Abstract
Autophagy is important for cell renewing for its contribution to the degradation of bulk cytoplasm, long-lived proteins, and entire organelles and its role in embryonic development is largely unknown. In our study, we investigated the function of autophagy in gastrulation of the chick embryo using both in vivo and in vitro approaches, especially in the EMT process, and we found that autophagy gene Atg7 was expressed on the apical side of the ectoderm and endoderm. Over-expression of Atg7 could enhance the expression of Atg8 and the E-cadherin, the latter of which is a crucial marker of the EMT process. We also found that the disturbance of autophagy could retard the development of chick embryos in HH4 with shorter primitive steak than that in the control group, which is a newly formed structure during EMT process. So we assumed that autophagy could affect EMT process by adhesion molecule expression. Moreover, more molecules, such as slug, chordin, shh et., which were all involved in EMT process, were detected to address the mechanism of this phenomena. We established that the inhibition of autophagy could cause developmental delay by affecting EMT process in gastrulation of chick embryos.
Abbreviations
| 3-MA | = | 3-Methyladenine |
| BF | = | bright-field |
| DAPI | = | 49-6-Diamidino-2-phenylindole |
| EB | = | embryoid bodies |
| E-Cad | = | E-cadherin |
| EMTs | = | epithelial-mesenchymal transitions |
| GFP | = | green fluorescent protein |
| HN | = | Hensen's node |
| MAPILC3(LC3) | = | microtubule-associated protein 1 light chain 3 |
| mTOR | = | mammalian target of rapamycin |
| N-Cad | = | N-cadherin |
| NT | = | neural tube |
| PBS | = | phosphate-buffered saline |
| PCD | = | Programmed cell death |
| PD | = | idiopathic Parkinson's Disease |
| PI3K | = | phosphoinositide-3-kinase |
| PPIA | = | peptidylprolyl isomerase A |
| PS | = | primitive streak |
| RAPA | = | Rapamycin |
| RT-PCR | = | reverse transcription PCR |
| shh | = | sonic hedgehog |
Introduction
Autophagy is a complex biochemical process, in which eukaryotic cells acquires nutrition and make themselves survive during nutrient stress by means of cellular autolysis and self-clearance. It is regulated by a series of Atg proteins including Atg7, an essential gene for autophagy in yeast. At present, lots of researches focus on the functions of Atg7 in autophagy either on the cellular or molecular level or oxidation stress under the starvation.Citation1,2 For instance, Atg7 was reported to have an unique pro-apoptotic function in response to lysosome dysfunction, and it is also able to regulates p53-dependent cell cycle and cell death pathways when nutrients are limited.Citation1,2 On the other hand, dysfunctional autophagy via Atg7 conditional knock-out is considered as one of the failing cellular mechanisms involved in the pathogenesis of idiopathic Parkinson's Disease (PD).Citation3 So far, numerous evidences about the autophagy function have mainly concentrated on nervous system, such as, the differentiation of neuroepithelial cells or the neurodegeneration and behavioral deficits by Atg7 conditional knockout in mouse neurons.Citation4,5 However, autophagy functions on other types of cells remain controversial and much less was known about the function of Atg7 on the development of embryos. It was reported that mice with targeted mutations Atg7 died within 1 day after birth, and the weight of the Atg7 KO mice was significantly lower than that of normal mice.Citation6 And there are many literatures about the effect of Atg7 on organogenesis like tooth morphogenesis.Citation7 Concerning about the possible functions and the mechanism of Atg7 in gastrulation of the embryo development, almost no research has been done so far.
Gastrulation is a crucial time for the establishment of 3 primary germ layers and the construction of rudimentary primary body axes. During gastrulation, cells move to new positions to interact with their new adjacent cells, so that neurulation and organogenesis will follow accordingly. As a vital events in this process, epithelial-mesenchymal transitions (EMTs) occur when mesoderm and hypoblast cells delaminate from epiblast in many animal species, especially on key phases of the development of higher animals.Citation8,9 In other word, EMT is a process that epithelial cells undergo a morphological switch from a polarized epithelium to a highly invasive fibroblastic or mesenchymal phenotype, which requires the coordination of multiple cellular events, including the loss of apical-basal polarity, disruption of adherens junctions, tight junctions, desmosomes and cytokeratin intermediate filaments, breakdown of cell–BM interaction and the alteration in cytoskeletal architecture through the modulation of related molecules.Citation10-12
Autophagy has been proved to be involved in embryogenesis, insect metamorphosis, glandular atresia, lumen formation and so on via its role on massive cell elimination in all these issues.Citation13 Beclin1 and Atg5, the 2 pro-autophagy genes, were confirmed to be indispensable for the clearance of dead cells during the cavitation process, which is also one of the vital phases in gastrulation, using in vitro model of aggregates of inner-cell-mass-derived embryonic stem cells (embryoid bodies, EB).Citation14,15 In the neurogenesis of embryo, it was demonstrated that Atg7 and Atg5 genes were necessary for motor function.Citation16,17 Likewise, a large amount of cells die in some special regions during gastrulation of the chick embryo, especially in the rostral germinal crescent and the lateral marginal zones in the epiblast. Afterwards, they formed a rostral-lateral arc in the epiblast, which remained the same from gastrulation to the early neurulation stage. Another region that cell death occurs frequently is the primitive streak, it is probably due to the recurrent alteration of cell-cell and cell-matrix interaction in the primitive streak.Citation18 Moreover, autophagy prominent is considered as the type II Programmed cell death (PCD) in various pathways for activating self-destruction and it is reflected by different morphologies.Citation19
Autophagy is regarded as a crucial mediator in tumor invasion, in which EMT also plays a key role. There have been some literatures to investigate the relationship between autophagy and EMTs using tumor cell lines. Recent studies showed that DEDD, which can bind PI3KC3 to activate autophagy, can attenuate EMT process.Citation20 Whereas breast cancer cells shows EMT phenotype along with the inducing of autophagy to resist cytotoxic T lymphocyte.Citation21 As a consequence, we have reasons to assume that autophagy does not only occur but also function in the period of embryonic gastrulation to some extent.
In order to investigate the role of autophagy or/and Atg7 in EMT process of embryonic gastrulation, an early chick embryo model was employed since it was able to offer us a typical in vivo model of EMT either in gastrulation or/and early stage of neurulation. Rapamycin (RAPA) is a well-established inducer of autophagy, since autophagy is negatively regulated by mTOR, whose activity can be inhibited by RAPA.Citation22-24 3-Methyladenine (3-MA) is a well-known specific inhibitor of autophagy, since it has been approved to inhibit endogenous protein degradation in isolated rat hepatocytes by about 60% without adverse effects on the degradation of an exogenous protein (asialofetuin), on protein synthesis, or on intracellular ATP levels. By targeting the class III PI3K, 3-Methyladenine has an impact on autophagosome formation, specifically upon the autophagic/lysosomal pathway of degradation.Citation25-27 In this study, we revealed that disturbance of autophagy by chemical autophagy inducer or inhibitor did interfere with the normal EMT process in chick embryo, resulting in a disorder germ layers compared to the normal EMT in control embryos. Next, we also determined the crucial gene expressions that played vital roles in the EMT modulation of normal chick embryo development, attempting to explore the correlation between EMT and autophagy in avian gastrula embryo.
