Abstract
There is considerable current interest in the function of epigenetic mechanisms in neuroplasticity with regard to learning and memory formation and to a range of neural diseases. Previously, we described replication timing on human chromosome 21q in the THP-1 human cell line (2n = 46, XY) and showed that several genes associated with neural diseases, such as the neuronal glutamate receptor subunit GluR-5 (GRIK1) and amyloid precursor protein (APP), were located in regions where replication timing transitioned from early to late S phase. Here, we compared replication timing of all known human glutamate receptor genes (26 genes in total) and APP in 6 different human cell lines including human neuron-related cell lines. Replication timings were obtained by integrating our previously reported data with new data generated here and information from the online database ReplicationDomain. We found that many of the glutamate receptor genes were clearly located in replication timing transition zones in neural precursor cells, but this relationship was less clear in embryonic stem cells before neural differentiation; in the latter, the genes were often located in later replication timing zones that displayed DNA hypermethylation. Analysis of selected large glutamate receptor genes (>200 kb), and of APP, showed that their precise replication timing patterns differed among the cell lines. We propose that the transition zones of DNA replication timing are altered by epigenetic mechanisms, and that these changes may affect the neuroplasticity that is important to memory and learning, and may also have a role in the development of neural diseases.
Introduction
Considerable research effort is currently being expended on elucidating the genetic and epigenetic bases of both neural diseases and neuroplasticity associated with learning and memory formation. Glutamate receptors, the synaptic receptors primarily located on membranes of neuronal cells, are of particular interest in this regard as their malfunction has been reported in a number of neurological conditions.Citation1-3 Activation of the receptors is required for basal excitatory synaptic transmission and also for many forms of synaptic plasticity such as long-term potentiation and long-term depression.Citation1-3 These mechanisms are believed to underlie learning and memory. Glutamate receptors also appear to have a central role in excitotoxicity and to be important to the development of many neurodegenerative diseases.Citation1-4
Several genes associated with neural diseases are located on human chromosome 21 such as the neuronal glutamate receptor subunit GluR-5 (GRIK1) that is related to juvenile absence epilepsy, and the amyloid precursor protein (APP) that is implicated in familial Alzheimer disease. APP is expressed in the synapses of neurons and has been suggested to be responsible for forming and repairing synapses.Citation5 In previous studies, we determined replication timing along human chromosome 21q at the sequence level in the acute monocytic leukemia cell line, THP-1 (2n = 46, XY).Citation6 We found that chromosomal regions showing switches in replication timing from early to late S phase (so-called transition regions) contained several disease-related genes including oncogenes and neural disease genes such as GRIK1 and APP.Citation6,7 These transition regions also appeared to coincide with genomically “unstable” regions that show an increased risk of damage due to DNA amplification or chromosome rearrangements.Citation6,Citation8-11
In mammalian cells, DNA replication proceeds in a precisely coordinated manner in which megabase-sized clusters of replicons are activated at distinct times during S phase.Citation6,9,10,12,13 The majority of the replicons are in the range of 50–450 kb. Up to 10 (or occasionally more) contiguous replicons may fire synchronously to form a megabase-sized domain that can be visualized cytogenetically by the replication banding method.Citation14-16 The pattern of replication bands along a chromosome arm is essentially equivalent to the G- or R-banding patterns. G bands are composed primarily of AT-rich sequences and correspond to late replicating zones (heterochromatin); T bands (a subgroup of R bands) are composed of GC-rich sequences and correspond to zones that replicate very early (euchromatin)Citation14-22; ordinary R bands are composed of both GC- and AT-rich sequences and replicate early (euchromatin).Citation6,Citation20-22
