Abstract
Over the last 10 years, significant progress has been made in understanding the genetics, enzymology and structural components of the wybutosine (yW) biosynthetic pathway. These studies have played a key role in expanding our understanding of yW biosynthesis and have revealed unexpected evolutionary ties, which are presently being unraveled. The enzymes catalyzing the 5 steps of this pathway, from genetically encoded guanosine to wybutosine base, provide an ensemble of amazing reaction mechanisms that are to be discussed in this review article.
Introduction
Post-transcriptional modifications are one of the processing events that result in functional RNA molecules. To date, more than 100 chemically distinct modifications are described and they are found across the 3 kingdoms of life.Citation1 Among all RNAs, tRNAs exhibit the largest number and the widest variety of modifications.Citation1 Some of them are generated by relatively simple biosynthetic reactions involving methylation, thiolation, pseudouridylation, or dihydrouridine formation, and are found in most tRNAs.Citation2 The Anticodon Stem Loop (ASL) region particularly displays more complex modifications, which are proposed to contribute to the stabilization of the codon-anticodon pair, thereby maintaining the translational fidelity with high efficiency.Citation2 The distinctive chemistry of the modified nucleosides located at the ASL, mainly at the wobble position 34 and purine at position 37, is crucial for the decoding process, which may explain why both positions present the largest variety of hyper-modifications found among all tRNAs.Citation2 The biosynthetic pathway leading to these hyper-modifications requires several enzymatic steps for which the enzymological data are especially rich, though challenging.Citation3,4
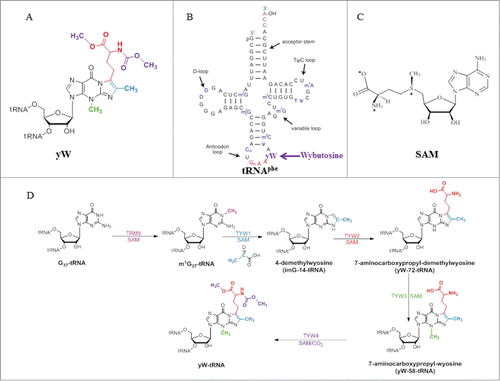
One of the most structurally complex tRNA modifications is the formation of the wybutosine base (yW) that contains a fluorescent tricyclic fused aromatic base derived from a genetically encoded guanosine residue ().Citation5 The yW and its derivatives are found at position 37 of eukaryotic and archaeal phenylalanine tRNA (tRNAPhe) () and their biosynthetic pathways have been described by excellent articles in great detail ().Citation6-8 In this review, we intend to describe the 5 chemical reactions that generate yW, from a mechanistic and structural perspective, and to highlight the unique chemical versatility of S-adenosylmethionine (SAM or AdoMet) in this pathway. In these 5 enzymatic reactions, SAM is utilized as a source of 3 different chemical entities. While Trm5, TYW3, and TYW4, all use SAM as a source of methyl group, TYW2 and TYW1 use SAM as a source of α-amino-α-carboxypropyl group and 5′-deoxyadenosine radical (), respectively.
Figure 1. (A) Chemical structure of wybutosine (yW), all appended groups are colored. (B) Secondary structure of yeast tRNAPhe. (C) Chemical structure of SAM. (D) Biosynthetic pathway of yW. Trm5 methylates G37 to produce m1G37; TYW1, a Radical-SAM enzyme, catalyzes the formation of 4-demethylwyosine (imG-14) using pyruvate as a co-substrate; TYW2 appends the α-amino-α-carboxypropyl moiety of SAM to produce 7-aminocarboxypropyl-demethylwyosine (yW-72); TYW3 methylates N4 of yW-72 to produce 7-aminocarboxypropyl-wyosine (yW-58); and TYW4 both transfers a carboxymethyl group and methylates yW-58 to yield yW.
The S-Adenosylmethionine (SAM)-Dependent tRNA-Methyltransferases Trm5, TYW3, and TYW4
The overwhelming majority of tRNA-methyltransferases use SAM as a methyl group donor.Citation9-11 SAM is an important biological sulfonium compound, which is involved in many essential biochemical processes.Citation12 It is one of the most widely used enzyme cofactors, second only to ATP.Citation13 SAM is biosynthesized during the reaction of methionine with ATP, which is catalyzed by SAM synthetase or methionine adenosyltransferase.Citation14 This reaction is stereospecific and only generates an S-configuration at the sulfur atom (). The strong preference for SAM over other methyl donors, such as folate, reflects the highly favorable thermodynamics of SAM-dependent methyl-transfer reactions. The methylation of nucleophiles is the consequence of an intrinsic chemical property of SAM, namely the strong electrophilic character of its methyl group. This property has been exploited by SAM-dependent tRNA-methyltransferases, which bring SAM into contact with nucleophilic groups of substrates.