Results
Atg7 mediated-autophagy promoted the expression of E-cadherin on the epiblast cells of gastrula embryos
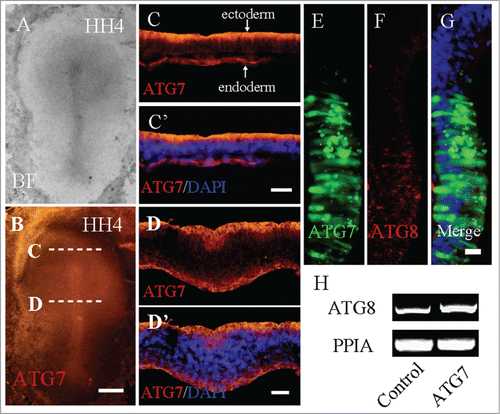
We would like to know whether autophagy is involved in the development of avian gastrula. Though Atg8 is considered as a biomarker of autophagy in situ, it is unfortunately undetectable unless Atg8 is overexpressed.Citation28 Since endogenous in situ expression of Atg8 protein is hard to be detected, we detected the in situ protein expression of another autophagy associated gene – Atg7 in HH4 embryos. From the view of whole-mount HH4 embryos, Atg7 is relatively strongly expressed in neural plate (). Consequently, we made sections at the anterior and middle of the primitive streak since it was across the neural plate region. In those transverse sections, we can clearly observe that Atg7 was expressed distinctly in the apical side of epiblast (ectoderm) and endoderm in both levels of sections (). In order to detect whether Atg7 could regulate the expression of Atg8, we detected the expression of Atg8 after co-electroporating Atg7 and pEGFP-N1 to the neural tube. The Atg8 expression was activated at the corresponding region (, red arrow), compared with the non-transfected region (, white arrow). Furthermore, ATG8 expression was confirmed by RT-PCR analysis after the transfection of ATG7 in the embryos of primitive streak stage. It indicated that Atg7-mediated autophagy did appear in the gastrulation of early embryonic development, and it might suggest that Atg7-mediated autophagy is involved in the cell polarity due to the localization of Atg7 expression. Both of ectoderm and endoderm are highly epithelium-like cells. Therefore, we determined if the promotion of autophagy could affect the adhension molecules in ectoderm.
Figure 1. Autophagy exists in HH4 chick embryo. A-D: Immunofluorescent staining was performed on whole-mount HH4 chick embryo to detect the expression of Atg7. The bright-field images of HH4 chick embryo (A) and the immunofluorescent image of Atg7 expression in the HH4 chick embryo (B). Transverse sections were carried out at the anterior primitive streak (C and C′) and the middle primitive streak (D and D′) levels indicated by the white dotted lines in B. E-G: Atg8 immunofluorescent staining was performed after co-transfected pEGFP-N1 and Atg7. The transfected region was indicated by GFP fluorescence (E) and the Atg8 expression was activated at the corresponding region (F-G, red arrow) compared with the non-transfected region (F, white arrow). H: Primitive streaks were collected for RT-PCR analysis after transfected with Control-GFP, or Atg7 respectively. In the Atg7 transfected embryos, Atg8 expression was promoted compared with Control-GFP embryos (N = 3). Abbreviations: BF, bright-field. Scale bars = 600 μm in A-B, 100 μm in C′-C″, 50 μm in D′-D″, and 20 μm in E-G.
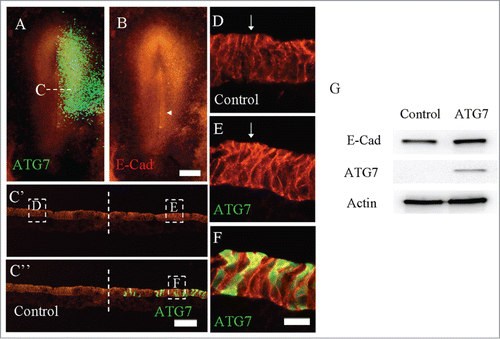
As a typical calcium-dependent adhesion molecule expressed in epithelium, E-Cadherin is the type-1 transmembrane proteins and plays a vital role in epithelial cells adhesion by holding epithelial tissues together. Likewise, E-Cadherin is also expressed strongly in epiblast layer of gastrula embryo. The expression of cadherin molecule switch, i.e., E-Cadherin down-regulation and N-Cadherin up-regulation, is regarded as an indispensable event for EMT in gastrulation.Citation29 In order to explore if Atg7 expression affects the EMT in chick gastrulation stage, we electroporated wild-type Atg7-GFP into half-side (right side) HH3 chick embryo while leaving another side (left right) as control one (). Using the electroporation method, we could guarantee that the gene transfection occurred in the epiblast (see ), which consists of the precursor cells prior to EMT. When cautiously comparing E-Cadherin expression between control and Atg7-GFP transfected side, we could find that E-Cadherin expression was slightly enhanced in right side of primitive streak of while embryo, especially besides the primitive streak as indicated by white arrow (), which could be more distinctly shown in the transverse sections. In , the expression of E-Cadherin on the side transfected with Atg7-GFP was stronger than that on control side (), implying that overexpression of Atg7 could result in enhancing E-Cadherin expression in epiblast, and as a result, EMT might be delayed in HH4 chick embryo.
Figure 2. Overexpression of Atg7 in epiblast enhanced the expression of E-Cadherin. Atg7 expression was determined using immunofluorescent staining following the electroporation of Atg7-GFP at half-side of HH4 chick embryo. A: The merge image of Atg7-GFP (green) and E-Cadherin immunofluorescent staining (red) in HH4 chick embryo. B: E-Cadherin immunofluorescent staining (red) in HH4 chick embryo. E-Cadherin expression was slightly promoted at the side of Atg7-GFP transfection as indicated by arrow. C′-C″: The transverse sections of transfected embryo at middle primitive streak level as indicated by the white dotted lines in A. C′ is E-Cadherin only; C″ is Atg7-GFP + E-Cadherin staining. D-E: High magnification images from the control side (D) and transfected side (E) as indicated by white line squares in C′ respectively. F: High magnification merge images from the transfected side as indicated by white line squares in C″. G: Western blot (WB) showing the expression of E-cadherin and Atg7 in HCT116 cells, which were transfected with either myc-Atg7 (Atg7-targeted) or myc-vector plasmids (control) respectively. Actin was used as a loading control. Scale bars = 500 μm in A-B and 80 μm in C′- C″ and 15 μm in D, E-E′.
We also could determine the E-Cad expression in protein level of HCT116 cells by Western blotting analysis, after the transfection of the myc-Atg7 plasmids or myc-vector plasmids as control, E-Cad expression was up-regulated in Atg7-transfected HCT116 cells compared to the control (). Thus, experimental results from both in vivo and in vitro indicate that over-expression of Atg7 could prompt the expression of E-Cad.
The disturbance of autophagy retarded the development of early gastrula embryos
Since the Atg7 overexpression could increase E-Cadherin expression in epiblast (), we assumed that the EMT process in gastrulation embryo might be influenced by autophagy. In order to verify our hypothesis, we employed RAPA, a well-known promoter for autophagy, in the culture medium to incubate early chick embryos. As is shown in , the embryos treated with RAPA showed obvious developmental delay (), compared to the normal groups (Fig. A-C). For example, at the 18th hour of incubation, the RAPA treated embryos were at HH3 stage () while the normal embryos were at HH4 stage with full length of primary streak (); at the 33rd hour of incubation, the normal group have reached to about HH9 (), whereas, the 3-MA-treated embryos still stuck on HH4 ().
Figure 3. The Disturbance of autophagy retards chick embryo development. The evaluation of chick embryo development was fulfilled following the treatment of either RAPA or 3-MA compared to control. A-I: The bright-field images of the developing embryos in control group (A-C), RAPA treated group (D-E) or 3-MA treated group (F-I) at 0 hour, 18 hour and 33 hour respectively. J: Bar chart showing the comparison of the length of primary streak in embryos at stage HH4 between the control, RAPA treated and 3-MA treated group. K: Bar chart showing the number of embryos in different stages at 33-hour incubation between the control group, RAPA treatment group and 3-MA treated group. L: Bar charts showing the pairs of somites at 33-hour incubation between the control group, 3-MA treated group and RAPA treated group. ***P < 0.001 indicating highly significant difference between RAPA-or 3-MA-treated and control embryos. Scale bars = 1000 μm in A,D,G, 700 μm in B,E,H, and 600 μm in C,F,I.