In the present study, we compared the replication timing of glutamate receptor genes and of large genes on chromosome 21q that are associated with neural diseases. Our earlier study used a single cell line; in the present work, we have extended this by analyzing other human cell lines of different origin and by integrating our previously reported dataCitation6-8,Citation23 with newly generated data and information from the online database ReplicationDomain.Citation24
Results
Replication timing of chromosome 21q in human cell lines
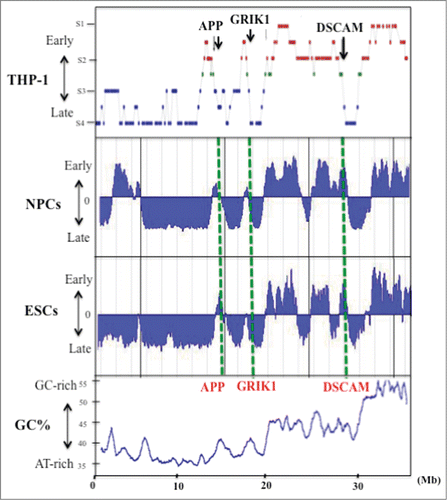
In our earlier study of chromosome 21q replication in THP-1 cells (a monocytic leukemia cell line; 2n = 46, XY), we quantified the level of newly replicated DNA at various loci by a PCR-based method.Citation6,25 Our analyses indicated that several neural disease genes located on human chromosome 21q and associated with Down syndrome, such as GRIK1 and APP, are located in regions where replication timing transitions from early to late S phase in THP-1 cells.Citation6,7 To determine whether this pattern of replication timing was also present in other cell types, we examined chromosome 21q in neural precursor cells (NPCs) and embryonic stem cells (ESCs) using information obtained from the online database ReplicationDomain that stores data on DNA replication timings (http://www.replicationdomain.com; ref. Twenty-four). The GC% distribution of human 21q is shown in along with replication timings in THP-1 cells, NPCs and ESCs (the NPCs and ESCs were derived from the BG01 cell line; and the NPCs had undergone neural differentiation from ESCs. Average replication timing data for each cell line is shown here.Citation24 We found that these cell lines showed essentially the same replication timing patterns for human 21q (). In particular, we found that genomic regions that covered a few to several megabases replicated at identical or similar times during S phase in these cell lines (). This observation provided molecular confirmation of earlier cytogenetic reports that early or late replicons cluster into megabase-sized domains in which multiple origins fire fairly synchronously at a specific time during S phase.Citation6,9,Citation12-16 In general, replication timing and GC% level were correlated on chromosome 21q in the 3 cell lines analyzed (). Early replicating zones were more GC-rich than late replicating zones; this was especially evident between the adjacent early and late replication zones. The switch from early to late replication occurred at positions identical to or near GC% transitions. This concordance between transitions in replication timing and GC% level is consistent with the predicted molecular characteristics for chromosome band boundaries, which correspond to the boundaries between euchromatin and heterochromatin ().Citation6,10,11,Citation26-29
Figure 1. GC% distribution and DNA replication timing of human chromosome 21q in the THP-1 cell line, and in NPCs and ESCs. Replication timing in chromosome 21q in THP-1 cells (data from Watanabe et al. 2002), NPCs and ESCs. The latter data were obtained by analysis of information held on ReplicationDomain (http://www.replicationdomain.com). The “y” axis on each graph indicates the estimated numerical value for DNA replication timing obtained from ReplicationDomain. The value “0” indicates medium replication timing. The upper horizontal line and the bottom horizontal line of each graph indicate the value of 2.4 (very early replication timing) and -2.4 (very late replication timing), respectively. The “x” axis indicates genomic position of the gene. The positions of 3 genes (APP, GRIK1 and DSCAM) are indicated by the green dotted lines. The bottom panel shows GC% distribution along chromosome 21q.
Replication timing of 3 large genes on chromosome 21q
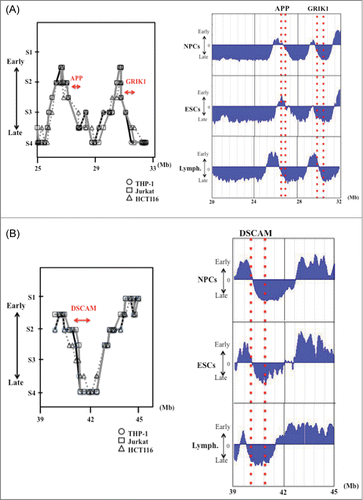
We next focused on replication timing patterns of APP (290 kb), GRIK1 (402 kb) and DSCAM (836 kb), 3 large genes on 21q that are associated with Down syndrome/familial Alzheimer disease. Six cell lines were compared: NPCs and ESCs, lymphoblastoid (GM06990), THP-1, Jurkat (human prototypical CD28+ T leukemia cells) and HCT116 (human colorectal cancer cells). The detailed average replication timings for NPCs, ESCs and lymphoblastoid cells were obtained from the online database ReplicationDomain. Replication timings in the THP-1, Jurkat and HCT116 cell lines were determined as described in the “Materials and Methods” (, Supplemental Tables 1 and 2). APP and DSCAM were located in the boundary zones for DNA replication timing switches in all cell lines (, Supplemental Table 2). However, although GRIK1 was clearly located in a DNA replication boundary zone in NPCs, it was located in a later replication timing zone in ESCs (, Supplemental Table 2).
