Abstract
Stem cells have a peculiar chromatin architecture that contributes to their unique properties, including uncommitted status, multi/pluripotency and self-renewal. We analyzed the effect of the de-regulation of the SWI/SNF chromatin remodeling complex in mesenchymal stromal cells (MSC) through the silencing and up-regulation of BRG1, which is the ATPase subunit of the complex. The altered expression of BRG1 promoted the senescence of MSC with suppression of the NANOG transcription, which is part of the transcriptional circuitry governing stem cell functions. To gain insight on the way NANOG was silenced, we evaluated how the de-regulated BRG1 expression affect the binding of activators and repressors on the NANOG promoter. We found 4 E2F binding motifs on NANOG promoter, which can be occupied by RB1 and RB2/P130. These are members of the retinoblastoma gene family. In MSC with a silenced BRG1, the relative binding of the 2 retinoblastoma proteins increased, and this was associated with the recruitment of DNMT1. This induced the methylation of CpG on the NANOG promoter. Opposingly, when a high level of BRG1 was present, the same E2F binding motifs were docking sites for BRG1, which induced chromatin compaction without CpG methylation but with increased histone deacetylation, associated with the presence of HDAC1 on E2F binding sites. Besides the sharp regulation of the NANOG expression, we evidenced, through proteomic analysis, that the de-regulation of the SWI/SNF function affected the expression of histones and other nuclear proteins involved in “nuclear architecture,” suggesting that BRG1 may act as global regulator of gene expression.
Introduction
Stem cells play a key role in the homeostasis of several tissues. For this reason, the blunted regenerative potential of tissues observed during aging may be viewed as a stem-cell disorder, where stem cells are lost or inactivated by senescence. This is a process through which the capacity of cell division, growth, and function is lost over time. Cells may respond to endogenous and exogenous stresses by undergoing senescence. Genomic stressors are damage events, which include i) the shortening of chromosome telomeres; ii) non-telomeric DNA damage; iii) excessive mitogenic signals, which may cause DNA damage; iv) and non-genotoxic stress, such as perturbations to chromatin organization.Citation1
Modulations of chromatin structure that often accompany the regulation of transcription can be achieved by chromatin remodeling complexes. These complexes carry out enzymatic activities, changing chromatin status by altering DNA–histone contacts within a nucleosome in an adenosine triphosphate (ATP)-dependent manner.Citation2,3
The mammalian SWItch/Sucrose NonFermentable (SWI/SNF) family is a class of ATP-dependent remodeling complexes.Citation4 It includes several members that share most of the same subunits–that is, the ATPase enzyme, in the brahma-related gene 1 (BRG1) or the BRM proteins and/or the presence of tissue-specific isoforms.Citation5,6 These complexes can either promote or impair transcription through their ability to slide nucleosomes along DNA, modify histone composition or the conformation of nucleosomes. In this way, they can contribute to the creation of regulatory regions and nucleosome-free regions on gene promoters. This allows for the binding of RNA polymerase and associated factors. Alternatively, they contribute to the formation of dense DNA strings on gene-regulatory elements, which impair polymerase binding.Citation5
Given the paramount importance of such phenomena, it is not surprising that the BRG1 activity must be tightly regulated, and unscheduled changes in its expression–even subtle modifications–may have a profound negative impact on cell functions.Citation7 In this context, we demonstrated that both the forced upregulation and the silencing of BRG1 in mesenchymal stromal cells (MSC) trigger cellular senescence (File S12). This phenomenon may be ascribed to the observation that de-regulated changes in chromatin status can trigger senescence.Citation8-11 Studies on the senescence of MSC are of great interest given their key physiological roles and their wide use in clinical trials for cellular therapy.Citation12–15
Pluripotency of embryonic stem cells (ESC) is maintained through various extrinsic and intrinsic influences. In the center of intrinsic stemness signaling, a triad of transcription factors–namely NANOG, Oct3/4 and Sox2–sustain ESC properties.Citation16 In detail, it has been demonstrated that NANOG counteracts differentiation but is ultimately dispensable for the maintenance of pluripotency in ESC.Citation17 Some data suggest that NANOG contributes to maintaining cells in an uncommitted state, blocking senescence. Indeed, cellular senescence, which is a barrier to the direct reprogramming of somatic cells into induced pluripotent stem cells (iPSC), may be overtaken by a forced NANOG expression.Citation18 Some genes of ESC stemness signaling are expressed in adult stem cells and may regulate self-renewal, pluripotency and commitment. We and other groups evidenced the expression of Oct3/4 and NANOG in MSC.Citation19-21
Of interest, the senescence of MSC, induced by the forced modification of the BRG1 level is accompanied by a strong down-regulation of the NANOG gene expression (File S11).Citation8,10 This is in good agreement with studies showing that NANOG may reverse the effect of organismal aging on MSC and which appears to have a key role in maintaining stemness and blocking senescence.Citation18,22,23
We decided to analyze in depth the effect of BRG1 silencing and up-regulation on the expression of NANOG, given its role in regulating stem-cell properties and acting as barrier for senescence. Chromatin remodelers act by modulating access to gene-regulatory regions (promoters, enhancers and silencers) of transcription activators and repressors. In this setting, we evaluated how de-regulated BRG1 expression may affect the binding of activators and repressors on the NANOG promoter. We decided to determine if the NANOG promoter has binding sites for the RB/E2F and P53 complexes and whether their binding may be affected eventually by changes in the BRG1 level. Our working hypothesis relies on our previous findings showing that BRG1-related senescence is associated with activation of RB- and P53-pathways.Citation8,10
Perturbation in BRG1 and NANOG activity may have profound impact on the gene expression programs in MSC, since BRG1 regulates gene expression at the chromatin level, and NANOG is at the top of the transcription-factor hierarchy, which controls stem-cell properties. For this reason, we completed our analysis on the effect of the de-regulated of BRG1 and NANOG expression in MSC by performing a comparative proteome analysis of nuclear-protein profiles in MSC with silenced and overexpressed BRG1. The aim of the study was to identify possible factors involved in the switch from the “healthy” to senescent phenotype.
Results
The proximal NANOG promoter has putative P53 and E2F binding sites
We used the Nucleotide Database Collection of the National Center for Biotechnology Information (NCBI) to identify the putative NANOG promoter. We selected a 6001 bp region, spanning −5700 to +300 of NANOG gene, and considered it a 5’-flanking region (from nt 7936295 to nt 7942295 of the sequence of GI 224589803 [http://www.ncbi.nlm.nih.gov/nuccore/224589803]), hereafter indicated as −5700 + 300.