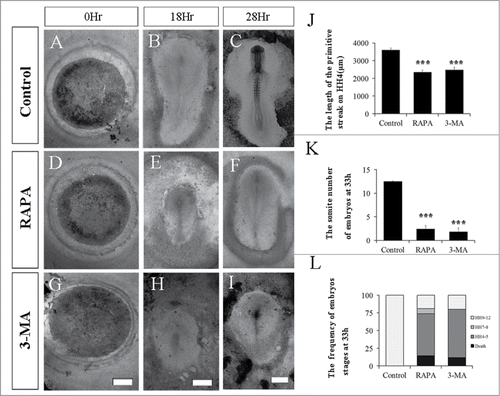
Then we employed 3-MA, a well-known inhibitor for autophagy, in the culture medium to incubate early chick embryos. Interestingly, the embryos treated with 3-MA also showed developmental delay () compared to the normal groups (). The statistics analysis was performed on the delay of embryo development with software SPASS, and demonstrated that there were significant differences between control and RAPA or 3-MA group on the length of the primary streak () at the 18th hour of incubation and the somite numbers of embryos () at incubation the 33rd hour of incubation (P<0.01). Moreover, at incubation the 33rd hour of incubation, the 3-MA-treated embryos also still stuck on HH4 ().
The disturbance of autophagy with RAPA or 3-MA resulted in disorder of ATG7 and EMT adhesion molecule expression in chick early embryo
After application of RAPA, the autophagy promoter, we checked Atg7 expression following the treatment of RAPA in early embryo development. At anterior primitive streak, we observed in both epiblast and hypoblast ATG7 were expressed (). At the middle primitive streak, in all the ectoderm ATG7 was expressed (). The ATG7 expression pattern in 3-MA-treated embryos is different from that of the normal embryos as we showed in small panels in (Atg7 expressed distinctly in the apical side of epiblast or ectoderm in both levels of sections).
Figure 4. RAPA treatment promoted the expression of ATG7 and E-Cadherin. Immunofluorescent staining against Atg7 and E-Cadherin were performed on the control and RAPA-treated HH4 chick embryos. A-B: The bright-filed images (A) and Atg7 immunofluorescent images (B) of the embryo respectively treated by RAPA at 33 hour. C-C′: The transverse sections of Atg7 expression (C) and Atg7 expression + DAPI staining (C′) respectively at the level indicated by dotted line C in B panel, the small panel in C′ is control section. D-D′: The transverse sections of Atg7 expression (D) and Atg7 expression + DAPI staining (D′) respectively at the level indicated by dotted line D in B panel, the small panel in D′ is control section. E-E′: E-Cadherin is expressed on the ectoderm cell membrane of control embryo (white arrow in E′); F-F′: E-Cadherin expression level was enhanced on ectoderm cell membrane and ectopic expression in nucleus after RAPA treatment (white arrow in F′). I: Primitive streaks were collected for RT-PCR analysis after being treated with RAPA for 33 h and the control group. In RAPA-treated embryos, E-Cad expression was increased and N-Cad expression was inhibited in comparison with control embryos (N = 20). H: E-Cadherin and N-Cadherin expression levels were detected by real-time PCR. E-Cadherin expression was enhanced and N-Cadherin expression was inhibited in 80 nM RAPA-treated embryos compared to the control. Error bars indicate mean ± s.d. ***P < 0.001 indicating highly significant difference between RAPA-treated and control embryos. I: Western blot shows that the expression of E-Cadherin in HCT116 cells from no RAPA treatment (control), or 100 nM RAPA treatment for 1 hour and 2 hours. Actin was used as a loading control. Scale bars = 600 μm in A-B and 500 μm in C-C′ and 500 μm in D-D′, E-E′.
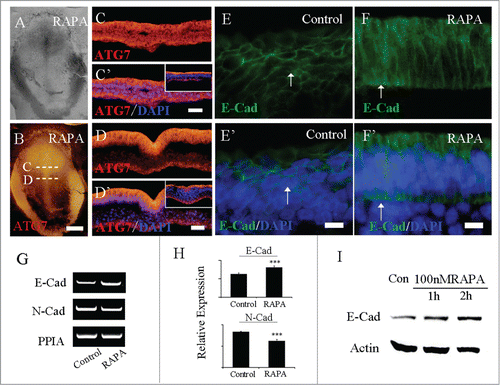
As mentioned above, over-expressing Atg7 could enhance the expression of E-Cadherin, so we speculated that the promotion of autophagy in gastrulation led to the defect of EMT, since EMT is a vital process in 3 germ layer development in gastrulation. To verify the hypothesis, we determined the adhension molecule expression following the treatment of RAPA by immunofluorescent staining and RT-PCR. As a typical epithelial cell maker, E-Cadherin is distinctly expressed on ectoderm cell membrane in control embryos. However, the E-Cadherin expression lost its normal expression pattern after being treated with 3-MA, and it is slightly enhanced on ectoderm cell membrane after being treated with 3-MA and it shows abnormal expression after RAPA treatment (). Furthermore, the extent of E-Cadherin expression was validated by semi-quantitative RT-PCR and quantitative PCR analysis after being treated with RAPA (). Meanwhile, we also determined the expression of N-Cadherin, another crucial Cadherin molecule known as mesoderm maker. Using RT-PCR and real-time PCR assays, we discovered that N-Cadherin expression was repressed by the treatment of RAPA (). The real-time PCR results demonstrated that the expression of E-cadherin in 80 nM RAPA-treated HH4 embryos was 1.3 times compared to the control group. The expression level of N-cadherin in 80 nM RAPA-treated HH4 embryos was 30% lower than the one in the control group. The results of real-time PCR were in accordance with the one from RT-PCR assay. To verify our hypothesis in vitro, we treated the HCT116 cells with 100 nM RAPA, which could increase autophagy. E-Cad expression was upregulated after RAPA treatment at 1 hour, compared to that in control. Whereas, E-Cad expression seemed to stay the same at hour 2 after RAPA treatment ().
Next, we checked the Atg7 expression following the treatment of 3-MA in early embryo development (). Surprisingly, what we observed was that the 3-MA treatment could induce the disordered expression pattern of Atg7 in gastrula embryo rather than enhance or reduce Atg7 expression (). More specifically, Atg7 expression became more scattered among ectoderm, mesoderm and endoderm in 3-MA treated embryo () rather than concentrated only on apical side of ectoderm and endoderm in control embryo ().
Figure 5. 3-MA treatment leads to the alteration of adhension molecule expression in gastrula embryo. Immunofluorescent staining against Atg7, E-Cadherin and Laminin were performed on the control and 3-MA-treated HH5 chick embryos, so did the RT-PCR assay of N-Cadherin expression. (A and B) The bright-filed images (A) and Atg7 immunofluorescent images (B) of the embryo respectively treated by 3-MA at 33 hour. C-C′: The transverse sections of Atg7 expression (C) and Atg7 expression + DAPI staining (C′) respectively at the level indicated by dotted line C in B panel, the small panel in C′ is control section. D-D′: The transverse sections of Atg7 expression (D) and Atg7 expression + DAPI staining (D′) respectively at the level indicated by dotted line D in B panel, the small panel in D′ is control section. (E and G) Immunofluorescent staining against E-Cadherin on the whole-mount control (E) and 3-MA-treated (G) HH4 chick embryos respectively. F, F′: The transverse sections at the level at the middle primitive streak as indicated by white dotted line in E. F is the E-Cadherin only; F′ is the E-Cadherin + DAPI staining. H, H′: The transverse sections at the level at the middle primitive streak as indicated by white dotted line in G. H is the E-Cadherin only; H′ is the E-Cadherin + DAPI staining. (I and K) Immunofluorescent staining against laminin on the whole-mount control (I) and 3-MA-treated (K) HH4 chick embryos respectively. J, J′: The transverse sections at the level at the middle primitive streak as indicated by white dotted line in I. J is the laminin only; J′ is the Laminin + DAPI staining. L, L′: The transverse sections at the level at the middle primitive streak as indicated by white dotted line in K. L is the Laminin only; L′ is the Laminin + DAPI staining. (M) RT-PCR showing Atg8 and N-Cadherin expression in the control and 3-MA-treated embryos. Abbreviations: E-Cad, E-Cadherin; N-Cad, N-Cadherin. Scale bars = 500 μm in A and B, 70 μm in C and C′, 40 μm in D and D′, 500 μm in E and G, 30 μm in F and F′, 600 μm in I and K and 30 μm in H and H′.