tRNA-methyltransferase Trm5
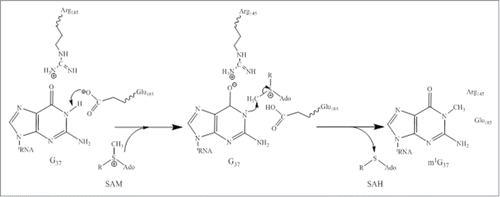
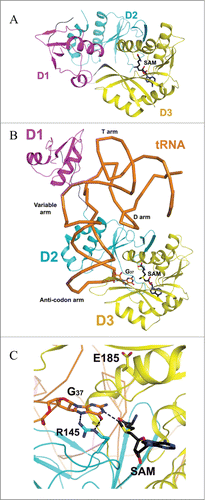
Trm5 is specific to eukaryotes and archaea, and is evolutionary unrelated to the bacterial counterpart TrmD.Citation15,16 Trm5 is a member of class I amino-methyltransferase family, which transfers the methyl group from SAM to nitrogen in widely different chemical environments.Citation17 The crystal structures of the enzyme from the archaeon Methanocaldococcus jannaschii in complex with its substrate tRNAs and SAM have been reported.Citation18,19 The overall structure consists of 3 domains (D1, D2 and D3) in which D1 is linked to D2 by a flexible loop comprising 8 residues (Glu66-Lys73), whereas D2 and D3 are tightly associated (). The arrangement of the 3 domains therefore suggests that D1 acts as the tRNA recognition entity similar to what has been reported for tRNA modification enzyme MiaA.Citation20 Unlike Trm5, MiaA is composed of 2 domains: the N-domain which is linked by a flexible loop to the C-terminal catalytic domain. Similar to N-domain of MiaA, D1 of Trm5 undergoes a drastic conformational change from apo to tRNA-bound structures (). While D1 recognizes both variable and T arms, forming the outer corner of L-shape tRNAs, the catalytic domains D2 and D3 both directly interact with the anticodon region comprising the anticodon stem-loop and the D arm of both tRNACysGCA and tRNACysGCA in the reported structures.Citation18 Interestingly, the structural analysis suggests that Trm5 has evolved to select and recognize L-shaped tRNAs as its substrates, and it thus functions as a tRNA tertiary structure checkpoint in the tRNA methylation process.Citation18 It is likely that Trm5 enzyme enhances reaction rates by simply promoting favorable orientation and spatial proximity of the tRNA substrate and methyl group of SAM. Recent considerations from the kinetic and site-directed mutagenesis data in light of the Trm5-tRNA-SAM ternary structures suggest that the conserved R145 is involved in stabilizing a proposed oxyanion at G37-O6 (), and E185 has been proposed to act as a general base to accept a proton from N1-G37, even though the side chain of E185 is approximately 12 Å away from N1-G37 ().Citation21
Figure 2. Crystal structure of archaeal Trm5. (A) Structure of Trm5 in complex with SAM (PDB id : 2YX1). The three domains of Trm5 are color coded and labeled. SAM is depicted as a stick model with black for carbon atoms. (B) Ternary structure of Trm5 in complex with tRNALeu and SAM (PDB id : 2ZZM). G37 of tRNALeu is shown as a stick model with orange for carbon atoms. (C) The active site of Trm5 in which the side chains of R145 and the proposed catalytic residue E185 are shown as stick models. are made using PyMOL (www. pymol. org).
tRNA-methyltransferase TYW3
The fourth step in the yW biosynthesis relies on TYW3, an S-AdoMet-dependent enzyme, which methylates the N3 position of the imidazo-purine ring to yield yW-58 (). The gene encoding this enzyme (tyw3) has first been identified by ribonucleome screening method in S. cerevisiae.Citation6 Later, archaeal TYW3 orthologues were identified using S. cerevisiae and Arabidopsis thaliana as representatives of eukaryotes.Citation8 TYW3 from different organisms was found to be a highly conserved protein. However, homologues of TYW3 from A. thaliana and Oryza sativa have been shown to contain an extended C-terminal domain of about 1000 amino acid residues. Interestingly, this extension is almost identical to the C-terminal domain of TYW4 and the entire sequence of TYW2. Thus, the plant enzymes appear to be a large fusion protein comprising TYW3, the C-terminal domain of TYW4, and the entire TYW2.
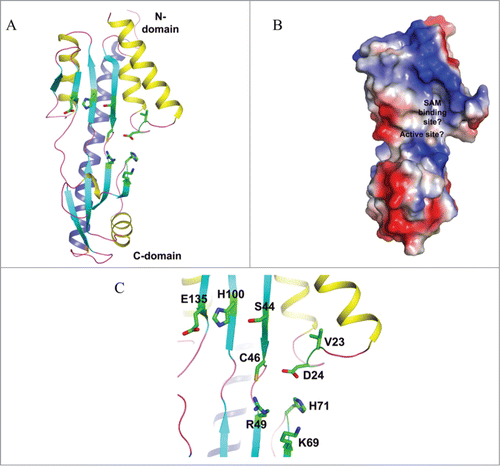
Curiously, the fourth step in yW biosynthesis is the only one in the pathway that escaped biochemical and enzymological investigations. However, 5 highly homologous crystal structures from Archaeoglobus fulgidus (PDB id: 2QG3), Sulfolobus solfataricus (PDB id: 1TLJ), Pyrococcus horikoshii (PDB ids: 2DRV, 2IT3, and 2IT2), have recently been deposited in Protein Data Bank (). According to PFAM (http://pid. nci. nih. gov/2011/110913/full/pid. 2011. Three. shtml), all these apo structures are annotated as TYW3-like methyltransferases. The crystal structures show that all of these proteins form head-to-head dimers in each crystal lattice. Whether the biological unit of these proteins is monomer or dimer remains to be investigated, and in the absence of a SAM-bound structure, we can only speculate the probable SAM binding pocket and the active site of these proteins (). As shown in , the representative protein is composed of 2 domains, and the C-terminal domain contains a long C-terminal helix that traverses the entire length of 2 domains (). There is a cavity at the interface of 2 domains () where several invariant residues reside (). The strictly conserved nature of these residues () suggests that they probably play important roles in catalysis. From a mechanistic point of view, the methylation of N3 atom is reminiscent of the reaction catalyzed by Trm5. We therefore anticipate that TYW3 enzyme uses a similar mechanism for N3 methylation, which generates yW-58 ().