Figure 2. DNA replication timing for genomic regions in and around the large genes APP and GRIK1 (A) and DSCAM (B) in THP-1, Jurkat and HCT116 cells, and in NPCs, ESCs and lymphoblastoid cells. Replication timing in THP-1, Jurkat and HCT116 cells were determined as described in the “Materials and Methods.” The “x” axis of each graph indicates the genomic position of the gene as given by the Ensembl Genome Brower (www.ensembl.org/). The position and the range of each gene are indicated by the red arrows. The PCR primer sets used in the present study and the replication timings are listed in Supplemental Table 1. Replication timings in NPCs, ESCs and lymphoblastoid cells were obtained by analysis of information held on ReplicationDomain (http://www.replication-domain.com). The “y” axis on each graph indicates the estimated numerical value for DNA replication timing obtained from ReplicationDomain. The value “0” indicates medium replication timing. The upper horizontal line and the bottom horizontal line of each graph indicate the value of 2.4 (very early replication timing) and -2.4 (very late replication timing), respectively. The “x” axis of each graph indicates the genomic position of the gene, which was obtained from ReplicationDomain. The position and range of each gene in NPCs, ESCs and lymphoblastoid cells are indicated by the dotted red lines. “Lymph.” indicates lymphoblastoid cells.
Replication timing of human glutamate receptor genes in relation to boundary zones
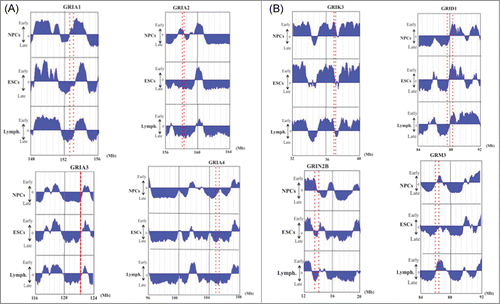
As described above, the glutamate receptor gene GRIK1 was located in a replication timing boundary zone in NPCs but in a later replication timing zone in ESCs (, Supplemental Table 2). This variation in the GRIK1 chromosomal milieu led us to speculate that it may be linked to the genetic/epigenetic basis of neuroplastic changes required for learning and memory formation as well as in the development of various neural diseases.Citation1-4,Citation30 To determine whether other members of the glutamate receptor gene family behave similarly, we examined the replication timing of all neuronal glutamate receptor genes (26 genes in total) in the human genome in NPCs, ESCs and lymphoblastoid cells (, ). We found that most of the genes were located in or near replication timing boundary zones in NPCs; however, in ESCs, the genes were often located in later replication timing zones or in late replication zones (). This effect is illustrated by the 4 genes encoding AMPA glutamate receptor, an ionotropic transmembrane receptor for glutamate that mediates fast synaptic transmission in the central nervous system (CNS). Analysis of their replication timings indicated that they were clearly located in transition zones in NPCs but not in ESCs (). Some large glutamate receptor genes (>200 kb) are also shown in . In total, we found that 23 of 26 glutamate receptor genes (88.46%) were located in or near replication timing transition zones in NPCs, whereas, only 18 genes (69.23%) were located in these zones in ESCs and 19 genes (73.08%) in lymphoblastoid cells (). Notably, many of these genes are large (>200 kb) ().
Table 1. DNA replication timing in NPCs, ESCs and lymphoblastoid cells and lengths of human neuronal glutamate receptor genes
Figure 3. DNA replication timings of selected neuronal glutamate receptor genes in NPCs, ESCs and lymphoblastoid cells. The position or the range (large genes) of each gene is indicated by a red dotted line or 2 dotted lines for large genes. (A) Replication timing of AMPA glutamate receptor family (GRIA1–4). (B) Examples of replication timing of other large (> 200 kb) glutamate receptor genes. The “y” axis on each graph indicates the estimated numerical value for DNA replication timing obtained from ReplicationDomain. Average replication timing data for each cell line is shown. The value “0” indicates medium replication timing. The upper horizontal line and the bottom horizontal line of each graph indicate the value of 2.4 (very early replication timing) and -2.4 (very late replication timing), respectively. “Lymph.” indicates lymphoblastoid cells.