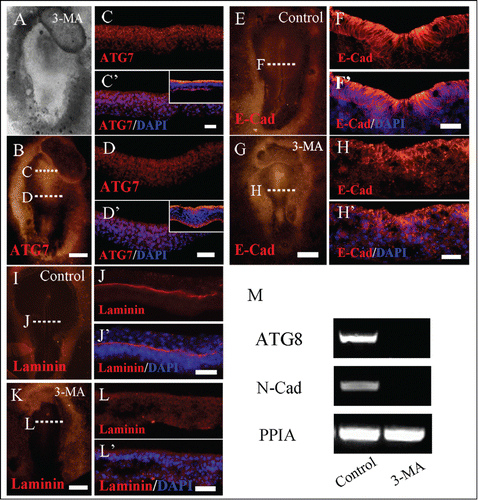
We then determined the adhension molecule expression following the treatment of 3-MA by immunofluorescent staining of whole-mount embryos and RT-PCR. We can see that ATG8 expression was reduced after the 3-MA treatment, indicating 3-MA could indeed inhibit autophagy in this case (). As a typical epithelial cell maker, E-Cadherin is distinctly expressed on epiblast layer in control embryo (). However, the E-Cadherin expression lost its normal expression pattern after being treated with 3-MA, showing the disorder of E-Cadherin expression in epiblast, even stronger expression in primary streak and in some mesoderm cells. It might be the reason for cell accumulations in the bilateral of the primitive groove (). Meanwhile, we also determined the expression of N-Cadherin by RT-PCR, it was significantly repressed by the treatment of 3-MA (). Laminin is a major ECM protein component of basal lamina and form sheets in the basal lamina of epithelium as shown in . In the embryo exposed to 3-MA, laminin became hardly detectable (), implying the lost of polarity in epiblast layer.
The alteration of EMT-related gene expression in gastrula embryo by suppression of autophagy
The aforementioned results suggest that EMT defect is involved in the dysplasia of gastrula embryo induced by the inhibition of autophagy. Moreover, the normal development of early embryo relies on the proper expression of crucial genes spatiotemporally in early gastrula embryo. Therefore, we detected the expressions of following genes appearing in primitive streak and Hensen's node by using in situ hybridization and RT-PCR respectively ().
Figure 6. Detecting EMT-related gene expression in gastrulating embryos following 3-MA treatment using in situ hybridization. Whole-mount chick embryo in situ hybridization of Slug, FGF8, Shh and chordin were carried out following 3-MA treatment. Western blotting for RhoA, Msx1 and Slug expression was performed as well. (A and B) Slug in situ hybridization in control (A) and 3-MA-treated (B) HH4 embryo respectively. (C–E) FGF8 in situ hybridization in control HH4 embryo (C), control HH6 embryo (D) and 3-MA-treated HH4 embryo (E) respectively. C′-C″: The transverse sections at the levels indicated by dotted lines in C respectively. D′-D″: The transverse sections at the levels indicated by dotted lines in D respectively. E′-E″: The transverse sections at the levels indicated by dotted lines in E respectively. (F and G) Shh in situ hybridization in control (F) and 3-MA-treated (G) HH5 embryo respectively. F′-F″: The transverse sections at the levels indicated by dotted lines in F respectively. G′-G″: The transverse sections at the levels indicated by dotted lines in G respectively. (H and I) Chordin in situ hybridization in control (H) and 3-MA-treated (I) HH4 embryo respectively. (J) Semi-quantitative RT-PCR was performed for detecting the expression of RhoA, Rac1, Cdc42, Msx1 and Slug in the control and 3-MA-treated embryos. Abbreviations: HN, Hensen's node; NT, neural tube; PS, primitive streak. Scale bars = 900 μm in A and B, 500 μm in C–E, 40 μm in C′ and C″, 40 μm in D′ and D″, 40 μm in E′ and E″, 800 μm in F and G, 40 μm in F′ and G′, 40 μm in F″ and G″ and 600 μm in H and I.
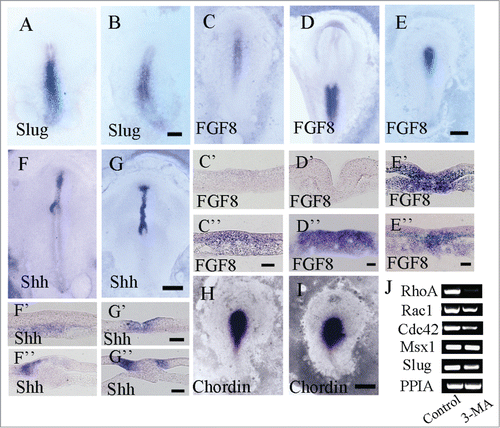
Slug is considered as a transcription factor involved in EMT regulation by suppressing E-Cadherin expression, and it is expressed in primitive streak in early avian embryo (). The autophagy inhibition by the exposure of embryo to 3-MA slightly reduced Slug expression () compared to that of control one ().
As one of FGF family members, FGF8 is also involved in the regulation of EMT in embryo development. Although the expression pattern of FGF8 in gastrula embryo is similar to that of slug expression (), on the whole-mount embryo, the responses to exposure of 3-MA were completely different. At the 18th hour of incubation, control embryos had reached HH4, and FGF8 was expressed in the primitive streak rather than in Hensen's node (); at the 28th hour, control embryos had reached HH8, and FGF8 was expressed in the primitive streak of the caudal side rather than on neural fold (); however, early embryo after being treated with 3-MA, seemed still in HH4 at the 28th hour of incubation (). The ectopic expression of FGF8 was more obvious in transverse sections, in which FGF8 was expressed slightly on the neural fold (), and strongly in Hensen's node rather than in primitive streak in normal embryo ().
Sonic hedgehog (Shh) is a gene related to asymmetric development of embryonic morphogenesis and is expressed in the Hensen's node. Firstly, Shh was expressed asymmetrically across the top of Hensen's node on HH4; subsequently, its expression patterns became gradually asymmetric until HH5 ().Citation30 After the treatment of 3-MA, we noted that these embryos still kept symmetrical expression of Shh () compared to the asymmetrical expression of Shh at similar stage of embryos (). The difference of Shh expression was more obvious in their transverse sections. From the view of the transverse section at the node level, we could see that the expression of Shh was expressed asymmetrically mainly on left mesoderm of node (), while that Shh was still expressed on both sides of Hensen's node in 3-MA-treat embryo although it was at later stage than control embryo (). Asymmetrical expression of Shh appeared in embryo after 18 hours of incubation in control (), but symmetrical expression of Shh remained to be seen in 3-MA-treated embryo after 33 hours of incubation (). The abnormal expression of Shh indicated that the asymmetric related gene expression might be influenced by the suppression of autophagy.
Chordin was detectable predominantly in Hensen's node in HH4 embryos (). The expression pattern of chordin in 3-MA treated embryo looks similar to the one in control groups from the view of whole embryo level () although embryo development delay was obvious.
The results of RT-PCR showed that RhoA, Rac1 and Cdc42 gene expression were down-regulated in 3-MA group (), while no changes were presented on Msx1 gene expression and slight reduction on Slug expression following the treatment of 3-MA.
Discussion
Autophagy (also named macroautophagy) is defined as the delivery of broken cytoplasmic components to lysosome for degradation in eukaryotic cells. As an important cellular response to stress, autophagy is indispensable for cell survival in response to many kinds of stresses, and there is no doubt that autophagy is also associated with cell death in several in vivo aspects. Accumulating evidences supported the notion that autophagy pathway in higher eukaryote might require more elaborate molecular machinery. Unfortunately, very little has been known recently about higher eukaryote autophagy components and functions.Citation31,32 Atg7, which encodes the single E1-like obligatory enzyme for activating Atg8 and Atg12, is one of crucial molecules in autophagy regulation, but its precise role in autophagy has not yet been fully understood. Juhász et al. reported that Atg7 is indispensable for maintaining normal levels of autophagy for stress survival and continuous cellular renewal as a core autophagy regulator, however, the flies lacking the Atg7 are able to be viable and fertile in spite of harsh defects in autophagy.Citation33 Loss of either Atg7 or Atg3 function has no effect on programmed reduction of cell size during intestine cell death. but Uba1 is indispensable for autophagy and reduction of cell size in Drosophila.Citation34 Since all cell migration, differentiation, proliferation and cell death are extremely active in embryonic gastrulation, it is speculated that autophagy might be involved the developmental process in the special period as well. However, until recently, almost no solid experimental evidence has been in literature.