Figure 4. Crystal structure of yeast TYW4. (A) Apo structure of TYW4 (PDB id : 2ZWA). The three domains of TYW4 are colored and labeled. The N-terminal helices are shown as yellow. (B) Structure of TYW4 in complex with SAM (PDB id : 2ZW9). SAM is depicted as a stick model with black for carbon atoms. The N-terminal helix is shown as yellow. (C) The active site of TYW4 in which the side chains of Y229 and R88, and SAM are shown as stick models. The flexible loop of C-domain is shown in magenta and is labeled. The substrate binding site is labeled.
tRNA-methyl-methoxycarbonyltransferase TYW4
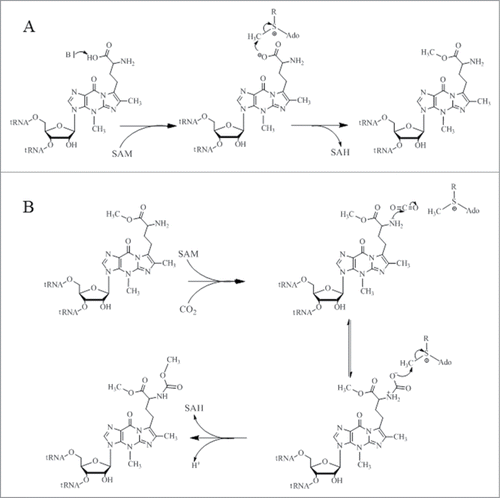
TYW4 catalyzes the final step in yW biosynthesis, i.e. the modification of the α-amino-α-carboxypropyl side chain attached by TYW2 to the C7 position of the tricyclic ring (see below) (). The gene encoding this enzyme (tyw4) has been identified by ribonucleome screening analysis in S. cerevisiae.Citation6 An in vitro biochemical analysis coupled to mass spectrometry showed that TYW4 is a bi-functional enzyme that catalyzes 2 reactions (i.e., methylation and methoxycarbonylation) of the α-amino-α-carboxypropyl side chain apparently within the same catalytic site.Citation22 Interestingly, the methoxycarbonylation reaction proceeds through the fixation of CO2, an oddity among all RNA modification mechanisms.
The apo and SAM-bound TYW4 structures have been obtained to resolution 2.5 Å and 2.7 Å, respectively. The overall structure of the enzyme consists of an N-terminal domain (1–350) and a C-terminal domain (371–694), connected by a linker (residues 351–370) (). The structures reveal that the N-terminal domain belongs to the class I SAM-dependent methyltransferases, while the C-terminal domain adopts a β-propeller fold, which has a flexible loop that resides near the yW-58 binding pocket. Upon SAM binding, the N-terminal α-helix undergoes a significant conformational change so as to efficiently shield the active site from the solvent ().Citation22
Figure 5. Crystal structure of a putative TYW3 from Archaeoglobus fulgidus. (A) Apo structure of TYW3 (PDB id : 2QG3). The secondary structural elements, β-strands, α-helices, and loops, are colored as cyan, yellow, and light-magenta. The C-terminal α-helix is colored as dark blue. The side chains of invariant residues are shown with stick models. (B) Electrostatic surface potential of TYW3. Blue, red, and white patches present the positively, negatively-charged, and neutral regions of the protein surface, respectively. The presumed SAM binding pocket and the active site are labeled. (C) The presumed SAM-binding and the active sites of TYW3. The side chains of strictly conserved residues are shown as stick models.
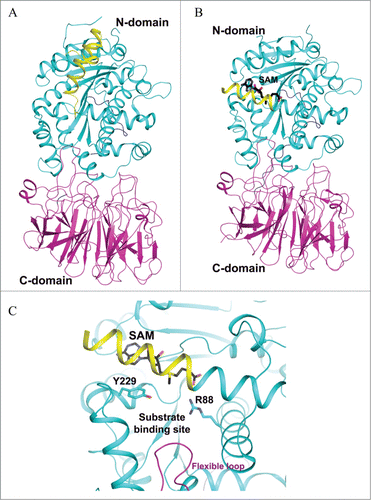
In the absence of a ternary structure of TYW4 in complex with SAM and a tRNA substrate, docking exercises for such a complex were performed with yW-58 as a substrate. In the resulting model, it was proposed that the α-amino-α-carboxypropyl of yW-58 adopts 2 different binding modes, which are responsible for the 2 reactions described above. For the methylation reaction, TYW4 is supposed to enhance the reaction rate by simply promoting favorable orientation and spatial proximity of the inherently nucleophilic oxygen atom of the α-carboxyl group toward the electrophilic methyl group of SAM. The amino acid residues Arg88 and Tyr229 have been proposed for this improved positioning ( and ). In contrast, for the methoxycarbonylation reaction, the hydroxyl group of Tyr229 is proposed to act as a general base catalyst to deprotonate the α-amino group of yW-58, while Arg88 would activate CO2 making it prone to be methylated by SAM ().