We evaluated the selected region with Proscan online software, which finds a putative RNA pol II promoter region in the eukaryotic genome with a small rate of false positives (http://www-bimas.cit.nih.gov/molbio/proscan/).Citation24 The results evidenced a putative promoter core region predicted on the forward strand in −226 to +24 with a TATA box found at +2 and an estimated transcription start sequence (TSS) at +32 (File S1). This result is in good agreement with the hypothetical TSS, which is reported by NCBI and suggests that the 5’-flanking sequence that we selected may contain NANOG promoter regulatory elements.
We used JASPAR, PATCH and ALIBABA2 software to analyze the 5’-flanking region of the NANOG gene to look for transcription factors that might participate in regulating its expression. We focused our attention on P53 and E2F binding motifs, since in our previous research, we evidenced that the BRG1 de-regulated expression is associated with the activation of retinoblastoma-like protein 2 RB2/P130- and P53-related pathways.Citation8,10 Moreover, there are reports showing that the mouse Nanog promoter may contain putative P53 binding sites, and the proximal human NANOG promoter may have E2F-motifs.Citation25,26
The JASPAR program found 2 P53 binding sites at −5576 and −3620 (; File S2). The PATCH analysis evidenced 3 putative binding sites at −3944, −3115 bp, and −44. The ALIBABA2 program did not find any P53 binding site (; File S3).
Figure 1. P53 and E2F binding sites on the NANOG promoter. A 6001 bp sequence of the human NANOG gene, from nt 7936295 to nt 7942295 of the NCBI GI 224589803 (http://www.ncbi.nlm.nih.gov/nuccore/224589803), was considered the 5’-flanking Transcription Start Sequence (TSS), which encompass the −5700 + 300 gene region. JASPAR, PATCH and ALIBABA2 programs were used to identify the putative transcription-factor binding site. Panel A shows the nucleotide positions of the P53 putative and validated binding sites. Panel B shows the E2F binding sites.
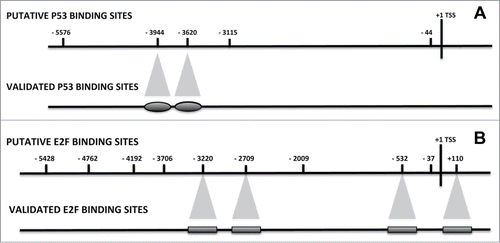
The search for E2F binding motifs evidenced several putative sites, as follows: −4192, −3706 and −532 according to JASPAR; −5428, −4762, −3220, −2709, −2009, −532, −37 and +110 according to PATCH (; Files S2 and S3). The ALIBABA2 program did not find any E2F binding motif.
We selected several couples of primers to perform CHIP analysis in order to verify if these regions were true functional binding motifs and if their occupancy was modified by the de-regulation of BRG1 expression (File S4).
We carried out CHIP assays on confluent MSC cultures–which did not express NANOG, as detected by RT-PCR–and on a low-passage proliferating MSC that showed a NANOG mRNA expression. We measured the relative binding of P53 and RB2/P130 on the above-identified P53 and E2F DNA-sequence motifs in the NANOG promoter by anti-P53 and anti-RB2 immunoprecipitation versus immunoprecipitation with a non-specific antibody control.
The CHIP assay for the identification of the binding of P53 on 5’-flanking region of the NANOG gene evidenced that 2 sites (−3944 and −3620) may be considered putative P53 binding sites as determined by the presence of P53 protein on these specific DNA sequences in the assay carried out on confluent MSC that did not express NANOG (). We observed the presence of P53 on the 2 binding sites also in the low-passage proliferating MSC. This is not unexpected, since even in young MSC cultures, there are some senescent cells and cells with damaged DNA, which are cell-cycle stalled.Citation27
The identification of the RB2/P130 binding site on the NANOG promoter evidenced 4 putative binding sites (−3220, −2709, −532 and +110), as determined by CHIP performed on the confluent MSC (). As for P53, a residual RB2 binding was also present in proliferating MSC cultures.
The retinoblastoma gene family also comprises the RB1 gene, which has been described as a major regulator of cell cycle, proliferation, differentiation, apoptosis and senescence.Citation27-29 We then decided to evaluate if this factor may occupy the same putative E2F binding sites of RB2/P130 or, alternatively, different binding regions. The identification of the RB1 binding site on the NANOG promoter evidenced that this protein occupies the same putative binding sites as RB2/P130 on confluent and proliferating MSC ().
CHIP is most useful to examine proteins directly bound to DNA, such as transcription factors, histones, and their covalent modifications. However, in some cases, it may be not effective to examine proteins that are indirectly associated with DNA (i.e., transcriptional coactivators and corepressors), such as RB2/P130 and RB1. For this reason, our analysis might have overlooked some E2F binding sites on the NANOG promoter. Nevertheless, those we identified may be considered strong E2F binding sites, since they were identified through indirect DNA-associated proteins.
De-regulated BRG1 expression is associated with the modification of P53, RB2/P130 and RB1 binding on the NANOG promoter
Once we identified the binding sites for P53, RB2/P130 and RB1 on the NANOG promoter, we carried out experiments to evaluate if the occupancy of these sites is influenced by BRG1 up- and downregulation. We silenced and up-regulated BRG1 in MSC, as we already reported.Citation8,10 On these samples, we analyzed the binding of P53 on the −3944 and −3620 binding sites (). CHIP-PCR analysis showed quantitative changes in P53 binding, only on the −3620 site in cells with silenced BRG1. No changes in P53 binding levels were detected following BRG1 upregulation.
Figure 2. CHIP analysis of the P53, RB1 and RB2 binding on the NANOG promoter. MSC with silenced or over-expressed BRG1 was used for CHIP analysis. The upper graph shows CHIP results in cells with silenced BRG1. In these cells, the P53, RB1 and RB2 binding on the corresponding P53 and E2F sites, respectively, was compared with control MSC cultures. Normalized data with input percent method in cells with silenced BRG1 were divided by normalized data in control cells. Ratio values are expressed as changes in the binding level (fold change). The numbers on the X-axis refer to the nucleotide positions of the identified binding sites. The lower graph shows the same analysis in cells with overexpressed BRG1. Three biological replicates were used for each experimental condition, * P ≤ 0.05.