In our initial detection of autophagy related gene expression, we disclosed that Atg7 was expressed in apical side of ectoderm and endoderm as well (). Since Atg7 could up-regulate Atg8 (), this implied that Atg7-induced autophagy was involved in embryo development. It also might imply that Atg7, the autophagy-related gene, play a role in the formation of epithelium-like cell polarity. Of course, we certainly require further experimental evidence to verify the hypothesis. As we all know, E-Cadherin expression in epiblast in gastrulation is the most important characteristic as it does in other epithelium cell to maintain the adhension of epithelial cells. Losing E-Cadherin expression in epiblast/epithelium may result in the failure of EMT initiation. Hence, in order to explore the correlation between Atg7 and E-Cadherin expression, we performed Atg7-GFP transfection on half-side HH3 chick embryo, followed by the detection of E-Cadherin expression () after overnight incubation. Highly magnification image of E-Cadherin expression showed it was enhanced on the side of Atg7-GFP transfection side () in comparison to control side (). Here, E-Cadherin was expressed in epiblast and nascent mesoderm cells ().
HCT116 cells are epithelial cells derived from a human colorectal carcinoma,Citation35 and this cell line has been widely used to study EMT process, the vital process in tumor invasion in cancer development.Citation36 In this study, HCT116 cells were employed to provide in vitro model to verify the in vivo experimental results. And we could reach the same conclusion in HCT116 cells by the transfection of Atg7 () as in gastrulating embryos. This phenomena might hint at the regulatory function of Atg7 on downregulation of E-Cadherin, a molecule essential in the establishment of homotypic adhesion junctions.Citation37 In contrast, the upregulation of E-Cadherin by Atg7 over-expression () prompts that autophagy is involved in regulating cell-cell adhesion maintained by cadherin molecules,Citation38 implying the novel functions of autophagy in EMT process via influencing adhension molecule expression like E-Cadherin. Taken together, this aforementioned result could indicate that gastrula embryo development might be interfered with the enhancement of E-Cadherin expression through the Atg7-dependent autophagy.
To hunt experimental evidence, early chick embryo during gastrulation development was exposed to RAPA and 3-MA, the specific inducer and inhibitor of autophagy. We surprisingly discovered that both RAPA and 3-MA treatment dramatically disrupted gastrulation of embryo development () compared to that of control embryo (). Next, Atg7 expression was examined in those RAPA or 3-MA treated embryos to compare Atg7 expression after the inhibition of autophagy, and disclosed that 3-MA treatment led to the disorder of Atg7 expression pattern (), in which Atg7 was also disturbed in all of ectoderm, mesoderm and endoderm rather than only in apical side of ectoderm and endoderm in normal gastrula embryo (). It indicated that the interruption of Atg7-related autophagy might be involved in the development of 3 germ layers in gastrula embryo, probably through influencing EMT process in gastrula period. Of course, we are also not able to eliminate another possibility that the damage of 3 germ layer development was aroused by other autophagy-related pathways, so that the abnormal expression of Atg7 was the result rather than the cause. Further experiments are required to solve the mystery.
Several reports demonstrated that autophagy was involved in the EMT progress of some cell lines or tumor tissues,Citation39-42 and the embryonic EMT was similar to the transformation of cell phenotype during carcinoma progression.Citation43 Thus, we did perform the evaluation of E-Cadherin, N-Cadherin and laminin expression following the treatment of RAPA and 3-MA (), and found that exposure to RAPA or 3-MA resulted in disorder of adhesion molecule expression in early chick embryo. One well-known fact is that the E-Cadherin down-regulation and N-Cadherin up-regulation are necessary conditions for EMT initiation. And the expression of laminin presented on basal side of epithelium should disappear as well prior to EMT. Thus, we could have reason to assume that our data on adhension molecule expression in is generally in accordance with those conditions indispensable for igniting EMT. In another word, we speculate that autophagy affects the early embryo development principally through regulating EMT, and this also hinted that we could research how the autophagy gene affected tumor invasion by use of chick gastrulation embryo.
Slug, the transcription factor, is able to promote formation of β-catenin-TCF-4 transcription complexes, which bind to the promoter of the TGF-β3 gene to increase its transcription, and thereby initiate EMT.Citation44 In this study, using in situ hybridization and RT-PCR, we disclosed that the Slug expression was reduced after autophagy inhibition treatment (). Similarly, Slug protein level was also abridged by autophagy inhibition with 3-MA (). It indicated that the suppression of Slug expression could be one of the reasons for MET disorder after autophagy inhibition. Hedgehog signaling is crucial factor during embryogenesis and its activation could leads indirectly to EMT initiation via FGF, Notch, TGFβ signaling cascades and miRNA regulatory networks.Citation45 Interestingly, we observed the abnormal gene expression of both Shh () and FGF8 () following the treatment of 3-MA. Exposure to 3-MA mainly resulted in FGF8 expression in Hensen's node () rather than in primitive streak at control embryo (). Likewise, asymmetrical Shh expression was not present in 3-MA-treated embryo () compared to the control embryo (). The autophagy inhibition-induced ectopic expression of Shh and FGF singling might also contribute to the abnormal development of gastrula embryo. Chordin is expressed at the rostral end of the primitive streak at early gastrula stage and subsequently present in Hensen's node.Citation46 The Chordin expression was not found to be much different from that in the control one after 3-MA treatment ().
Cdc42, Rac1 and RhoA are Rho family small GTPases, which has been reported to be implicated in maintaining the polarization and regulating the directional migration.47,48 The inhibition of Rac1 and Rho but not Cdc42 could lead to the abnormal expression of E-Cadherin,Citation49 Therefore, we could speculate that the suppression of Rac1 and Rho () is one of the major reasons which lead to the abnormal E-Cadherin expression in 3-MA treated embryos () in our study. We could apply same cause to explain the similar phenomena in RAPA treated embryos (). Lucy Erin O’Brien et al. reported that dominant-negative Rac1 transfection affected the assemble of Laminin on basolateral membrane, which in turn lead to the invert of polarity in epithelial cells.Citation50 The reduced-expression of Rac1 in 3-MA treated embryos may contribute to the lost expression of Laminin, which has a crucial effect on maintaining the epithelial tissue structure in vivo. Laminin is a glycoprotein expressed only in basement membrane.Citation51 The loss of expression of Laminin subunits could not maintain the basement membrane to assemble and differentiate, which result in the disability of epiblast polarity.Citation52 Nakaya et al. reported that RhoA and Net1 played vital roles in the basement membrane (BM) breakdown, the first recognizable step in EMT, by controlling disruption of basal membrane-extracellular matrix interaction and consequently to BM breakdown.Citation12 It's known that the activity of Cdc42 and Rac1 could affect the actin-based structures, such as lamellipodia and filopodia,47,53 suggesting 3-MA could influence EMT process through dysfunctional actin assemble. Meanwhile, we did find that Cdc42 expression were reduced by autophagy inhibition (), and the activity of Cdc42 can also have influences on the microtubule-organizing center (MTOC) and Golgi, which was relative to the polarity in the front of the migrating cells.Citation53 However, the mechanism on how autophagy affect the Rho family of small GTPase was unknown. Further study is definitely required. Taken together, the 3-MA-inhibited reduction of RhoA, Rac1 and Cdc 42 expression may be responsible for the disruption of epiblast layer basal membrane structure and losing Laminin expression so that normal EMT could not be ignited. Citation48 Meanwhile, the expression of Msx1, one of a larger family of homeobox genes, was not affected by 3-MA treatment.
It is worth noting that in some in vitro experiments, 3-MA act as an inhibiter of autophagy at first, but it could become a promoter of autophagy when 3-MA is applied at suboptimal concentrations and for prolonged time.Citation54 However, we did observe the downregulation of Atg8 and Atg7, the key proteins in autophagy, using immunofluorescence staining () or RT-PCR () after 3-MA treatment, implying that 3-MA could act as an effective autophagy inhibitor in this experiment.