tRNA-aminocarboxypropyltransferase TYW2
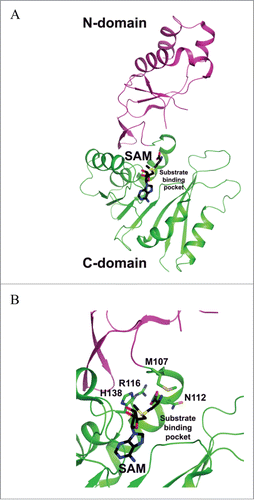
TYW2 catalyzes the transfer of the 3-amino-3-carboxypropyl group (acp) from SAM to the C7 position of the tricyclic core structure of imG-14 base, forming yW-72 (yW minus 72 Da) ().Citation6,23 Reactions in which acp-group is derived from SAM for substrate modification have also been reported for several biosynthetic pathways including diphthamide,Citation24 the antibiotic nocardicin,Citation25 diacylglyceryl-O-4′-(N, N, N, -trimethyl) homoserine,Citation26 and acp3U modified nucleoside in tRNAs.Citation27 Reaction mechanisms have yet to be delineated for the latter 3 biosyntheses. In the case of wybutosine, the detailed mechanism of acp-group transfer catalyzed by TYW2 is also poorly understood. However, it has been suggested that the acp-group transfer proceeds via an ionic mechanism based on crystal structures of the enzyme in complex with SAM and 5′-deoxy-5′-methylthioadenosine (MeSAdo) and by mutagenesis studiesCitation23 (). Thus, TYW2 likely transfers the acp-group through a single-displacement mechanism in which the inversion of the configuration of the methylene carbon occurs during the nucleophilic attack by C7 in the imG-14 base. Generally, nucleophilic attacks by aromatic carbon atoms are more demanding than those by polarized nitrogen and oxygen atoms. For example, it is well known that the SAM-dependent C5 methylation of pyrimidine by E. coli Fmu methyltransferase involves activation of the nucleo-base through covalent-bond formation between a conserved Cys-thiol of the enzyme and the C6 carbon of the pyrimidine substrate.Citation28 This covalent-bond formation increases the nucleophilicity of the C5 carbon and thus facilitates its alkylation. Although TYW2 catalyzes the alkylation of aromatic carbon atom, the published structure showed no conserved Cys residues near the catalytic site.Citation23 It was therefore suggested that the nucleophilicity of the C7 carbon atom might be increased via wyosine tautomerization.Citation29
Figure 7. Crystal structure of P. horikoshii TYW2. (A) SAM-complexed structure of TYW2 (PDB id : 3A25). The two domains of TYW2 are color coded and labeled. SAM and the substrate binding pocket are labeled. (B) The active site of TYW2 in which the side chains of M107, N112, R116, N112, and SAM are shown as stick models. SAM and the location of the substrate binding pocket are labeled.
A completely different mechanism for C-C bond on the aromatic ring has recently been unveiled during the investigation of diphtamide biosynthesis in the archaeon Pyrococcus horikoshii strain.Citation24 Indeed, structural and biochemical data showed that Dph2, the enzyme involved in the acp-group transfer from SAM to the histidine residue in the translation elongator factor 2 (eEF2) is a SAM-dependent, [4Fe-4S]-containing enzyme.Citation24 The reduced [4Fe–4S]+ cluster provides one electron to reductively cleave the Cγ, Met–S bond of SAM and generate a 3-amino-3-carboxypropyl radical along with methylthioadenosine.Citation24 The acp radical was then suggested to attack the histidine residue in the translation elongation factor 2 (eEF2).Citation24 Despite the fact that acp-group transfers from SAM are very similar in both Dph2 and TYW2, it is intriguing that these enzymes use completely different strategies to achieve alkylation of an aromatic ring.
tRNA-4-demethylwyosine synthase TYW1
The second step in yW biosynthesis is chemically the most challenging reaction: the imidazoline ring of yW is built on the m1G37 backbone of tRNA. This suggests that in addition to the tRNA substrate and SAM co-substrate, a second co-substrate, which is a source of the inserted 2-carbon unit, is also involved in the reaction.Citation6 TYW1, the enzyme that catalyzes the transformation of m1G37 into ImG-14 has previously been classified as a Radical-SAM enzyme due to the presence of a conserved CysxxxCysxxCys motif in its aminoacid sequenceCitation6 In 2007, 2 apo structures of TYW1 from Methanococcus jannaschii and Pyrococcus horikoshii have been reportedCitation30,31 (). At that time, no in vitro assay was available, as the identity of the second co-substrate was unknown. In 2011, with the discovery that pyruvate is the 2-carbon donor for the reaction, the investigation of the mechanism was made possible.Citation32 More recently, important insights into the mechanisms of TYW1 have been obtained.Citation33 First, reconstituted TYW1 was shown to contain 2 oxygen-sensitive [4Fe−4S]Citation2+/+ clusters, each ligated by only 3 cysteine residues that are absolutely required for activity.Citation33 Second, the N-terminal [4Fe-4S] cluster (cluster II), bound to the polypeptide by a CysX12CysX12Cys motif, contains a non-cysteinyl iron that is capable to bind and activate the pyruvate co-substrate.Citation33 Third, using a tRNAPhe transcript from S. cerevisiae, which was labeled with (13C2H3)-methyl-SAM on m1G in a Trm5-dependent reaction, m1G was shown to be the true substrate of the initial Ado• reaction that leads to the formation of imG-14 (T. Molle et al., to be published). These findings place substantial constraints on the mechanism by which the imidazoline ring is formed, as depicted by a working model in Scheme 4.
Figure 8. Crystal structures of TYW1. (A) Apo structure of archaeal TYW1 (PDB id : 2Z2U). The side chains of invariant cysteine residues are depicted as stick models and are labeled. (B) Apo structure of P. horikoshii TYW1 (PDB id : 2YX0). The side chains of invariant cysteine residues are depicted as stick models and are labeled.
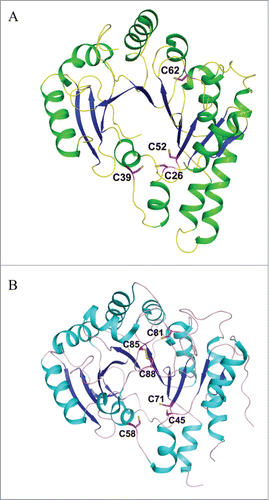
Figure 9. Proposed catalytic reaction mechanism of holo-TYW1. Radical-SAM cluster I and cluster II are sketched as cubes, non-cysteinyl irons are shown in blue (cluster I) and red (cluster II), (A): SAM and pyruvate binding to cluster I and II respectively (reaction 1). Reduction of cluster I (reaction 2). Reductive cleavage of SAM in presence of m1G37-tRNA.