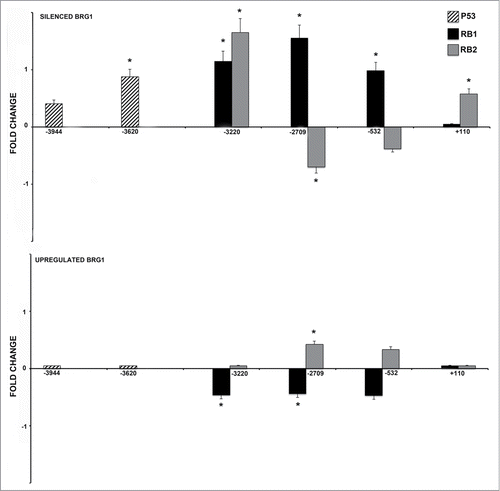
Changes in BRG1 levels affected greatly the binding of RB2/P130 and RB1 on the E2F sites of the NANOG promoter. Silencing of BRG1 induced an increase in the binding capacity of RB2/P130 on 2 sites (−3220, +110) and of RB1 on 3 sites (−3220, −2709, −532). Of note, each of the 2 proteins showed specific changes in binding capacity, as for the −2709 and – 532 sites. This suggests that their activities may be only partially overlapping. ().
Differences in RB2/P130 and RB1 binding were observed in cells overexpressing BRG1. RB1 binding showed a complementary profile to that observed in cells with silenced BRG1. We detected a net decrease of the RB1-binding capacity on the same sites that showed increased binding in cells with silenced BRG1 (−3220, −2709, −532). A mild increase in RB2 binding occurred on −2709 and −532 sites ().
Partners of RB2/130 and RB1 on the NANOG promoter
Previous CHIP analysis suggested that silencing of NANOG expression was mainly associated with quantitative changes in the binding of RB2/P130 and RB1 on E2F binding sites rather than on P53. To get further insights on the way RB2/P130 and RB1 may participate in silencing NANOG expression, we evaluated if these proteins may attract other co-factors on the NANOG promoters. The RB1 and RB2/P130 proteins regulate gene expression by attracting on E2F binding sites the chromatin-remodeling enzymes (BRG1, BRM), the histone deacetylases and the DNA methyltransferases.Citation28,30,31 In different settings, the retinoblastoma proteins may require only some of these factors for signaling to critical downstream effectors and the subsequent regulation of several aspects of cell biology (proliferation, differentiation, apoptosis, senescence).
We evaluated the presence of DNMT1 on the NANOG promoter through indirect binding on E2F sites. CHIP analysis of DNMT1 indirect binding on E2F sites evidenced the same quantitative changes we observed in RB1 binding profiles with an increased binding in cells with silenced BRG1 and a complete absence of binding in cells with upregulated BRG1 (). Of great interest, changes occurred on the same E2F sites of RB1 (−3220, −2709, −532). DNMT1 methylates CpG residues and is involved in maintaining the methylation pattern in the newly synthesized DNA strand that is essential for epigenetic inheritance.Citation32 We evaluated if the methylation pattern of the NANOG promoter was affected by changes in DNMT1 binding on the E2F sites. We identified CpG regions in the NANOG promoter by the METHPRIMER software (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi) and by previous findingsCitation33 (File S5). We did not evidence CpG islands according the software criteria (Island size > 100, GC Percent > 50.0, Obs/Exp > 0.60). Nevertheless, there were some CpG-enriched sequences, such as those in the region encompassing −554 nt and −325 nt (File S5). We selected this region and used MS-Real Time PCR to identify changes in the methylation status. The selected CpG region evidenced a strong methylation in cells with silenced BRG1, as compared with the control and cells overexpressing BRG1. In detail, the methylation-specific amplicons were 50% increased in cells with silenced BRG1, compared with control cells, whereas in cells overexpressing BRG1, we detected a 55% decrease.
Figure 3. CHIP analysis of the DNMT1, HDAC1 and acetylated H3 binding on the NANOG promoter. MSC with silenced or overexpressed BRG1 was used for CHIP analysis. Panel A shows DNMT1 binding on the E2F site in control cultures and in cells with silenced and over-expressed BRG1. The numbers on the X-axis refer to the nucleotide positions of the identified binding sites. The horizontal bar represents the CpG regions we analyzed with MS-PCR. Panel B shows the HDAC1 binding, and Panel C shows the acetylated H3 binding (H3Ac) on the NANOG promoter in the same experimental conditions reported in A. Real Time PCR data are expressed as arbitrary units. Three biological replicates were used for each experimental condition, * P ≤ 0.05. Y-axis represent cell total DNA bound/input sample according the percent input procedure described in methods.
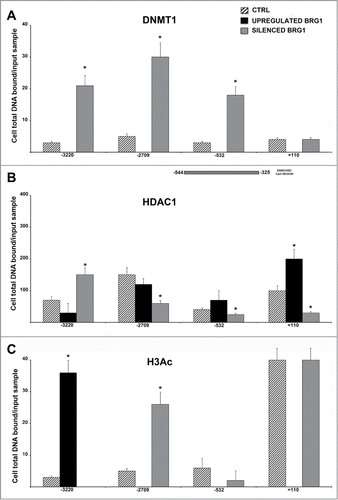
We then determined if RB2/P130 and/or RB1 may recruit HDAC1 on E2F binding sites. CHIP for the HDAC1 binding site on the NANOG promoter evidenced that this factor is present on the 4 putative E2F binding sites (−3220, −2709, −532 and +110), as determined by CHIP performed on the confluent MSC (control cultures) (). A residual HDAC1 binding was present in proliferating MSC cultures (data not shown). Silencing and up-regulation of BRG1 had a different effect on the relative HDAC1 occupancy. Silencing of BRG1 induced a decreased HDAC1 binding on 3 sites (−2709, −532 and +110) and increased HDAC1 presence on the -3220 site (). In cells with up-regulated BRG1, we found almost a reciprocal pattern with an increased HDAC1 binding in the −532 and +110 sites and a diminution on the −3220 site ().
Changes in HDAC1 binding may affect the histone acetylation level on the NANOG promoter. We performed CHIP assay to detect the presence of acetylated H3 in the regions of the NANOG promoter encompassing the identified E2F binding sites (). The increase in HDAC1 binding we observed in cells with upregulated BRG1, resulting in a complete absence of H3 acetylation on 3 E2F sites on the NANOG promoter (). In cells with silenced BRG1, the absence of H3 acetylation was observed only on the −3220 sites ().
The SWI/SNF ATPase function is manifested through one of 2 subunits, BRG1 or brahma (BRM). Although both BRG1 and BRM can function as the central ATPase in the SWI/SNF complex, each defines a discrete complex with unique biochemical activity. We evaluated if BRG1 and BRM are recruited on E2F1 sites of the NANOG promoter.
In basal conditions (confluent MSC), we detected the presence of both BRG1 and BRM on the 4 E2F binding sites. In cells over-expressing BRG1, we evidenced a net increase of BRG1 occupancy on the E2F binding sites (). This was associated with a complete absence of BRM on the same sites (). We found a minimal BRG1 binding on NANOG promoter in cells with silenced BRG1, but data were below LOQ (limit of quantification). In cells with silenced BRG1, the E2F binding sites were occupied by BRM ().