Next, we focused on the relationship between replication timing and DNA methylation in the genomic regions where replication timing transitioned from early to late S phase in ESCs compared to NPCs. We analyzed DNA methylation changes in CpG islands located in the 5′-upstream regions of the glutamate receptor genes GRIA2, GRIA4 and GRIK1. These genes were clearly located in replication transition zones in NPCs but not in ESCs (). The methylation status of these 3 genes was examined using bisulfite DNA sequencing in 20 individual clones from ESCs and NPCs. This analysis showed the GRIK1 5′-upstream CpG island had 21.8% methylation in ESCs and 12.5% methylation in NPCs (). Likewise, both GRIA2 and GRIA4 had significantly lower rates of methylation in NPCs than ESCs (). These findings are consistent with a close correlation between late replication timing and DNA hypermethylation.
Table 2. DNA methylation of 5′-upstream genomic regions of GRIK1, GRIA2 and GRIA4 genes in NPCs and ESC
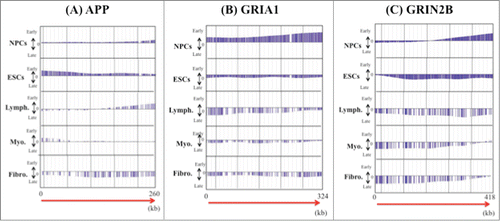
We also compared replication timing patterns in glutamate receptor genes and APP in 5 cell lines (). The genes varied in their precise replication timing patterns in these cell lines (). Interestingly, the relationship between the direction of transcription (5′ to 3′) and replication timing (early to late) in these genes varied among the cell lines ().
Figure 4. Examples of the precise replication timing patterns of large genes (>200 kb). (A) APP (260kb), (B) GRIA1 (324 kb) and (C) GRIN2B (418 kb). Replication timing profiles in 6 cell lines (NPCs and ESCs, lymphoblastoid, myoblast and fetal lung fibroblast) were obtained from the online database ReplicationDomain. The “y” axis on each graph indicates the estimated numerical value for DNA replication timing obtained from ReplicationDomain. The value “0” indicates medium replication timing. The upper horizontal line and the bottom horizontal line of each graph indicate the value of 2.4 (very early replication timing) and -2.4 (very late replication timing), respectively. The direction of transcription (5′ to 3′) in the genes is indicated by the red arrow. “Lymph.,” “Myo.” and “Fibro.” indicate lymphoblasts, myoblasts and fetal lung fibroblasts, respectively.
Discussion
In order to gain more insights into the molecular pathology of neural diseases such as Down syndrome and Alzheimer disease, and into neuroplastic changes associated with memory formation, we initiated a study of DNA replication timing of human chromosome 21q. To carry out a more precise analysis of replication timing, we FACS sorted cells at different cell cycle phases, namely, G1/S boundary, G2/M boundary, and various times in S phase, as well as BrdU labeling at several time-points (30 min, 90 min etc.) (data not shown). The results of this analysis confirmed those obtained by FACS sorting of cells into 4 fractions of S phase after BrdU labeling for 60 min. We found that 3 large genes associated with neural disease genes (APP, GRIK1 and DSCAM) were located in the boundary zones where DNA replication timing switched from early to late S phase. Interestingly, the glutamate receptor gene GRIK1 was clearly located in a replication transition zone in NPCs but not in ESCs where it was located in a later replication timing zone. A survey of the other glutamate receptor genes in the human genome showed that they too were concentrated in or near transition zones in NPCs but not in ESCs where they were often located in later replication timing zones or in late replication zones. It has been shown that early replicating zones tend to be formed of “looser” chromatin structures than late replicating zones.Citation9,11,12,14,15,18 Therefore, a change in relative chromatin compaction likely occurs within the transition regions for replication timing.