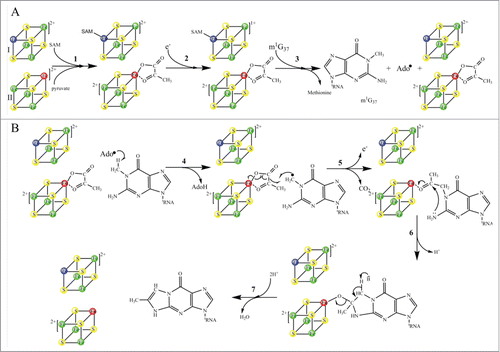
In this model, the reaction starts with the binding of SAM to cluster I and pyruvate to cluster II (Scheme 4A, reaction 1). In presence of electrons, cluster I-SAM complex is reduced and cluster II-pyruvate complex remains oxidized (Scheme 4A, reaction 2). When the substrate m1G37-tRNAs is added to the reaction mixture, the reductive cleavage of SAM occurs, which generates the canonical Ado• radical ( reaction 3) with concomitant release of methionine. In the following step, Ado• initiates H-atom abstraction from the methyl group of m1G37-tRNA (, reaction 4), which results in formation of the substrate radical, while pyruvate remains bound to oxidized cluster II. Addition of this substrate radical to the C2 of pyruvate initiates the homolytic cleavage of the C1–C2 bond and release of one electron and CO2 (, reaction 5). The last 2 steps of the mechanism consist in the formation of the tricyclic ring system through nucleophilic attack of the nitrogen atom on the C3 of the pyruvate and elimination of H2O (, reactions 6 and 7). Alternatively, reactions 5 and 6 of could be reversed, that is, the initial nucleophilic attack of the exocyclic G37 amino group on the cluster II-activated carbonyl is followed by radical coupling. A variant of this mechanism has been proposed in which pyruvate initially forms a Schiff base with a conserved and essential lysine (K41) upstream of the Radical-SAM cluster.Citation32,34 However, no evidence for such an adduct could be obtained either by UV-vis spectroscopy or by reductive trapping as commonly used for characterization of Schiff bases in PLP-containing enzymes.Citation35 On the contrary, preliminary Electron Paramagnetic Resonance (EPR) data obtained with the K41A mutant suggest that K41 is not at bonding distances of cluster II and rather plays an essential role in controlling the redox state of cluster II (T. Molle et al., to be published).
Several questions remain unsolved in this model, which are the focus of ongoing studies in our group. Two pressing questions to be addressed are: 1) What is the molecular basis of the interaction between cluster II and pyruvate; 2) How does cluster II control its redox potential. In this regard, parallel studies of another group of Radical-SAM enzymes involving radical insertion such as methylthiotransferases may help in deciphering the complex mechanism at work in TYW1.
Additional Pathways
Archaeal specific modifications : Involvement of aTrm5a and TYW3 enzymes
One recent report gives additional insights into the large variety of wyosine derivatives found in Archae.Citation36 Three families of methyltransferases that are homologous to Trm5 have been identified (aTrm5a-c) of which aTrm5a has the ability to methylate not only the genuine coded guanine, as found for Trm5, but also the imG-14 wyosine intermediate so as to give rise to isowyosine imG2 (7-methyl, imG-14). A classical methylation of the latter by TYW3 brings about 7-methylwyosine mimG (4,7-dimethyl, imG-14). How these enzymes mediate the transfer of a methyl group either to a nitrogen atom (Trm5) or a carbon (aTrm5a) awaits further biochemical and structural characterization.
tRNA-hydroxylase enzyme TYW5
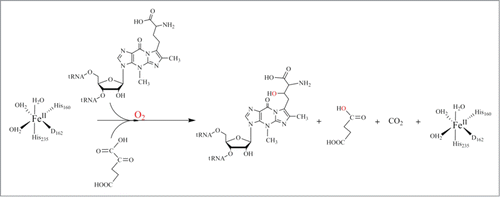
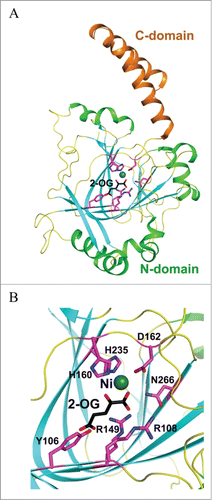
The hydroxywybutosine (OHyW), a hydroxyl derivative of yW, is one of the additional modifications found in eukaryotes including human. It has been reported that JumonjC-related enzyme (JmjC) catalyzes the hydroxylation of yW to hydroxywybutosine (OHyW).Citation37 The JmjC-domain-containing protein, TYW5 (tRNA-yW-synthesising enzyme 5), catalyzes the formation of OHyW nucleoside, using Fe(II) ion and 2-oxoglutarate as cofactors. The crystal structure of human TYW5(hTYW5), alone and in complex with 2-oxoglutarate and Ni(II) ion, as an inactive substitute for Fe(II), have recently been reported to resolution 2.5 Å and 2.8 Å respectively Citation38 (). The structures reveal that the catalytic domain consists of a β-jellyroll fold, a hallmark of the JmjC domain and other Fe(II)/2-oxoglutarateoxygenases.Citation38 The active site is located at the center of the β-jellyroll fold and consists of a highly conserved His-X-(Asp/Glu)-Xn-His signature motif.Citation39 These conserved residues provide 3 chemical groups to chelate the Fe(II) ion, while 2 additional chelating groups are supplied by the C1 carboxylate and C2 ketone groups of 2-oxoglutarate cofactor. In all these enzymes, the oxidative decomposition of 2-oxoglutarate to CO2 and succinate leads to formation of a highly active Fe(IV)-oxo species that has a great potential for substrate hydroxylation (Scheme 5).