Figure 4. CHIP analysis of BRG1 and BRM binding on the NANOG promoter along with CHART PCR. MSC with silenced or overexpressed BRG1 was used for CHIP analysis. Panel A shows BRG1 binding on the E2F site in control cultures and in cells with silenced and overexpressed BRG1. The numbers on the X-axis refer to nucleotide positions of the identified binding sites. Panel B shows the BRM binding on the NANOG promoter in the same experimental conditions reported in A. Real-time PCR data are expressed as arbitrary units. Three biological replicates were used for each experimental condition, * P ≤ 0.05. Y axis represent cell total DNA bound/input sample according the percent input procedure described in methods. Panel C shows the CHART-PCR analysis to identify heterochromatic regions in the NANOG promoter. This assay evaluates chromatin accessibility by real-time PCR. The more the chromatin is compact, the less it is amenable to digestion by the micrococcal nuclease (MNase). Undigested DNA will provide more copies of target sequences for PCR amplification. We amplified 2 regions (Proximal and Distal) of the NANOG promoter following treatment of DNA with MNase. The upper graph shows results in cells with silenced BRG1. In these cells, the level of amplified DNA, representing heterochromatic DNA, was compared with the control MSC cultures. Changes in the heterochromatic DNA level are expressed as fold changes. The lower graph shows the same analysis in cells with overexpressed BRG1. Three biological replicates were used for each experimental condition, * P ≤ 0.05.
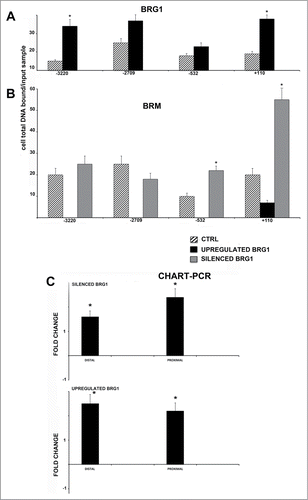
An increased or decreased presence of BRG1 on the NANOG promoter may alter nucleosome structure and induce changes in chromatin structure, either allowing or impairing access to transcription factors and related proteins. We measured chromatin remodeling by the CHART-PCR assay, which determines the accessibility of selected DNA regions to digestion with a micrococcal nuclease. An increase in accessibility is associated with an open-chromatin status; opposingly, the decrease is related with a compaction of chromatin. Both in cells with up-regulated and silenced BRG1, we observed a significant compaction of the NANOG promoter regions that encompass E2F binding sites, as compared with control cells ().
Quantitative proteomic analysis of nuclear proteins in senescent MSC with de-regulated BRG1 expression
In the experiments above-reported, we demonstrated that the de-regulated BRG1 expression induced the silencing of the NANOG gene through sharp modifications of the nucleosome status on gene regulatory elements. This result could be in line with previous findings, evidencing that BRG1 may be involved in tuning a few genes.Citation6 By way of contrast, other studies suggest that BRG1 may act as a global regulator of gene expression.Citation3
In order to evaluate if BRG1 de-regulation, besides fine-tuning gene expression, may also have a wide impact on chromatin, which in turn may globally affect gene transcription, we performed a comprehensive comparative nuclear proteome analysis in MSC, with silenced or upregulated BRG1, by exploiting an iTRAQ labeling-based strategy (File S6). To reduce the dynamic range of the samples and increase the probability of identifying low-abundance proteins, proteomic analysis was performed on enriched nuclear fractions.
To assess the enrichment of the nuclear fraction, western blots were performed with anti-GAPDH as a cytoplasmic marker, and anti-H1.2 as a nuclear marker (File S10). Up-regulated (upBRG1) and silenced (siBRG1) BRG1 samples and their respective controls were then digested with trypsin and labeled with the iTRAQ reagents. The resulting labeled peptide mixtures were then combined and fractionated according to their pI by using OFFGEL electrophoresis. Each of the 12 fractions per group was analyzed by high-resolution LC-MS/MS. Overall, 3577 unique peptides were assigned to 704 proteins by the EasyProt algorithm (File S6). Isobaric quantification was further performed allowing the quantification of 371 (upregulated BRG1) and 410 (silenced BRG1) non-redundant proteins with a minimum of 2 peptides per protein and a confidence threshold of 95% (P < 0.05). In particular, 106 proteins showed a significant increased (≥1.5) or decreased (≤0.6) ratio and were considered differentially expressed in upBRG1 vs. control (File S7). Furthermore, 282 proteins were found differentially expressed in siBRG1 v. control (File S8). In particular, 40 and 144 proteins were found to be down-regulated in upBRG1 and siBRG1, respectively, while 66 and 138 proteins were upregulated in upBRG1 and siBRG1, respectively.
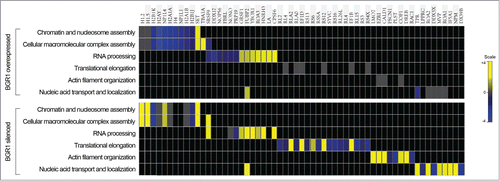
To investigate the functional annotation of proteins quantified in upBRG1 and siBRG1, a gene-ontology-enrichment analysis was performed by using the on-line DAVID software. The clusterization according to biological processes revealed that most of the identified proteins were involved in a nucleosome, chromatin and cellular macromolecular complex assembly, RNA processing, translational elongation and nucleic acid transport and localization (). Several histone variants, core components of nucleosomes–which are known to play a central role in transcription regulation, DNA repair, DNA replication and chromosomal stability were decreased in upregulated BRG1, including the strong-down-regulation of the core histone macro-H2A.1. This variant histone replaces conventional H2A in a subset of nucleosomes resulting in transcription repression by preventing the binding of transcription factors and by interfering with the activity of remodeling SWI/SNF complexes.Citation34 In addition, a significant upregulation of histone H1.2 and H1.5 was observed in silenced BRG1. Therefore, histone variants H1.2 and core histone macro-H2A.1, presenting the major changes in their expression levels, were further validated by Western blot analysis that confirmed our proteomic results (Fig. S10).
Figure 5. Heat map view representing the functional classification of differentially expressed proteins in upregulated and silenced BRG1, identified by LC-MS/MS analysis. The assessment of enriched biological processes for up- (yellow) and down- (blue) regulated proteins was performed according to gene ontology annotations by using the DAVID software.
Discussion
Stem cells have a peculiar chromatin architecture that contributes to unique properties, including uncommitted status, (multi)pluripotency and their potential to self-renew.
The chromatin of stem cells has numerous hallmarks of highly active chromatin (euchromatin), including an altered higher-order structure, an accumulation of activating histone modifications, an abundance of chromatin-remodeling factors, a reduction of DNA methylation and a hyperdynamic interaction with chromatin proteins.
