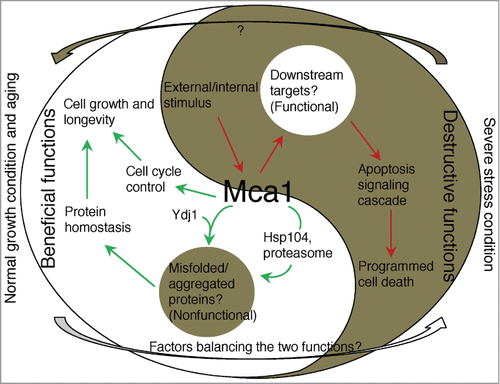
Figure 1. The Yin and Yang of the yeast Mca1. The yeast Mca1 induces PCD under certain conditions (dark-half), probably by targeting unknown functional components crucial for cell survival (bright-island). It also plays a beneficial role in cell cycle control and by potentially removing nonfunctional misfolded proteins (dark-island), helps cells to maintain protein homeostasis to ensure cell growth and longevity together with chaperones and proteasomes (bright-half).
It has been widely debated whether there are evolutionary benefits for a unicellular organism, like yeast, to harbor self-terminating genes and if metacaspases possess other functions unrelated to cellular execution. To approach such possibilities, we performed an unbiased synthetic genetic (lethality/sick) array analysis (SGA)Citation6 to provide us with clues regarding these questions. Since synthetic genetic interaction identifies functional relationships between genes, a genome-wide analysis of such interactions of MCA1 can provide a global map of all its functional partners. In this screen, we identified the MCA1 genetic network and almost all strong interactors buffering against the loss of Mca1. We found that Mca1 buffers against deficiencies in chromosome segregation and chromatin remodeling functions, which is in line with its cell cycle control function observed previously.Citation4 The other Mca1 interacting group is involved in protein quality control (PQC) processes. Strikingly, the strongest and only clear hit we attained from the non-essential gene deletion collection (about 4200 deletion strains) was the major yeast Hsp40 chaperone YDJ1. This indicates that MCA1 buffers against the loss of function of the Ydj1 chaperone and thus might play a direct role in PQC functions.Citation1 From another imaging-based screen of a mini array that consists of yeast chaperones and chaperone-related/interacting mutants, Mca1 was found to be required for proper segregation of heat-induced protein aggregates between mother and daughter cells; specifically the MCA1 deletion affected aggregate removal in daughter cells and in turn, Mca1 overproduction enhanced aggregate removal in daughter cells.
Lee and colleagues have suggested that Mca1 associates with heat-induced protein aggregates and chaperones.Citation5 We observed that Mca1 not only co-localize with heat-induced aggregates but also associates with aggregates formed upon aging. Due to the intimate link between protein aggregates partitioning/PQC and aging,Citation7 we then addressed the question whether Mca1 plays a role in the yeast aging process. A previous study has shown that disruption of Mca1 can increase cell survival during prolonged periods of cell culturing, i.e. stationary phase-induced growth arrest (chronological aging).Citation3 We instead examined the replicative lifespan of yeast cells (number of daughters a yeast mother cell can generate before it reaches senescence). Surprisingly, we found that the lack of Mca1 shortened rather than prolonged lifespan and that elevated Mca1 level drastically extended lifespan to about 46–56%. Such lifespan extending effects were found to require the presence of the Hsp104 disaggregase, a fully functional pool of proteasomes, and, in part, the Hsp40, Ydj1. Together, these data suggest that the removal of damaged and misfolded/aggregated proteins is key to the function of Mca1 in lifespan control ().
A direct follow-up question is whether the Mca1 catalytic activity is required for these functions. The yeast Mca1 harbors the conserved caspase cysteine catalytic siteCitation2 and this site was previously found to be required for both the full activity of PCD inductionand aggregate clearance functions.Citation3,5 Our data shows that a catalytically inactive Mca1 is ineffective in counteracting the accumulation of aging-induced aggregates and in extending lifespan. Without a functional Ydj1, overproduction of a catalytically inactive Mca1 caused a reduced rather than prolonged lifespan.Citation1
These results and results from other researchers have raised some challenging questions: One is how cells control and balance the beneficial (PQC) and destructive (PCD) functions of Mca1 (); the other is how, exactly, Mca1 exerts these functions through its potential physiological targets (). Hunting for Mca1 downstream substrates under different growth conditions will shed light on these questions and advance our understanding of the Mca1 related PQC and PCD pathways.
References
- S. M. Hill, et al. Science 2014; 344: 1389; PMID:24855027; http://dx.doi.org/10.1126/science.1252634
- Uren AG, et al. Mol cell 2000; 6: 961; PMID:11090634
- Madeo F. et al. Mol cell 2002; 9: 911; PMID:11983181; http://dx.doi.org/10.1016/S1097-2765(02)00501-4
- Lee RE, et al. PloS one 2008; 3: e2956; http://dx.doi.org/10.1371/journal.pone.0002956
- Lee RE et al. PNAS 2010; 107: 13348; PMID:20624963; http://dx.doi.org/10.1073/pnas.1006610107
- Tong AH et al. Science 2001; 294: 2364; PMID:11743205; http://dx.doi.org/10.1126/science.1065810
- Liu B, et al. Cell 2010; 140, 257; PMID:20141839; http://dx.doi.org/10.1016/j.cell.2009.12.031