Cytogenetic analyses have shown that replication banding patterns and R- and G-banding patterns are tissue invariant.Citation6,Citation14-16 Additionally, the global correlation of replication timing with GC% level suggests that megabase-sized early or late replicating domains should be common in different cell types. However, despite the tissue related invariance of the megabase-sized banding patterns, replicon sized segments that harbor genes that are tissue specific in expression are known to replicate early in these tissues even when the chromosomal segment is located within a late replicating G-band zone.Citation7,14,15,31
Thus far, the mechanisms that control replication timing and their relationships to chromatin structure are unclear. Several recent studies have identified factors that regulate the timing of origin activation. It is generally accepted that in eukaryotes, the firing of DNA replication origins is highly coordinated during S phase, although the control that establishes genome-wide replication timing domains has not yet been elucidated. Yamazaki et al.Citation32 showed that Rif1 (Rap1-interacting-factor-1) is a vital modulator of the replication timing program in human cells. Rif1 colocalizes specifically with mid-S replication foci, and its depletion results in the loss of mid-S replication foci, highly perturbed replication timing, and changes in replication timing domains. In addition, Rif1 strongly interacts with nuclear-insoluble structures and modulates chromatin loop sizes. These findings indicate that Rif1 controls replication timing domain structures by regulation of higher-order chromatin structures.Citation32,33
Our analysis of large glutamate receptor genes and of APP indicated differences in replication timings between different cell lines, suggesting that the replication timing of genes located in or near transition zones are altered by epigenetic mechanisms (). The activation of glutamate receptors is responsible for basal excitatory synaptic transmission and for many forms of synaptic plasticity, such as long-term potentiation and long-term depression; these effects of glutamate receptor activation are thought to underlie learning and memory. We therefore propose that the transition zones of DNA replication timing, which might be altered by epigenetic mechanisms in response to environmental factors, are associated with the epigenetic molecular basis of neuroplastic changes, such as the synaptic plasticity involved in learning and memory formation, and neural diseases associated with mutation of glutamate receptor genes or APPCitation1-5,Citation30 ().
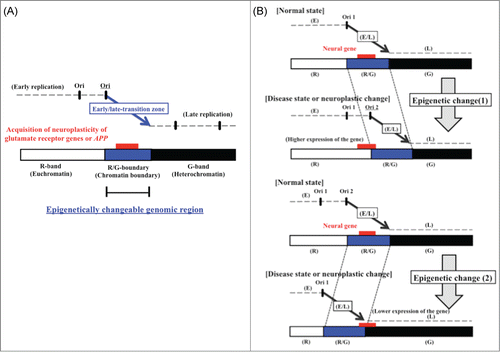
Figure 5. (A) Model of high-risk/high-return regions of the human genome located in transition regions of replication timing as the epigenetic molecular basis of neuroplastic changes. The model proposes that modifications of the chromatin structure during development of neural diseases (or neuroplastic changes) affect the timing of firing of replication origins. (B) Postulated changes in replication timing of a neural disease gene located in a transition zone. The panels illustrate how replication timing might switch from mid S phase to early or late S phase due to an increase (top panel) or decrease (bottom panel) in the number of active early replication origins at the edge of the early replication zone. Additionally, the chromatin environment of the neural disease gene might switch from that of an R/G chromosome band boundary to an R band or to a G band. Stalling of the replication fork in the vicinity of neural disease genes might also induce chromosomal amplifications (such as triplet repeat expansions) or chromosome rearrangements that affect gene function, possibly through influencing the rate of expression. The position of the neural disease gene (large gene) is indicated by the red rectangle. E, early replication zone; L, late replication zone; E/L, early/late-switch region; R, R band; G, G band; R/G, R/G band boundary; Ori, replication origin.