Chromatin remodeling factors can modify the balance between euchromatin and heterochromatin by acting as main regulators of gene expression in stem cells, and even subtle alterations in their functions may greatly affect stem-cell identity.
The SWI/SNF chromatin remodeling complexes include several members that share most of the same subunits, such as the ATPase enzyme BRG1. These complexes can either promote or impair transcription, and their activity must be tightly regulated, since unscheduled changes in its expression may have a profound negative impact on cell functions.
We analyzed the effect of the de-regulation of the SWI/SNF chromatin remodeling complex in MSC through silencing and the up-regulation of BRG1. The altered expression of the BRG1-promoted senescence of MSC with the complete suppression of NANOG transcription is part of the core transcriptional circuitry governing stem-cell functions, as well as through its anti-senescence activity.Citation18,22,23 Our data cannot determine if NANOG silencing is a pre-requisite for senescence or is a consequence.
Some clues may be derived from the studies of Han and colleagues, who evidenced that in late passage, MSC NANOG ectopic expression significantly increases the proliferation and decreases the percentage of senescent cells.Citation22 In this scenario, our result further reinforces the hypothesis that NANOG, among the several “stemness” genes, may play a key role in counteracting MSC senescence. Indeed, in our previous findings, in senescent MSC, we observed a residual expression of several genes involved in the regulation of stem-cell properties, such as OCT3, KLF4, and BMI1, while NANOG was completely silenced.Citation8,10,35
The suppression of the NANOG transcription in both conditions (silencing and the overexpression of BRG1) suggested that the regulation of this gene relies upon a careful control of BRG1 activity. This prompted us to analyze the NANOG promoter in order to dissect the way BRG1 can accomplish its task. For the first time, we identified 4 putative E2F binding sites on the NANOG promoter, which, through the recruitment of RB/E2F repressive complexes, may block transcription. The E2F binding sites are occupied both by RB1 and RB2/P130 in cells with silenced or overexpressed BRG1. Their relative binding is, however, different: RB1 binding significantly increased in cells with silenced BRG1 and dropped following BRG1s forced expression. RB2/P130 binding was augmented on 2 of the 4 E2F sites in MSC with silenced BRG1 and remained substantially unchanged in cells with BRG1 overexpression.
In MSC with silenced BRG1, the relative binding of the 2 retinoblastoma proteins was associated with the recruitment of DNMT1 and HDAC1. This induced the methylation of CpG dinucleotides and histone deacetylation on the NANOG promoter. Opposingly, in the presence of a high level of BRG1 (overexpression), the same E2F binding motifs are docking sites for BRG1 recruitment, which induced chromatin compaction without the contribution of DNMT1 and HDAC1.
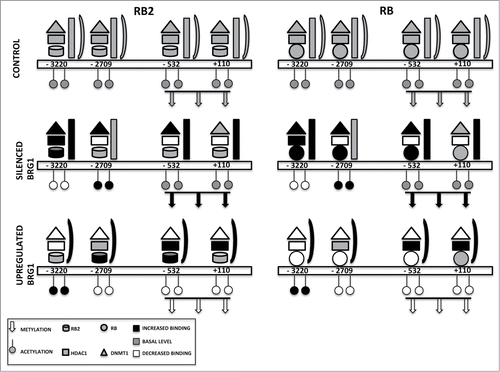
Our results allowed us to speculate that RB1 may be mainly involved in recruiting DNMT1 and HDAC1 on E2F sites, while RB2/P130 could contribute to engaging BRG1 on the NANOG promoter. The data we presented may be recapitulated in the model shown in , showing that the inhibition of NANOG transcription may occur through different pathways, depending on the level of BRG1. In addition, an untargeted analysis of the enriched nuclear fraction allowed the identification of candidate proteins that potentially correlate with BRG1 de-regulation, several of which are nuclear proteins involved in RNA processing, translational elongation, macromolecular complex assembly and nucleosome and chromatin assembly.
Figure 6. Binding on the NANOG promoter of retinoblastoma proteins and associated factors. The picture depicts the occupancy of 4 E2F binding sites on the NANOG promoter in a basal condition (CONTROL) and in cells with silenced or overexpressed BRG1. For clarity, RB2 binding is reported on the left with the RB1 binding on the right, but the 2 proteins may be present at the same time on the NANOG promoter. According to our model, in cells with silenced BRG1, the inhibition of NANOG transcription may occur mainly through recruitment of DNMT1 through binding with RB1 and/or RB2. This may promote the methylation of CpG dinucleotides in the NANOG promoter. In these cells, the lack of BRG1 is compensated by increased BRM binding. In cells overexpressing BRG1, the DNMT1 and RB1 binding on the NANOG promoter is drastically reduced, while the presence of HDAC1 and RB2 persists on E2F sites. In this condition, the inhibition of NANOG transcription may occur mainly through histone deacetylation.
The major mechanisms modulating chromatin structure involve ATP-dependent nucleosome remodeling complexes, the covalent modification of histones, histone chaperones and histone-variant exchange.Citation36,37 Indeed, the deposition and replacement of histones are important to maintain and alter the overall chromatin structure.Citation36,37 Accordingly, the de-regulation of the BRG1 protein level greatly affects the expression of chromatin proteins, including histones and non-histone proteins. These findings suggest that BRG1 de-regulation may affect nucleosome architecture by regulating the so-called histone code at both the translational and post-translational levels. In line with this evidence, several histone chaperones were also differentially expressed following BRG1 de-regulation. Among them, the histone chaperone FACT (facilitates chromatin transcription) was downregulated in upBRG1. In has been reported that FACT is a chromatin-structure modulator involved in transcription and DNA replication, having the ability to bind histones, DNA, and intact nucleosomes.Citation38,39
The nucleosome-binding activity of the FACT complex is regulated by by poly(ADP-ribose) polymerase-1 (PARP-1),Citation40 also found down-regulated in upBRG1, suggesting regulatory roles for these proteins in chromatin remodeling. Furthermore, the multitasking protein SET, endowed with pro- and anti-apoptotic function, transcription, nucleosome assembly and histone chaperoning,Citation41 was up-regulated in both upBRG1 and siBRG1.