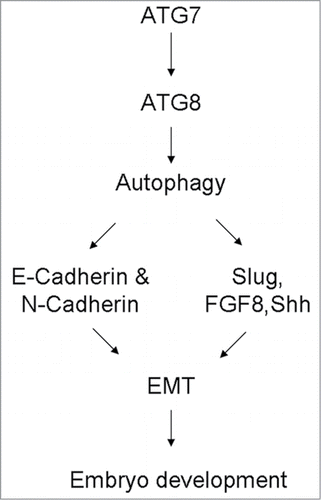
In sum, we first demonstrated that ATG7-dependent autophagy was involved in the development of embryonic gastrulation by means of modulation of EMT process through regulating the cell adhesion and some essential genes expressions such as RhoA, Rac1, Cdc42, Slug, Shh and FGF8 (), so that excess, loss or metathesis of autophagy would lead to the disorder of 3 germ layer development. There is no doubt that more precise experimental evidence would be supplied before completely exploring the function of autophagy in early embryonic development.
Material and Methods
Avian embryos and manipulation
Fertilized leghorn eggs obtained from the Avian Farm of South China Agriculture University, were incubated in a humidified incubator (Yiheng Instruments, Shanghai, China) at 38°C with humidity until the required Hamburger and Hamilton (HH) stages of chick embryo.Citation55
For the RAPA (LC Labs, R5000) or 3-Methyladenine (3-MA; Sigma, M9281) treated early chick embryos, adding 5 mM 3-MA or 40 nM RAPA in the culture medium of Early Chick (EC) culture and incubated with embryos for the required time.
For the early gene transfection, HH3 chick embryos in EC (Early Chick) culture Citation56 were employed to maintain the embryos during and after electroporation. About 1 μl of GFP expression plasmid DNA (pEGFP-N1, Clontech) or Atg7 (pCMV-Myc-Atg7)-GFP expression plasmid DNA (a kindly gift from Dr. Toren's Laband) with a 1.5 μg/μl concentration was microinjected in the space between the vitelline membrane and the epiblast prior to electroporation. Electroporation was performed using an electroporator (BTX-ECM399) and a pair of home-made platinum electrodes arranged in a parallel fashion. Two pulse of 10v voltage was delivered between the electrodes as previously described.Citation57 For embryonic one-sided gene transfection, the polarity of the pulses was kept constant. The survival ratio of embryo after the transfection is close to 100%, as long as the embryos are in good condition, i.e., growing normally so long as the embryos were covered by enough fluid as conducting medium. The transfected embryos were then incubated at 37°C with 70% humidity until the desired developmental stage was reached. During incubation, the fluorescence of GFP was directly observed without performing anti-GFP immunostaining.
Cell culture
The human colon cancer cell line (HCT116 cell line) grew at 37°C and in a humidified air CO2 (19:1) atmosphere in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco BRL), 100 mg of penicillin, 100 mg of streptomycin, 2 mM-glutamine, 4 mM sodium pyruvate and 100 μM non-essential amino acids.
Immunofluorescent staining
Whole-mount chick embryos were immunofluorescently stained to reveal the expression of Atg7, E-Cadherin (E-Cad) and Laminin proteins as previously described.Citation58,59 Generally, the embryos were fixed with 4% paraformaldehyde (PFA) at 4°C overnight, and unspecific immunoreactions was blocked with 2% Bovine Serum Albumin (BSA) + 1% Triton-X + 1% Tween 20 in PBS for 2 hours at room temperature, followed by a brief wash in PBS. The embryos were incubated with primary monoclonal antibody mixture raised against Atg7 (Sigma, 1:100), E-Cad (BD Transduction Laboratories, 1:100) and Laminin (Developmental Studies Hybridoma Bank, 1:100) overnight at 4°C with shaking. After extensive washing, the embryos were incubated in specific secondary antibody conjugated to Alexa Fluor 488 dye (Alexa Fluor 555 goat anti-mouse IgG; Invitrogen, 1:1000) overnight at 4°C on a shaker to visualize the primary antibodies. After immunofluorescent staining, all the embryos were counterstained with DAPI (49-6-Diamidino-2-phenylindole, Invitrogen, 5 mg/ml) for 1 hour at room temperature. Subsequently, the embryos were sectioned on a cryostat microtome (Leica CM1900). The sections were mounted in mounting solution (Mowiol 4–88, Sigma) on glass slides and sealed with coverslips. All immunofluorescent staining were performed in replicates where at least 5–6 embryos were used.
In situ hybridization
Whole-mount in situ hybridization of chick embryos was performed according to a standard in situ hybridization protocol.Citation60 Briefly, Digoxigenin-labeled probes were synthesized for ShhCitation61 and Slug.Citation62 The whole-mount stained embryos were photographed and then frozen sections were prepared at a thickness of 15–20 μm on a cryostat microtome (Leica CM1900).
Photograph
After immunofluorescent staining, the whole-mount embryos were photographed using stereoscope fluorescence microscope (Olympus MVX10) with imaging software (Image-Pro Plus 7.0). The sections of the embryos were photographed using an epi-fluorescent microscope (Olympus IX51, Leica DM 4000B) at 200 or 4006 magnification with the Olympus software package Leica CW4000 FISH.
RNA isolation, RT-PCR and Real-time PCR
Gene expressions were semi-quantitatively assessed utilizing reverse transcription-polymerase chain reaction (RT–PCR) as previously reported,Citation63 and some of them were quantitatively assessed by real-time PCR. Total RNA was extracted from brain samples using TRIzol reagent according to the protocol of the manufacturer (Invitrogen, Carlsbad, CA, USA). A 5 μg amount of total RNA was reversely transcribed into cDNA at 42°C for 1 hour in 20 μL of reaction mixture containing iscript reverse transcriptase (BIO-RAD, Hercules, CA) with oligo (dT) and random hexamer primers (BIO-RAD, Hercules, CA) and followed by PCR amplification. PCR was carried out with 1 μL of cDNA, 12.5 μL of DreamTaq Green PCR master mix(2X) (Thermo scientific, Foster City, California), containing dreamTaq DNA polymerase, dATP, dCTP, dGTP, dTTP and MgCl2, mixed with 1 μM forward primer, 1 μM reverse primer in a total volume of 25 μL. The cDNA was amplified using specific primers with 30 cycles at 94°C for 30 s, an annealing temperature of 60°C for 30 s, and then 72°C for 30 s, with final incubation at 72°C for 7 min. The PCR primers for chicken N-Cadherin mRNA were (F) 5′-AGATTCTGGAAATCCACATGC-3′ and (R) 5′-CTTCCTTCATAGTCAAAGACT-3′, with 148 bp product size. The PCR primers for chicken E-Cadherin mRNA were (F) 5′-CGCTTCCCCGTGTTGGT-3′ and (R) 5′-GGCCGTTTTGTTGAGACGAC-3′, with 251 bp product size. The PCR primers for chicken RhoA mRNA were (F) 5′-GCAGCCATTCGAAAAAGCT-3′ and (R) 5′-TTTATAAGAGAAGGCACCCG-3′, with 153 bp product size. The PCR primers for chicken Slug mRNA were (F) 5′-CCAATGACCTCTCTCCGCTTTCTG-3′ and (R) 5′-ATCGCTAATGGGACTTTCTGAACCG-3′, with 116 bp product size. The PCR primers for chicken Msx1 mRNA were (F) 5′-AGACTTCTCCGCTCCCTTCATCC-3′ and (R) 5′- TGCCTTTGTGCCCTTTCTCTGC-3′, with 130 bp product size. The PCR primers for chicken Atg8 mRNA were (F) 5′- CGAGCAAAGAGTTGAAGA-3′ and (R) 5′-CCACCTGCGTGTCCTA-3′, with 353 bp product size. The PCR primers for chicken Rac1 mRNA were (F) 5′-GCCCCAACACTCCCATCATT-3′ and (R) 5′-TGGGGGAGGGTGACTTTACA-3′, with 281 bp product size. The PCR primers for chicken Cdc42 mRNA were (F) 5′-AGAAGACTCGCAGGTGTGTG-3′ and (R) 5′-ATGGTGCATCCAGGGGAAAG-3′, with 688 bp product size. The primers for the chicken housekeeping gene PPIA mRNA were (F) 5′-TGACAAGGTGCCCATAACAG-3′ and (R) 5′-TTCTCGTCGGCAAACTTCTC-3′, with 183 bp product size. The PCR products were fractionated on a 2.5% agarose gel and visualized by ethidium bromide staining. The band intensity of ethidium bromide fluorescence was measured using an image analysis system. The real-time PCR used the MiniOpticon Real-Time PCR System, Thermo Scientific Mixiama SYBR Green qPCR kit plus iscript Reverse Transcriptase, the same as it mentioned in RT-PCR. Specific primers are used in the thermal cycle: 50°C for 2 minutes, 94°C for 10 minutes, followed by 40 cycles of 94°C for 15 seconds, 60°C 0e real-time PCR used the MiniOpticon Real-Time PCR System, Thermo Scientific Mixiama SYBR Green qPCR kit plus iscript Reverse Transcriptase, the same AGATTCTGGAAATCCACATGC-3′ and (R) 5′-GCCAACAATCCGGTCAACAT-3′. The real-time PCR primers for chicken E-cadherin primers were (F) 5′-CGCTTCCCCGTGTTGGT-3′ and (R) 5′-GCACACTGAAGCTGAAGGTC-3′. The real-time PCR primers for chicken housekeeping gene PPIA were (F) 5′-TGACAAGGTGCCCATAACAG-3′and (R) 5′-GCGTAAAGTCACCACCCTGA-3′.