Some neural diseases in humans are associated with expansions in DNA repeats, such as fragile X syndrome which shows expansion of CGG repeats in the FMR1 gene during early development. Recently, Gerhardt et al.Citation34 examined replicated DNA at the FMR1 locus in wild type and fragile X syndrome ESCs and found that expanded repeats are instable because of the interaction of (CGG)n hairpin-like structures and the replication fork. The hairpins can cause the DNA polymerase to pause and slide off, and/or induce a change in direction of the replication fork which results in the CGG strand serving as the lagging strand template. Fork stalling and the bypassing of replication origins due to the expansion of repeats during embryogenesis may predispose to the development of neural disease.Citation34,35
In the present study, we analyzed the DNA methylation changes in the 5′-upstream CpG island regions of 3 glutamate receptor genes (GRIA2, GRIA4 and GRIK1). These genes were clearly located in the transition zones of early replication timing in NPCs but not in ESCs () and were more highly methylated in the NPCs than EPCs. Thus, the level of DNA methylation was correlated with replication timing. We also identified sequence motifs or similar sequences for the cell-type specific DNA binding protein SATB1 (Special AT-rich sequence Binding protein 1) in the GRIK1, GRIA2 and GRIA4 loci. SATB1 sequences have been reported to be involved in controlling the change in replication timing in and around transition regions.Citation36
Previously, we proposed a model for replication timing of genes located in the transition regions.Citation9-11 Here, we have modified this proposal to focus on epimutation of neural diseases as well as neuroplastic changes linked with memory formation. We propose that there is an interaction between chromatin conformation or replication timing and the expression of neural disease genes located in early/late-switch zones (). In our model, an increase (or decrease) in the number of early acting replication origins alters the timing of replication from mid S phase to early S phase (or mid S phase to late S phase) (). In addition, the chromatin environment of the neural disease gene would change from that of an R/G band boundary (the boundary between euchromatin and heterochromatin) to an R band (euchromatin) or to a G band (heterochromatin). The transition zone, which is shown by a thick arrow, is expected to be a large origin-free region between early- and late-replicating domains, which correspond to unstable genomic regions.Citation9,10,Citation37-39 Only the replication fork that starts at the edge of the early zone is predicted to be able to continue replicating over a period of hours or to pause at specific sites in the replication-transition region until it meets the fork initiated from the adjacent later-replicating zone. A pause during replication is known to increase the risk of DNA breaks and rearrangements.Citation9,10,11,Citation40-42 Therefore, the model also hypothesizes that a switch in replication timing increases the risk of replication errors, such as stalling of the replication fork, which might induce chromosomal amplifications (such as the triplet repeat expansions often identified in mutations of neural disease genes) and chromosomal rearrangements (such as translocations). Such de novo rearrangements might then adversely affect expression of closely positioned neural disease genes (). Overall, our model proposes an intimate interrelationship of replication timing, chromosome rearrangements and abnormal expression of neural disease genes. Finally, on the basis of our results, we propose that transition regions of replication timing correspond to the high-risk/high-return regions of human genome, which correlate with genomically unstable regions; these regions are important to the epigenetic mechanisms regulating neuroplastic changes, such as long-term potentiation and long-term depression. If our model proves accurate, then it also suggests that it might be feasible to detect neural diseases much earlier than currently by use of replication timing assays as part of an epigenetic investigation.Citation9-11
Materials and Methods
Cell culture, cell cycle fractionation and isolation of newly replicated DNA