Besides proteins involved in nucleosome and chromatin assembly, several lamins–components of nuclear lamina–as well as members of the nuclear lamina-associated protein family were overexpressed following BRG1 silencing. Among them, Lamin-B1, Lamin-B2, Prelamin-A/C and Emerin are involved in the organization of the inner-nuclear membrane, providing a framework for the nuclear envelope as well as interactions with chromatin.Citation42,43 In particular, Lamin A and C plays an important role in nuclear assembly, chromatin organization, and nuclear membrane and telomere dynamics.Citation44 Interestingly, it has been reported that Prelamin-A/C can accelerate cell senescence by inducing DNA damage in vascular smooth-muscle cells, leading to mitotic failure, genomic instability, and premature senescence.Citation45
Overall, these findings suggest that BRG1, besides acting on proximal gene regulatory elements, may function as a global regulator of gene expression through the regulation of the expression of nuclear proteins involved in “nuclear architecture.” This scenario could explain why even subtle alterations in BRG1 expression may disrupt the normal functioning of MSC and trigger senescence.
Material and Methods
MSC cultures
Human bone marrows were obtained from healthy donors after informed consent. We separated cells on the Ficoll density gradient (GE Healthcare, Italy), and the mononuclear cell fraction was collected and washed in PBS. We seeded 1–2.5 × 105 cells/cm2 in α-MEM containing 10% FBS and bFGF. After 72 hours, non-adherent cells were discarded, and adherent cells were further cultivated to confluency. On these cells (passage 0), we carried out silencing and overexpression experiments, as we already reported.Citation8,10
Chromatin immunoprecipitation (CHIP) assay
We used the Simple CHIP Enzymatic Chromatin IP kit (Cell Signaling Technology, MA, USA) and followed the manufacturer's instructions. In brief, to crosslink proteins to DNA, we fixed cells 0.068% ethylene glycolbis [succinimidyl succinate] (EGS) for 20 minutes and then with 1% formaldehyde in PBS.Citation46 We then treated samples with 125 mM glycine and incubated them in a buffer containing DTT and protease inhibitors. This step was followed by micrococcal nuclease digestion and sonication. The lysates were centrifuged, and supernatants were collected in clean tubes. Five μg of antibodies directed against the proteins of interest or normal IgG was added to the supernatants that were incubated overnight at 4°C on a rotating device. Following antibody incubation, protein G Agarose beads were added to samples and further incubated for 2 h at 4°C. DNA samples were then eluted from the antibody/protein G beads by incubation in a specific elution buffer for 30 minutes at 65°C and transferred to new tubes. Samples were further treated with proteinase K and purified on spin columns. The obtained immunoprecipitated DNA samples were used for Real-Time PCR amplification.
The sequence of human NANOG promoter and Primer Express software (Applied Biosystems, CA, USA) was used to design primer pairs for CHIP analysis (see File S4). PCR amplification was performed by using DNA Engine Opticon II real-time PCR (Bio-Rad, Hercules, CA, USA). Reactions were performed according to the manufacturer's instructions using ITaq Universal SYBR Green supermix (Bio-Rad, CA, USA); melting curves (65°C–94°C) were generated to determine whether there were any spurious amplification products. The real-time PCR efficiency was calculated for each primer pair using a dilution series and Bio-Rad analysis software. Quantitative IP efficiency was evaluated with the Percent Input Method according the following equation: Percent Input = 2% × 2CT(2%Input Sample) – CT(IP Sample).
We used the following antibodies for CHIP analysis: anti-Rb1 (clone 4H1, Cell Signaling Technology, MA, USA); Anti-Rb2 (Abcam, UK); anti-P53 (clone DO-1, Santacruz, CA, USA); anti-HDAC1 (clone 10E2, Abcam, UK); Anti-acetyl-H3 (clone C5B11, Cell Signaling, MA, USA); anti-Brm/Smarca2 (Abcam, UK); anti-Brg1 (clone H88, Santacruz, CA, USA); anti-DNMT1 (clone H300, Santacruz, CA, USA).
CHART-PCR
To evaluate the chromatin status of the NANOG promoter, we evaluated its accessibility with CHART-PCR.Citation47 In brief, we collected cells with scraping and incubated in ice for 10 minutes in a buffer containing DTT and protease inhibitors. This step was followed by micrococcal nuclease digestion for 5 min at 37°C. DNA was then extracted from pellets with a MasterPure DNA purification kit (Epicentre Biotechnologies, IL, USA) according to the manufacturer's instructions.
We used 2 primer pairs to amplify a proximal (−1073, −897) and distal (−2411, −2220) region of the NANOG promoter. The real-time PCR efficiency was calculated for each primer pair using a dilution series and Bio-Rad analysis software. Appropriate regions of the GAPDH gene of Lycopersicon esculentum were used as reference for quantitative evaluation (see File S5). Each real-time PCR reaction was repeated at least 3 times, and amplification profiles were determined according to the ΔΔCT method for relative quantification (BioRad Software; Bio-Rad, CA, USA).
MS-PCR
Methylation-specific PCR is a method for analysis of DNA methylation patterns. For performing MS-PCR, we modified DNA with the bisulfite method and then carried out PCR with 2 primer pairs, which detect methylated and unmethylated DNA, respectively. In brief, DNA was extracted with the MasterPure DNA purification kit (Epicentre Biotechnologies, IL, USA) according to the manufacturer's instructions. We performed bisulfite conversion and cleanup of DNA with the Epitect Bisulfite kit (Qiagen Italy, Italy). Bisulphite-modified DNA was used as a template for fluorescence-based, real-time, quantitative, Methylation-Specific PCR.Citation48 Primer pairs encompassing CpG regions of the NANOG promoter were selected with Methprimer softwareCitation49 (File S5). Quantitation of the methylation status was performed according published methods.Citation48
Western blots
Cells were lysed in a buffer containing 0.1% Triton for 30 minutes at 4°C. 40 μg of each lysate was electrophoresed in a polyacrylamide gel and electroblotted onto a nitrocellulose membrane. All the primary antibodies were used according to the manufacturers’ instructions. Immunoreactive signals were detected with a horseradish peroxidase–conjugated secondary antibody (Santacruz, CA, USA) and reacted with an ECL plus reagent (GE Healthcare, Italy).