Figure 11. Crystal structures of human TYW5. (A) Structure of hTYW5 in complex with Ni2+ and 2-oxoglutarate (PDB id : 3AL6). The two domains are depicted with different colors. Ni ion is shown as a dark green sphere, whereas 2-oxoglutarate (2-OG) and side chains of residues in the active site are shown with stick models. (B) The active site of TYW5. The side chains of invariant residues in the active site, Ni ion, and 2-OG are depicted as stick models and are labeled.
Conclusion
In 2004, we published a review article highlighting the amazing biological versatilities of SAM contrasting with its main known chemical use as a methylating reactant.Citation12 Indeed, in the cellular context, SAM is used as a source of methylene groups (in the synthesis of cyclopropyl fatty acids,Citation40 and rRNA modificationCitation41), amino groups (in the synthesis of 7, 8-diaminoperlagonic acid, a precursor of biotinCitation42), ribosyl groups (in the synthesis of epoxyqueuosine, a modified nucleoside in tRNAsCitation43,44), aminopropyl groups (in the synthesis of ethylene and polyaminesCitation45), and 3-amino-3-carboxypropyl group (in the synthesis of acp3U, a modified nucleoside in tRNA Citation46 and in the biosynthetic pathway of diphthamide Citation24). It is remarkable that this unique situation is mirrored in one biosynthetic pathway, that of yW, which is described in the present review article. Indeed, in the 5 enzymatic reactions leading to yW, SAM is utilized as a source, of methyl group by Trm5, TYW3, and TYW4 (), of α-amino-α-carboxypropyl group by TYW2 (), and of 5′-deoxyadenosine radical by TYW1 that critically plays in initiating complex radical-mediated transformations (). Recent reports on Radical-SAM enzymes suggest further unexpected usage of SAM, for example, as a carbide donor in maturation of nitrogenase.Citation47
As shown in this review, the mechanistic and structural enzymology of tRNA modifications can be considered a well investigated domain, as the research in this field has been occurring at an unprecedented rate. Recent published work on complex biosynthetic pathways leading to queuosine,Citation48 methylthioadenosine Citation49 in tRNA and methyladenosine in rRNA Citation41 suggest that we are only at the beginning of an exciting story.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by ANR Blanc-2010 grant INSERAD to PP-L, and Région Rhône-Alpes grants CIBLE 2011–2014 to TM. NIH grant 2U54GM75026 to the Northeast Structural Genomics Consortium.
References
- Machnicka MA, Milanowska K, Oglou OO, Purta E, Kurkowska, M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, et al. MODOMICS: a database of RNA modification pathways-2013 update. Nucleic Acids Res 2013; 41, D262-7; PMID:23118484; http://dx.doi.org/10.1093/nar/gks1007
- Agris PF, Vendeix FAP, Graham WD tRNA's wobble decoding of the genome: 40 years of modification. J Mol Biol; 2007 366, 1-13; PMID:17187822; http://dx.doi.org/10.1016/j.jmb.2006.11.046
- Atta M, Fontecave M, Mulliez E.2009 In Grosjean H (ed.), DNA and RNA modification enzymes: structure mechanism, function and evolution. Landes Bioscience, Austin, pp. pp. 347-57.
- Iwata-Reuyl,D An embarrassment of riches: the enzymology of RNA modification. Curr Opin Chem Biol2008; 12, 126-33; PMID:18294973; http://dx.doi.org/10.1016/j.cbpa.2008.01.041
- Thiebe R, Poralla K, Origin of nucleoside-Y in yeast transfer rnaphe. FEBS Lett1973; 38, 27-8; PMID:4589554; http://dx.doi.org/10.1016/0014-5793(73)80504-6
- Noma A, Kirino Y, Ikeuchi Y, Suzuki T Biosynthesis of wybutosine, a hyper-modified nucleoside in eukaryotic phenylalanine tRNA. EMBO J2006; 25, 2142-54; PMID:16642040; http://dx.doi.org/10.1038/sj.emboj.7601105
- Urbonavicius J, Droogmans L, Armengaud J Grosjean H.2009 In Grosjean H, e. (ed.), DNA and RNA Modification Enzymes: Structure,Mechanism, Function and Evolution. Landes Biosci., Austin, TX:, pp. pp. 423-35.
- de Crecy-Lagard V, Brochier-Armanet C, Urbonavicius J, Fernandez B, Phillips G, Lyons B, Noma A, Alvarez S, Droogmans L, Armengaud J, et al. Biosynthesis of wyosine derivatives in tRNA: an ancient and highly diverse pathway in archaea. Mol Biol Evol 2010; 27:2062-77; PMID:20382657; http://dx.doi.org/10.1093/molbev/msq096
- Hori H Methylated nucleosides in tRNA and tRNA methyltransferases. Front Genet 2014; 5:144; PMID:24904644; http://dx.doi.org/10.3389/fgene.2014.00144
- Watanabe K, Nureki O, Fukai S, Ishii R, Okamoto H, Yokoyama S, Endo Y, Hori H Roles of conserved amino acid sequence motifs in the SpoU (TrmH) RNA methyltransferase family. J Biol Chem 2005; 280:10368-77; PMID:15637073; http://dx.doi.org/10.1074/jbc.M411209200