THP-1 (2n = 46, XY; a human acute monocytic leukemia cell line), Jurkat (a human prototypical CD28+ T cell leukemia cell line) and HCT116 (human colorectal cancer cell line) cells were obtained from the Health Science Research Resources Bank (Tokyo, Japan).Citation43 BG01 (human ES cell line) cells were cultured according to the supplier's instructions (BresaGen, Athens, GA)Citation44 and were differentiated to NPCs as described previously.Citation45,46 These human cell lines were labeled for 60 min with 75 μM 5′-bromo-2′-deoxyuridine (BrdU; Roche, Basel, Switzerland). The BrdU labeling, cell cycle fractionation and isolation of newly replicated DNA were performed according to the methods described by Hansen et al.Citation47,48 but with minor modifications.Citation6-8,Citation25,28 The BrdU-labeled cells were washed with cold phosphate-buffered saline, fixed in 70% ethanol for 60 min, pelleted and resuspended in 70% ethanol at 4°C. Cells were then resuspended in a staining bufferCitation6,25 and incubated 30 min at room temperature. Cells were fractionated into 6 groups on the basis of cell cycle phase, G1, S1 through S4 and G2/M, with an EPICS Elite Cell Sorter (Beckman Coulter, Brea, CA). Equal numbers of cells (4 × 104) were collected in microfuge tubes containing lysis buffer and then incubated for 2 hr at 50°C. To provide controls for recovery of BrdU-labeled DNA, a mixture of [14C] thymidine-labeled (5 × 104 dpm), BrdU-labeled and [3H] deoxycytidine-labeled (5 × 104 dpm) Chinese hamster ovary cell (CHO) DNA was added to each fraction. DNA samples were purified by phenol/chloroform extraction and ethanol precipitation and dissolved in 460 μl TE containing sheared salmon testis DNA (0.5 mg/ml). The mixture was then sonicated into fragments with an average size of approximately 1 kb. These fragments were heat denatured for 3 min at 95°C and cooled on ice. After addition of 56 μl 10 × immunoprecipitation bufferCitation6,25 and 80 μl 25 μg/ml anti-BrdU mouse monoclonal antibody (Becton-Dickinson Immunocytometry, Franklin Lakes, NJ), the samples were incubated at room temperature for 20 min with constant rotation. Antibody-bound BrdU DNA was precipitated by addition of 15 μl 2.5 mg/ml anti-mouse IgG (Sigma-Aldrich, St. Louis, MO), and the mixture was incubated for 20 min at room temperature. After centrifugation, the pellet was washed with 1 × immunoprecipitation buffer, resuspended in 200 μl digestion bufferCitation6,25 and incubated overnight at 37°C. An additional 100 μl digestion buffer was added, followed by overnight incubation at 37°C. The sample was subjected to phenol/chloroform extraction, and after addition of 20 μg yeast tRNA, DNA was precipitated with ethanol and dissolved in TE. The recovery and purity level of the BrdU-DNA was checked by monitoring the [3H] and [14C] counts of the CHO DNA.Citation6,25,27
PCR-based quantification of replicated DNA
Quantitative PCR was used to examine the replication timing of individual loci.Citation6,25 Locus-specific primers were selected according to the following criterion: a single PCR product of the predicted size was amplified from THP-1 (or Jurkat, HCT116) genomic DNA but not from CHO DNA. The primer pair for a loci was added to a single tube containing a constant amount of BrdU-labeled DNA from each cell-cycle fraction, which had been calibrated on the basis of the [3H] count as described previously.Citation6,25,27 In addition to a locus-specific primer, each reaction contained a constant amount of plasmid pKF3 DNA and pKF3-specific primers for assessment of PCR efficiency.Citation6,25 The reaction buffer contained 0.5 U AmpliTaq Gold (Applied Biosystems, Foster City, CA) in 100 μl of the manufacturer's reaction buffer. Amplification conditions were one cycle of 95°C for 9 min to activate the AmpliTaq Gold followed by 32 cycles of 94°C for 30 s, 55°C for 1 min and 72°C for 1 min and a final cycle at 72°C for 10 min. Gel electrophoresis and FluorImager-normalized quantification were performed as described previously.Citation6-8,Citation25,28 Replication timing of each locus was assigned after quantification of at least 3 independent PCR products from reactions in which the annealing temperature, the ratio of BrdU-labeled DNAs to pKF3 DNA and the locus-specific primer pair were altered.
Assignment of replication timing during S phase
The amount of locus-specific PCR product was normalized to that of the plasmid-specific PCR product. Replication timing was measured with a fluorescence scanning system (Fluorimager SI, Molecular Dynamics; Sunnyvale, CA) and assigned on the basis of 6 cell-cycle fractions (G1, S1-S4, G2/M) based on the highest amount of replicated DNA after normalization to the amplified plasmid DNA.Citation6-8,Citation25,28 When the difference in the amount of replicated DNA between 2 consecutive cell-cycle fractions was less than 10%, the replication timing was defined as an intermediate period (e.g. S2.5 is between S2 and S3).Citation6-8,Citation25,28