Proteomic analysis by LC-MS/MS
Proteomic analysis was performed by applying an iTRAQ labeling strategy as previously described.Citation50
Bioinformatics analyses
An assessment of significantly enriched biological processes was performed according to gene ontology annotations by using the DAVID software v6.7 (Database for Annotation, Visualization and Integrated Discovery) with the application of the Fisher exact test (EASE Score Threshold 0.001).Citation51,52 The heat map representing the functional classification of up- and downregulated proteins was constructed by clustering significant functional terms according to the DAVID enriched biological processes by using the TIBCO Spotfire software (Somerville, MA, USA) (see File S9 for detailed description of methods).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
995053_Supplementary_Materials.zip
Download Zip (9.5 MB)Supplemental Material
Supplemental data for this article can be accessed on the publisher's website
References
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007; 8:729-40; PMID:17667954; http://dx.doi.org/10.1038/nrm2233
- Martens JA, Winston F. Recent advances in understanding chromatin remodeling by Swi/Snf complexes. Curr Opin Genet Dev 2003; 13:136-42; PMID:12672490; http://dx.doi.org/10.1016/S0959-437X(03)00022-4
- Trotter KW, Archer TK. The BRG1 transcriptional coregulator. Nucl Recept Signal 2008; 6:e004; PMID:18301784
- Saha A, Wittmeyer J, Cairns BR. Chromatin remodelling: the industrial revolution of DNA around histones. Nat Rev Mol Cell Biol 2006; 7:437-47; PMID:16723979; http://dx.doi.org/10.1038/nrm1945
- de la Serna IL, Ohkawa Y, Imbalzano AN. Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat Rev Genet 2006; 7:461-73; PMID:16708073; http://dx.doi.org/10.1038/nrg1882
- Hendricks KB, Shanahan F, Lees E. Role for BRG1 in cell cycle control and tumor suppression. Mol Cell Biol 2004; 24:362-76; PMID:14673169; http://dx.doi.org/10.1128/MCB.24.1.362-376.2004
- Takeuchi JK, Lou X, Alexander JM, Sugizaki H, Delgado-Olguin P, Holloway AK, Mori AD, Wylie JN, Munson C, Zhu Y, et al. Chromatin remodelling complex dosage modulates transcription factor function in heart development. Nat Commun 2011; 2:187; PMID:21304516; http://dx.doi.org/10.1038/ncomms1187
- Alessio N, Squillaro T, Cipollaro M, Bagella L, Giordano A, Galderisi U. The BRG1 ATPase of chromatin remodeling complexes is involved in modulation of mesenchymal stem cell senescence through RB-P53 pathways. Oncogene 2010; 29:5452-63; PMID:20697355; http://dx.doi.org/10.1038/onc.2010.285
- Jiang N, Du G, Tobias E, Wood JG, Whitaker R, Neretti N, Helfand SL. Dietary and genetic effects on age-related loss of gene silencing reveal epigenetic plasticity of chromatin repression during aging. Aging 2013; 5:813-24; PMID:24243774
- Napolitano MA, Cipollaro M, Cascino A, Melone MA, Giordano A, Galderisi U. Brg1 chromatin remodeling factor is involved in cell growth arrest, apoptosis and senescence of rat mesenchymal stem cells. J Cell Sci 2007; 120:2904-11; PMID:17666433; http://dx.doi.org/10.1242/jcs.004002
- Schellenberg A, Lin Q, Schuler H, Koch CM, Joussen S, Denecke B, Walenda G, Pallua N, Suschek CV, Zenke M, et al. Replicative senescence of mesenchymal stem cells causes DNA-methylation changes which correlate with repressive histone marks. Aging 2011; 3:873-88; PMID:22025769
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006; 98:1076-84; PMID:16619257; http://dx.doi.org/10.1002/jcb.20886
- Galderisi U, Giordano A. The gap between the physiological and therapeutic roles of mesenchymal stem cells. Med Res Rev 2014; 34:1100-26; PMID:24866817; http://dx.doi.org/10.1002/med.21322
- Jiang SS, Chen CH, Tseng KY, Tsai FY, Wang MJ, Chang IS, Lin JL, Lin S. Gene expression profiling suggests a pathological role of human bone marrow-derived mesenchymal stem cells in aging-related skeletal diseases. Aging 2011; 3:672-84; PMID:21808097
- Wagner W, Bork S, Lepperdinger G, Joussen S, Ma N, Strunk D, Koch C. How to track cellular aging of mesenchymal stromal cells? Aging 2010; 2:224-30; PMID:20453259
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005; 122:947-56; PMID:16153702; http://dx.doi.org/10.1016/j.cell.2005.08.020
- Theunissen TW, Silva JC. Switching on pluripotency: a perspective on the biological requirement of Nanog. Philos Trans R Soc Lond B Biol Sci 2011; 366:2222-9; PMID:21727127; http://dx.doi.org/10.1098/rstb.2011.0003
- Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Ait-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, et al. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev 2011; 25:2248-53; PMID:22056670; http://dx.doi.org/10.1101/gad.173922.111
- Katsara O, Mahaira LG, Iliopoulou EG, Moustaki A, Antsaklis A, Loutradis D, Stefanidis K, Baxevanis CN, Papamichail M, Perez SA. Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev 2011; 20:1549-61; PMID:21204633; http://dx.doi.org/10.1089/scd.2010.0280
- Mimura S, Kimura N, Hirata M, Tateyama D, Hayashida M, Umezawa A, Kohara A, Nikawa H, Okamoto T, Furue MK. Growth factor-defined culture medium for human mesenchymal stem cells. Int J Dev Biol 2011; 55:181-7; PMID:21305471; http://dx.doi.org/10.1387/ijdb.103232sm
- Squillaro T, Alessio N, Cipollaro M, Renieri A, Giordano A, Galderisi U. Partial silencing of methyl cytosine protein binding 2 (MECP2) in mesenchymal stem cells induces senescence with an increase in damaged DNA. FASEB J 2010; 24:1593-603; PMID:20065105; http://dx.doi.org/10.1096/fj.09-143057
- Han J, Mistriotis P, Lei P, Wang D, Liu S, Andreadis ST. Nanog reverses the effects of organismal aging on mesenchymal stem cell proliferation and myogenic differentiation potential. Stem Cells 2012; 30:2746-59; PMID:22949105; http://dx.doi.org/10.1002/stem.1223
- Huna A, Salmina K, Jascenko E, Duburs G, Inashkina I, Erenpreisa J. Self-Renewal Signalling in Presenescent Tetraploid IMR90 Cells. J Aging Res 2011; 2011:103253; PMID:21629737; http://dx.doi.org/10.4061/2011/103253
- Prestridge DS. Predicting Pol II promoter sequences using transcription factor binding sites. J Mol Biol 1995; 249:923-32; PMID:7791218; http://dx.doi.org/10.1006/jmbi.1995.0349
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 2005; 7:165-71; PMID:15619621; http://dx.doi.org/10.1038/ncb1211