- Hou YM, Perona JJ. Stereochemical mechanisms of tRNA methyltransferases. FEBS Lett 2010; 584:278-86; PMID:19944101; http://dx.doi.org/10.1016/j.febslet.2009.11.075
- Fontecave M, Atta M, Mulliez E. S-adenosylmethionine: nothing goes to waste. Trends Biochem Sci 2004; 29:243-9; PMID:15130560; http://dx.doi.org/10.1016/j.tibs.2004.03.007
- Cantoni GL. Biological Methylation - Selected Aspects. Annu Rev Biochem 1975; 44:435-51; PMID:1094914; http://dx.doi.org/10.1146/annurev.bi.44.070175.002251
- Markham GD, Hafner EW, Tabor CW, Tabor H. S-Adenosylmethionine synthetase from escherichia-coli. J Biol Chem 1980; 255:9082-92; PMID:6251075
- Motorin Y, Helm M. RNA nucleotide methylation. Wiley Interdiscip Rev-RNA 2011; 2:611-31; PMID:21823225; http://dx.doi.org/10.1002/wrna.79
- Christian T, Hou Y-M. Distinct Determinants of tRNA recognition by the TrmD and Trm5 Methyl transferases. J Mol Biol 2007; 373:623-32; PMID:17868690; http://dx.doi.org/10.1016/j.jmb.2007.08.010
- Christian T, Gamper H, Hou YM. Conservation of structure and mechanism by Trm5 enzymes. RNA 2013; 19:1192-9; PMID:23887145; http://dx.doi.org/10.1261/rna.039503.113
- Goto-Ito S, Ito T, Kuratani M, Bessho Y, Yokoyama S Tertiary structure checkpoint at anticodon loop modification in tRNA functional maturation. Nat Struct Mol Biol 2009; 16:1109-15; PMID:19749755; http://dx.doi.org/10.1038/nsmb.1653
- Goto-Ito S, Ito T, Ishii R, Muto Y, Bessho Y, Yokoyama S Crystal Structure of archaeal tRNA(m1G37)methyltransferase aTrm5. Proteins 2008; 72:1274-89; PMID:18384044; http://dx.doi.org/10.1002/prot.22019
- Chimnaronk S, Forouhar F, Sakai J, Yao M, Tron CM, Atta M, Fontecave M, Hunt JF, Tanaka I. Snapshots of dynamics in synthesizing N-6-isopentenyladenosine at the tRNA anticodon. Biochemistry 2009; 48:5057-65; PMID:19435325; http://dx.doi.org/10.1021/bi900337d
- Christian T, Lahoud G, Liu CP, Hoffmann K, Perona JJ, Hou YM. Mechanism of N-methylation by the tRNA m(1)G37 methyltransferase Trm5. RNA 2010; 16:2484-92; PMID:20980671; http://dx.doi.org/10.1261/rna.2376210
- Suzuki Y, Noma A, Suzuki T, Ishitani R, Nureki O. Structural basis of tRNA modification with CO2 fixation and methylation by wybutosine synthesizing enzyme TYW4. Nucleic Acids Res 2009; 37:2910-25; PMID:19287006; http://dx.doi.org/10.1093/nar/gkp158
- Umitsu M, Nishimasu H, Noma A, Suzuki T, Ishitani R, Nureki O Structural basis of AdoMet-dependent aminocarboxypropyl transfer reaction catalyzed by tRNA-wybutosine synthesizing enzyme, TYW2. Proc Natl Acad Sci U S A 2009; 106:15616-21; PMID:19717466; http://dx.doi.org/10.1073/pnas.0905270106
- Zhang Y, Zhu XL, Torelli AT, Lee M, Dzikovski B, Koralewski RM, Wang E, Freed J, Krebs C, Ealick SE, et al. Diphthamide biosynthesis requires an organic radical generated by an iron-sulphur enzyme. Nature 2010; 465:891-U894; PMID:20559380; http://dx.doi.org/10.1038/nature09138
- Reeve AM, Breazeale SD, Townsend CA Purification, characterization, and cloning of an S-adenosylmethionine-dependent 3-amino-3-carboxypropyltransferase in nocardicin biosynthesis. J Biol Chem 1998; 273:30695-703; PMID:9804844; http://dx.doi.org/10.1074/jbc.273.46.30695
- Klug RM, Benning C. Two enzymes of diacylglyceryl-0-4 '-(N,N,N,-trimethyl)homoserine biosynthesis are encoded by btaA and btaB in the purple bacterium Rhodobacter sphaeroides. Proc Natl Acad Sci U S A 1998; 98:5910-15; PMID:11331765; http://dx.doi.org/10.1073/pnas.101037998
- Ohashi Z, Maeda M, MccloskeJa, Nishimur S. 3-(3-Amino-3-carboxypropyl)uridine - novel modified nucleoside isolated from escherichia-coli phenylalanine transfer ribonucleic-acid. Biochemistry 1974; 13:2620-25; PMID:4598734; http://dx.doi.org/10.1021/bi00709a023
- Foster PG, Nunes CR, Greene P, Moustakas D, Stroud RM. The first structure of an RNA m(5)C methyltransferase, Fmu, provides insight into catalytic mechanism and specific binding of RNA substrate. Structure 2003; 11:1609-20; PMID:14656444; http://dx.doi.org/10.1016/j.str.2003.10.014
- Itaya T, Mizutani A, Takeda M; Shioyama C. Studies Towards the synthesis of the fluorescent bases of phenylalanine transfer ribonucleic-acids - synthesis of 7-methylwye isolated from extremely thermophilic archaebacteria. Chem Pharm Bull (Tokyo) 1989; 37:284-91; PMID:2472901; http://dx.doi.org/10.1248/cpb.37.284
- Goto-Ito S, Ishii R, Ito T, Shibata R, Fusatomi E, Sekine S, Bessho Y, Yokoyama S. Structure of an archaeal TYW1, the enzyme catalyzing the second step of wye-base biosynthesis. Acta Crystallogr D-Biol Crystallogr 2007; 63:1059-68; PMID:17881823; http://dx.doi.org/10.1107/S0907444907040668
- Suzuki Y, Noma A, Suzuki T, Senda M, Senda T, Ishitani R, Nureki O. Crystal structure of the radical SAM enzyme catalyzing tricyclic modified base formation in tRNA. J Mol Biol 2007; 372:1204-14; PMID:17727881; http://dx.doi.org/10.1016/j.jmb.2007.07.024