Western blotting
HCT116 cells, transfected with Atg7(pCMV-Myc-Atg7)-GFP or myc-vector plasmids, were lysed with Nonidet P-40 Lysis buffer (1.0% Nonidet P-40, 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM EDTA) supplemented with protease inhibitor tablet (Roche) and phosphatase inhibitors (1 mM Na3VO3, 1 mMb-glycerolphosphate, 10 mMNaF) for 15 min on ice prior to clarification by centrifugation at 16100 3 g for 15 min. Protein lysates were resolved on precast Tris-Glycine SDS gels (Invitrogen) and transferred onto nitrocellulose membranes. Immunoblot analysis was performed with the antibody E-cadherin (BD) and Atg7.
Statistical analysis
Experimental values were given as means ± SD. Statistical analysis of the data was performed using the SPSS 18.0 statistical software. One-way analysis of variance (ANOVA) was applied to analyze for difference in data of biochemical parameters among the different groups, followed by Dunnett's significant post hoc test for pairwise multiple comparisons. Differences were considered as statistically significant at P < 0.05, or P < 0.01.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Dr. Toren's Laband for kindly supplying ATG7 (pCMV-Myc-ATG7)-GFP expression plasmid.
Additional information
Funding
References
- Walls KC, Ghosh AP, Franklin AV, Klocke BJ, Ballestas M, Shacka JJ, Zhang JH, Roth KA. Lysosome dysfunction triggers Atg7-dependent neural apoptosis. J Biol Chem 2010; 285:10497-507; PMID:20123985; http://dx.doi.org/10.1074/jbc.M110.103747
- Lee IH, Kawai Y, Fergusson MM, Rovira, II, Bishop AJ, Motoyama N, Cao L, Finkel T. Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science 2012; 336:225-8; PMID:22499945; http://dx.doi.org/10.1126/science.1218395
- Friedman LG, Lachenmayer ML, Wang J, He LQ, Poulose SM, Komatsu M, Holstein GR, Yue ZY. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of alpha-synuclein and LRRK2 in the brain. J Neurosci 2012; 32:7585-93; PMID:22649237; http://dx.doi.org/10.1523/JNEUROSCI.5809-11.2012
- Shehata M, Matsumura H, Okubo-Suzuki R, Ohkawa N, Inokuchi K. Neuronal stimulation induces autophagy in hippocampal neurons that is involved in AMPA receptor degradation after chemical long-term depression. J Neurosci 2012; 32:10413-22; PMID:22836274; http://dx.doi.org/10.1523/JNEUROSCI.4533-11.2012
- Vazquez P, Arroba AI, Cecconi F, de la Rosa EJ, Boya P, de Pablo F. Atg5 and ambra1 differentially modulate neurogenesis in neural stem cells. Autophagy 2012; 8:187-99; PMID:22240590; http://dx.doi.org/10.4161/auto.8.2.18535
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 2005; 169:425-34; PMID:15866887; http://dx.doi.org/10.1083/jcb.200412022
- Yang J-w, Zhu L-x, Yuan G-h, Chen Y-x, Zhang L, Zhang L, Chen Z. Autophagy appears during the development of the mouse lower first molar. Histochem Cell Biol 2013; 139:109-18; PMID:23052835; http://dx.doi.org/10.1007/s00418-012-1016-2
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2:442-54; PMID:12189386; http://dx.doi.org/10.1038/nrc822
- Chuai M, Weijer CJ. Regulation of cell migration during chick gastrulation. Current Opin Genet Dev 2009; 19:343-9; PMID:19647425; http://dx.doi.org/10.1016/j.gde.2009.06.007
- Grünert S, Jechlinger M, Beug H, <Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis.pdf>. Nat Rev Mol Cell Biol 2003; 4:657-65; PMID:12923528
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Current Opin Cell Biol 2005; 17:548-58; PMID:16098727; http://dx.doi.org/10.1016/j.ceb.2005.08.001
- Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol 2008; 10:765-75; PMID:18552836; http://dx.doi.org/10.1038/ncb1739
- Qu XP, Zou ZJ, Sun QH, Luby-Phelps K, Cheng PF, Hogan RN, Gilpin C, Levine B. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell 2007; 128:931-46; PMID:17350577; http://dx.doi.org/10.1016/j.cell.2006.12.044
- Di Bartolomeo S, Nazio F, Cecconi F. The role of autophagy during development in higher eukaryotes. Traffic 2010; 11:1280-9; PMID:20633243; http://dx.doi.org/10.1111/j.1600-0854.2010.01103.x
- Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A 2003; 100:15077-82; PMID:14657337; http://dx.doi.org/10.1073/pnas.2436255100
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006; 441:885-9; PMID:16625204; http://dx.doi.org/10.1038/nature04724
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature 2004; 432:1032-6; PMID:15525940; http://dx.doi.org/10.1038/nature03029
- Sanders EJ, Torkkeli PH, French AS. Patterns of cell death during gastrulation in chick and mouse embryos. Anat Embryol 1997; 195:147-54; PMID:9045984; http://dx.doi.org/10.1007/s004290050033
- Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ 2001; 8:569-81; PMID:11536007; http://dx.doi.org/10.1038/sj.cdd.4400852
- Lv Q, Hua F, Hu Z-W. DEDD, a novel tumor repressor, reverses epithelial-mesenchymal transition by activating selective autophagy. Autophagy 2012; 8:1675-6; PMID:22874565; http://dx.doi.org/10.4161/auto.21438
- Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AK, et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res 2013; 73:2418-27; PMID:23436798; http://dx.doi.org/10.1158/0008-5472.CAN-12-2432
- Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J. Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy 2010; 7:1115-31; http://dx.doi.org/10.4161/auto.7.10.16608
- Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ 2009; 16:46-56; PMID:18636076; http://dx.doi.org/10.1038/cdd.2008.110
- Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem 1998; 273:3963-6; PMID:9461583; http://dx.doi.org/10.1074/jbc.273.7.3963
- Aburto MR, Sanchez-Calderon H, Hurle JM, Varela-Nieto I, Magarinos M. Early otic development depends on autophagy for apoptotic cell clearance and neural differentiation. Cell Death Dis 2012; 3:e394; PMID:23034329; http://dx.doi.org/10.1038/cddis.2012.132
- Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Nat Acad Sci U S A 1982; 79:1889-92; PMID:6952238; http://dx.doi.org/10.1073/pnas.79.6.1889
- Chi PI, Huang WR, Lai IH, Cheng CY, Liu HJ. The p17 nonstructural protein of avian reovirus triggers autophagy enhancing virus replication via activation of phosphatase and tensin deleted on chromosome 10 (PTEN) and AMP-activated protein kinase (AMPK), as well as dsRNA-dependent protein kinase (PKR)/eIF2 alpha signaling pathways. J Biol Chem 2013; 288:3571-84; PMID:23233667; http://dx.doi.org/10.1074/jbc.M112.390245
- Martinet W, De Meyer GR, Andries L, Herman AG, Kockx MM. In situ detection of starvation-induced autophagy. J Histochem Cytochem: Off J Histochem Soc 2006; 54:85-96; PMID:16148314; http://dx.doi.org/10.1369/jhc.5A6743.2005