- O'Connor MD, Wederell E, Robertson G, Delaney A, Morozova O, Poon SS, Yap D, Fee J, Zhao Y, McDonald H, et al. Retinoblastoma-binding proteins 4 and 9 are important for human pluripotent stem cell maintenance. Exp Hematol 2011; 39:866-79.e1; PMID:21689726; http://dx.doi.org/10.1016/j.exphem.2011.05.008
- Alessio N, Bohn W, Rauchberger V, Rizzolio F, Cipollaro M, Rosemann M, Irmler M, Beckers J, Giordano A, Galderisi U. Silencing of RB1 but not of RB2/P130 induces cellular senescence and impairs the differentiation potential of human mesenchymal stem cells. Cell Mol Life Sci 2013; 70:1637-51; PMID:23370776; http://dx.doi.org/10.1007/s00018-012-1224-x
- Galderisi U, Cipollaro M, Giordano A. The retinoblastoma gene is involved in multiple aspects of stem cell biology. Oncogene 2006; 25:5250-6; PMID:16936744; http://dx.doi.org/10.1038/sj.onc.1209736
- Jori FP, Melone MA, Napolitano MA, Cipollaro M, Cascino A, Giordano A, Galderisi U. RB and RB2/p130 genes demonstrate both specific and overlapping functions during the early steps of in vitro neural differentiation of marrow stromal stem cells. Cell Death Differ 2005; 12:65-77; PMID:15459751; http://dx.doi.org/10.1038/sj.cdd.4401499
- Pradhan S, Kim GD. The retinoblastoma gene product interacts with maintenance human DNA (cytosine-5) methyltransferase and modulates its activity. EMBO J 2002; 21:779-88; PMID:11847125; http://dx.doi.org/10.1093/emboj/21.4.779
- Zhang HS, Gavin M, Dahiya A, Postigo AA, Ma D, Luo RX, Harbour JW, Dean DC. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell 2000; 101:79-89; PMID:10778858; http://dx.doi.org/10.1016/S0092-8674(00)80625-X
- Svedruzic ZM. Dnmt1 structure and function. Prog Mol Biol Transl Sci 2011; 101:221-54; PMID:21507353; http://dx.doi.org/10.1016/B978-0-12-387685-0.00006-8
- Nettersheim D, Biermann K, Gillis AJ, Steger K, Looijenga LH, Schorle H. NANOG promoter methylation and expression correlation during normal and malignant human germ cell development. Epigenetics 2011; 6:114-22; PMID:20930529; http://dx.doi.org/10.4161/epi.6.1.13433
- Angelov D, Molla A, Perche PY, Hans F, Cote J, Khochbin S, Bouvet P, Dimitrov S. The histone variant macroH2A interferes with transcription factor binding and SWI/SNF nucleosome remodeling. Mol Cell 2003; 11:1033-41; PMID:12718888; http://dx.doi.org/10.1016/S1097-2765(03)00100-X
- Galderisi U, Helmbold H, Squillaro T, Alessio N, Komm N, Khadang B, Cipollaro M, Bohn W, Giordano A. In vitro senescence of rat mesenchymal stem cells is accompanied by downregulation of stemness-related and DNA damage repair genes. Stem Cells Dev 2009; 18:1033-42; PMID:19099372; http://dx.doi.org/10.1089/scd.2008.0324
- Sarma K, Reinberg D. Histone variants meet their match. Nat Rev Mol Cell Biol 2005; 6:139-49; PMID:15688000; http://dx.doi.org/10.1038/nrm1567
- Kamakaka RT, Biggins S. Histone variants: deviants? Genes Dev 2005; 19:295-310; PMID:15687254; http://dx.doi.org/10.1101/gad.1272805
- Belotserkovskaya R, Oh S, Bondarenko VA, Orphanides G, Studitsky VM, Reinberg D. FACT facilitates transcription-dependent nucleosome alteration. Science 2003; 301:1090-3; PMID:12934006; http://dx.doi.org/10.1126/science.1085703
- Winkler DD, Muthurajan UM, Hieb AR, Luger K. Histone chaperone FACT coordinates nucleosome interaction through multiple synergistic binding events. J Biol Chem 2011; 286:41883-92; PMID:21969370; http://dx.doi.org/10.1074/jbc.M111.301465
- Huang JY, Chen WH, Chang YL, Wang HT, Chuang WT, Lee SC. Modulation of nucleosome-binding activity of FACT by poly(ADP-ribosyl)ation. Nucleic Acids Res 2006; 34:2398-407; PMID:16682447; http://dx.doi.org/10.1093/nar/gkl241
- Chakravarti D, Hong R. SET-ting the stage for life and death. Cell 2003; 112:589-91; PMID:12628178; http://dx.doi.org/10.1016/S0092-8674(03)00151-X
- Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol 2005; 6:21-31; PMID:15688064; http://dx.doi.org/10.1038/nrm1550
- Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 2008; 22:832-53; PMID:18381888; http://dx.doi.org/10.1101/gad.1652708
- De Vos WH, Houben F, Hoebe RA, Hennekam R, van Engelen B, Manders EM, Ramaekers FC, Broers JL, Van Oostveldt P. Increased plasticity of the nuclear envelope and hypermobility of telomeres due to the loss of A-type lamins. Biochim Biophys Acta 2010; 1800:448-58; PMID:20079404; http://dx.doi.org/10.1016/j.bbagen.2010.01.002
- Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, Shroff R, Skepper J, Shanahan CM. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation 2010; 121:2200-10; PMID:20458013; http://dx.doi.org/10.1161/CIRCULATIONAHA.109.902056
- Zeng PY, Vakoc CR, Chen ZC, Blobel GA, Berger SL. In vivo dual cross-linking for identification of indirect DNA-associated proteins by chromatin immunoprecipitation. BioTechniques 2006; 41:694, 6, 8; PMID:17191611; http://dx.doi.org/10.2144/000112297
- Cruickshank M, Fenwick E, Abraham LJ, Ulgiati D. Quantitative differences in chromatin accessibility across regulatory regions can be directly compared in distinct cell-types. Biochem Biophys Res Commun 2008; 367:349-55; PMID:18164259; http://dx.doi.org/10.1016/j.bbrc.2007.12.121
- Parrella P, la Torre A, Copetti M, Valori VM, Barbano R, Notarangelo A, Bisceglia M, Gallo AP, Balsamo T, Poeta ML, et al. High specificity of quantitative methylation-specific PCR analysis for MGMT promoter hypermethylation detection in gliomas. J Biomed Biotechnol 2009; 2009:531692; PMID:19503806; http://dx.doi.org/10.1155/2009/531692
- Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics 2002; 18:1427-31; PMID:12424112; http://dx.doi.org/10.1093/bioinformatics/18.11.1427
- Severino V, Malorni L, Cicatiello AE, D'Esposito V, Longobardi S, Colacurci N, Miraglia N, Sannolo N, Farina A, Chambery A. An integrated approach based on multiplexed protein array and iTRAQ labeling for in-depth identification of pathways associated to IVF outcome. PLoS One 2013; 8:e77303; PMID:24146976; http://dx.doi.org/10.1371/journal.pone.0077303
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4:44-57; PMID:19131956; http://dx.doi.org/10.1038/nprot.2008.211
- Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 37:1-13; PMID:19033363; http://dx.doi.org/10.1093/nar/gkn923