- Young AP, Bandarian V. Pyruvate is the source of the two carbons that are required for formation of the imidazoline ring of 4-demethylwyosine. Biochemistry 2011; 50:10573-5; PMID:22026549; http://dx.doi.org/10.1021/bi2015053
- Perche-Letuvee P, Kathirvelu V, Berggren G, Clemancey M, Latour JM, Maurel V, Douki T, Armengaud J, Mulliez E, Fontecave M, et al. 4-Demethylwyosine Synthase from Pyrococcus abyssi Is a Radical-S-adenosyl-L-methionine Enzyme with an Additional [4Fe-4S](+2) Cluster That Interacts with the Pyruvate Co-substrate. J Biol Chem 2012; 287:41174-85; PMID:23043105; http://dx.doi.org/10.1074/jbc.M112.405019
- Young AP, Bandarian V Radical mediated ring formation in the biosynthesis of the hypermodified tRNA base wybutosine. Curr Opin Chem Biol 2013; 17:613-8; PMID:23856057; http://dx.doi.org/10.1016/j.cbpa.2013.05.035
- Tang KH, Harms A, Frey PA. Identification of a novel pyridoxal 5'-phosphate binding site in adenosylcobalamin-dependent lysine 5,6-aminomutase from Porphyromonas gingivalis. Biochemistry 2002; 41:8767-76; PMID:12093296; http://dx.doi.org/10.1021/bi020255k
- Urbonavicius J, Meskys R, Grosjean H. Biosynthesis of wyosine derivatives in tRNA(Phe) of Archaea: role of a remarkable bifunctional tRNA(Phe):m1G/imG2 methyltransferase. Rna 2014; 20:747-53; PMID:24837075; http://dx.doi.org/10.1261/rna.043315.113
- Noma A, Ishitani R, Kato M, Nagao A, Nureki O, Suzuki T. Expanding Role of the Jumonji C Domain as an RNA Hydroxylase. J Biol Chem 2010; 285:34503-7; PMID:20739293; http://dx.doi.org/10.1074/jbc.M110.156398
- Kato M, Araiso Y, Noma A, Nagao A, Suzuki T, Ishitani R, Nureki O Crystal structure of a novel JmjC-domain-containing protein, TYW5, involved in tRNA modification. Nucleic Acids Res 2011; 39:1576-85; PMID:20972222; http://dx.doi.org/10.1093/nar/gkq919
- Hausinger RP. FeII/alpha-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol 2004; 39:21-68; PMID:15121720; http://dx.doi.org/10.1080/10409230490440541
- Taylor FR, Cronan JE Jr. Cyclopropane fatty acid synthase of Escherichia coli. Stabilization, purification, and interaction with phospholipid vesicles. Biochemistry 1979; 18:3292-300; PMID:380648; http://dx.doi.org/10.1021/bi00582a015
- Grove TL, Benner JS, Radle MI, Ahlum JH, Landgraf BJ, Krebs C, Booker SJ. A radically different mechanism for S-adenosylmethionine-dependent methyltransferases. Science 2011; 332:604-07; PMID:21415317; http://dx.doi.org/10.1126/science.1200877
- Stoner GL, Eisenberg MA. Biosynthesis of 7, 8-diaminopelargonic acid from 7-keto-8-aminopelargonic acid and S-adenosyl-L-methionine. The kinetics of the reaction. J Biol Chem 1975; 250:4037-43; PMID:1092682
- Iwata-Reuyl D. Biosynthesis of the 7-deazaguanosine hypermodified nucleosides of transfer RNA. Bioorg Chem 2003; 31:24-43; PMID:12697167; http://dx.doi.org/10.1016/S0045-2068(02)00513-8
- Van Lanen SG, Kinzie SD, Matthieu S, Link T, Culp J, Iwata-Reuyl D. tRNA modification by S-adenosylmethionine : tRNA ribosyltransferase-isomerase - Assay development and characterization of the recombinant enzyme. J Biol Chem 2003; 278:10491-9; PMID:12533518; http://dx.doi.org/10.1074/jbc.M207727200
- White MF, Vasquez J, Yang SF, Kirsch JF. Expression of apple 1-aminocyclopropane-1-carboxylate synthase in escherichia-coli - kinetic characterization of wild-type and active-site mutant forms. Proc Natl Acad Sci U S A 1994; 91:12428-32; PMID:7809054; http://dx.doi.org/10.1073/pnas.91.26.12428
- Nishimura S, Taya Y, Kuchino Y, Oashi Z. Enzymatic synthesis of 3-(3-amino-3-carboxypropyl)uridine in Escherichia coli phenylalanine transfer RNA: transfer of the 3-amino-acid-3-carboxypropyl group from S-adenosylmethionine. Biochem Biophys Res Commun 1974; 57:702-08; PMID:4597321; http://dx.doi.org/10.1016/0006-291X(74)90603-2
- Wiig JA, Hu YL, Lee CC, Ribbe MW. Radical SAM-dependent carbon insertion into the nitrogenase M-cluster. Science 2012; 337:1672-5; PMID:23019652; http://dx.doi.org/10.1126/science.1224603
- McCarty RM, Krebs C, BandarianV. Spectroscopic, steady-state kinetic, and mechanistic characterization of the radical SAM enzyme QueE, which catalyzes a complex cyclization reaction in the biosynthesis of 7-deazapurines. Biochemistry 2013; 52:188-98; PMID:23194065; http://dx.doi.org/10.1021/bi301156w
- Forouhar F, Arragain S, Atta M, Gambarelli S, Mouesca JM, Hussain M, Xiao R, Kieffer-Jaquinod S, Seetharaman J, Acton TB, et al. Two Fe-S clusters catalyze sulfur insertion by radical-SAM methylthiotransferases. Nat Chem Biol 2013; 9:333-8; PMID:23542644; http://dx.doi.org/10.1038/nchem-bio.1229