- Takeichi M. Self-organization of animal tissues: cadherin-mediated processes. Dev Cell 2011; 21:24-6; PMID:21763603; http://dx.doi.org/10.1016/j.devcel.2011.06.002
- Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. Cell movements at Hensen's node establish left/right asymmetric gene expression in the chick. Science 2009; 324:941-4; PMID:19359542; http://dx.doi.org/10.1126/science.1172478
- Tian Y, Li Z, Hu W, Ren H, Tian E, Zhao Y, Lu Q, Huang X, Yang P, Li X, et al. C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell 2010; 141:1042-55; PMID:20550938; http://dx.doi.org/10.1016/j.cell.2010.04.034
- McPhee CK, Logan MA, Freeman MR, Baehrecke EH. Activation of autophagy during cell death requires the engulfment receptor Draper. Nature 2010; 465:1093-6; PMID:20577216; http://dx.doi.org/10.1038/nature09127
- Juhasz G, Erdi B, Sass M, Neufeld TP. Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in Drosophila. Genes Dev 2007; 21:3061-6; PMID:18056421; http://dx.doi.org/10.1101/gad.1600707
- Chang TK, Shravage BV, Hayes SD, Powers CM, Simin RT, Wade Harper J, Baehrecke EH. Uba1 functions in Atg7- and Atg3-independent autophagy. Nat Cell Biol 2013; 15:1067-78; PMID:23873149; http://dx.doi.org/10.1038/ncb2804
- Ford J, Jiang M, Milner J. Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res 2005; 65:10457-63; PMID:16288037; http://dx.doi.org/10.1158/0008-5472.CAN-05-1923
- Otabe H, Nibuya M, Shimazaki K, Toda H, Suzuki G, Nomura S, Shimizu K. Electroconvulsive seizures enhance autophagy signaling in rat hippocampus. Prog Neuro-Psychopharmacol Biol Psychiat 2014; 50:37-43; PMID:24316174; http://dx.doi.org/10.1016/j.pnpbp.2013.11.012
- Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, de Herreros AG. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000; 2:84-9; PMID:10655587; http://dx.doi.org/10.1038/35000034
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development 1988; 102:639-55; PMID: 3048970
- Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo Y, Song Z, Zheng Q, Xiong J. Autophagy promotes hepatocellular carcinoma cell invasion through activation of epithelial-mesenchymal transition. Carcinogenesis 2013; 34:1343-51; PMID:23430956; http://dx.doi.org/10.1093/carcin/bgt063
- Akalay I, Janji B, Hasmim M, Noman MZ, Thiery JP, Mami-Chouaib F, Chouaib S. EMT impairs breast carcinoma cell susceptibility to CTL-mediated lysis through autophagy induction. Autophagy 2013; 9:1104-6; PMID:23635487; http://dx.doi.org/10.4161/auto.24728
- Lv Q, Wang W, Xue J, Hua F, Mu R, Lin H, Yan J, Lv X, Chen X, Hu ZW. DEDD interacts with PI3KC3 to activate autophagy and attenuate epithelial-mesenchymal transition in human breast cancer. Cancer Res 2012; 72:3238-50; PMID:22719072; http://dx.doi.org/10.1158/0008-5472.CAN-11-3832
- Lv Q, Hua F, Hu ZW. DEDD, a novel tumor repressor, reverses epithelial-mesenchymal transition by activating selective autophagy. Autophagy 2012; 8:1675-6; PMID:22874565; http://dx.doi.org/10.4161/auto.21438
- Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol 2010; 21 Suppl 7:vii89-92; PMID:20943648; http://dx.doi.org/10.1093/annonc/mdq292
- Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell 2008; 19:4875-87; PMID:18799618; http://dx.doi.org/10.1091/mbc.E08-05-0506
- Katoh Y, Katoh M. Hedgehog signaling, epithelial-to-mesenchymal transition and miRNA (review). Int J Mol Med 2008; 22:271-5; PMID:18698484
- Lawson A, Volas J-F, Schoenwolf GC. Classification Scheme for Genes Expressed During Formation and Progression of the Avian Primitive Streak. Anat Rec 2001; 262:221-6; PMID:11169917; http://dx.doi.org/10.1002/1097-0185(20010201)262: 2<221::AID-AR1019>3.0.CO;2-F
- Otabe H, Nibuya M, Shimazaki K, Toda H, Suzuki G, Nomura S, Shimizu K. Electroconvulsive seizures enhance autophagy signaling in rat hippocampus. Prog Neuro-Psychopharmacol Biol Psychiat 2014; 50:37-43; PMID:24316174; http://dx.doi.org/10.1016/j.pnpbp.2013.11.012
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell 2004; 7:871-83; PMID:15572129; http://dx.doi.org/10.1016/j.devcel.2004.10.017
- Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol 1997; 137:1421-31; PMID:9182672; http://dx.doi.org/10.1083/jcb.137.6.1421
- O'Brien LE, Jou TS, Pollack AL, Zhang QH, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell biol 2001; 3:831-8; PMID:11533663; http://dx.doi.org/10.1038/ncb0901-831
- Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin-a glycoprotein from basement membranes. J Biol Chem 1979; 254:9933-7; PMID:114518
- Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol 2002; 157:1279-90; PMID:12082085; http://dx.doi.org/10.1083/jcb.200203073
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 2003; 302:1704-9; PMID:14657486; http://dx.doi.org/10.1126/science.1092053
- Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 2010; 285:10850-61; PMID:20123989; http://dx.doi.org/10.1074/jbc.M109.080796
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dynam: An Off Pub Am Assoc Anatomists 1992; 195:231-72; PMID:1304821; http://dx.doi.org/10.1002/aja.1001950404
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dynam: An Off Pub Am Assoc Anatomists 2001; 220:284-9; PMID:11241836; http://dx.doi.org/10.1002/1097-0177(20010301)220: 3<284::AID-DVDY1102>3.0.CO;2-5
- Yang X, Dormann D, Munsterberg AE, Weijer CJ. Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev Cell 2002; 3:425-37; PMID:12361604; http://dx.doi.org/10.1016/S1534-5807(02)00256-3
- Yang X, Chrisman H, Weijer CJ. PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development 2008; 135:3521-30; PMID:18832396; http://dx.doi.org/10.1242/dev.023416
- Yue Q, Wagstaff L, Yang X, Weijer C, Munsterberg A. Wnt3a-mediated chemorepulsion controls movement patterns of cardiac progenitors and requires RhoA function. Development 2008; 135:1029-37; PMID:18256196; http://dx.doi.org/10.1242/dev.015321
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature 1995; 375:787-90; PMID:7596411; http://dx.doi.org/10.1038/375787a0
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 2003; 40:65-79; PMID:14527434; http://dx.doi.org/10.1016/S0896-6273(03)00565-8
- Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science (New York, NY 1994; 264:835-9; http://dx.doi.org/10.1126/science.7513443
- He R-R, Li Y, Li X-D, Yi R-N, Wang X-Y, Tsoi B, Lee KKH, Abe K, Yang X, Kurihara H. A New Oxidative Stress Model, 2,2-Azobis(2-Amidinopropane) Dihydrochloride Induces Cardiovascular Damages in Chicken Embryo. PloS One 2013; 8:e57732; PMID:AMBIGUOUS