Sodium bisulfite DNA sequencing
DNAs from ESCs and NPCs (derived from the BG01 cell line) were extracted with a QIAamp DNA Micro Kit (QIAGEN, Valencia, CA) and quantified using the NanoDrop 8000 Spectrophotometer (Thermo Scientific, Wilmington, DE). DNA aliquots were modified with bisulfite using the EZ DNA Methylation Kit™ (Zymo Research, Orange, CA).Citation49-51 The 5′ CpG island regions of the glutamate receptor genes were amplified, cloned into the pCR®4-TOPO vector using the TOPO TA cloning kit for sequencing (Invitrogen, Carlsbad, CA), and sequenced as previously described.Citation49-51
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
967585_Supplemental_Materials.zip
Download Zip (20.3 KB)Acknowledgments
The authors thank Professor David M. Gilbert of the Department of Biological Science at Florida State University for producing the online database ReplicationDomain. We also thank Professor Toshimichi Ikemura at Nagahama Institute of Bio-Science and Technology for organizing the previous study of replication timing on human chromosome 21q.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Additional information
Funding
References
- Ozawa S, Kamiya H Tsuzuki, K. Glutamate receptors in the2288; mammalian central nervous system. Prog Neurobiol 1998; 54:581-618; PMID:9550192; http://dx.doi.org/10.1016/S0301-0082(97)00085-3
- Simeone TA, Sanchez RM, Rho JM. Molecular biology and ontogeny of glutamate receptors in the mammalian central nervous system. J Child Neurol 2004; 19:343-60; PMID:15224708; http://dx.doi.org/10.1177/088307380401900507
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci 2008; 9, 423-36; PMID:18464791; http://dx.doi.org/10.1038/nrn2379
- Debanne D, Daoudal G, Sourdet V, Russier M. Brain plasticity and ion channels. J Physiol Paris 2003; 97:403-14; PMID:15242652; http://dx.doi.org/10.1016/j.jphysparis.2004.01.004
- Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med 2013; 2:1-10 http://dx.doi.org/10.1101cshperspect.a00629
- Watanabe Y, Fujiyama A, Ichiba Y, Hattori M, Yada T, Sakaki Y, Ikemura T. Chromosome-wide assessment of replication timing for human chromosomes 11q and 21q: disease-related genes in timing-switch regions. Hum Mol Genet 2002; 11:13-21; PMID:11772995; http://dx.doi.org/10.1093/hmg/11.1.13
- Watanabe Y, Shibata K, Ikemura T, Maekawa M. Replication timing of extremely large genes on human chromosomes 11q and 21q. Gene 2008; 421:74-80; PMID:18620035; http://dx.doi.org/10.1016/j.gene.2008.06.016
- Watanabe Y, Ikemura T, Sugimura H. Amplicons on human chromosome 11q are located in the earlylate-switch regions of replication timing. Genomics 2004; 84:796-805; PMID:15475258; http://dx.doi.org/10.1016/j.ygeno.2004.08.001
- Watanabe Y, Maekawa M. Spatiotemporal regulation of DNA replication in the human genome and its association with genomic instability and disease. Curr Med Chem 2010; 17:222-33; PMID:20214565; http://dx.doi.org/10.2174/092986710790149756
- Watanabe Y, Maekawa M. Methylation of DNA in cancer. Adv Clin Chem 2010; 52:145-68; PMID:21275343; http://dx.doi.org/10.1016/S0065-2423(10)52006-7
- Watanabe Y, Maekawa, M. RG-band boundaries: genomic instability and human disease. Clin Chim Acta 2013; 419:108-12; PMID:23434413; http://dx.doi.org/10.1016/j.cca.2013.02.011
- Craig JM, Bickmore WA. Chromosome bands–flavours to savour. Bioessays 1993; 15:349-54; PMID:8343145; http://dx.doi.org/10.1002/bies.950150510
- Berezney R, Dubey DD, Huberman JA. Heterogeneity of eukaryotic replicons, replicon clusters, and replication foci. Chromosoma 2000; 108:471-84; PMID:10794569; http://dx.doi.org/10.1007/s004120050399
- Holmquist G, Gray M, Porter T, Jordan J. Characterization of Giemsa dark- and light-band DNA. Cell 1982; 31:121-9; PMID:7159923; http://dx.doi.org/10.1016/0092-8674(82)90411-1
- Holmquist GP. Evolution of chromosome bands: molecular ecology of noncoding DNA. J Mol Evol 1989; 28:469-86; PMID:2549255; http://dx.doi.org/10.1007/BF02602928
- Drouin R, Holmquist GP, Richer CL. High-resolution replication bands compared with morphologic G- and R-bands. Adv Hum Genet 1994; 22:47-115; PMID:7762454; http://dx.doi.org/10.1007/978-1-4757-9062-7_2
- Ikemura T, Aota S. Global variation in G+C content along vertebrate genome DNA: possible correlation with chromosome band structures. J Mol Biol 1988; 203:1-13; PMID:3054117; http://dx.doi.org/10.1016/0022-2836(88)90086-1
- Bernardi G. The isochore organization of the human genome. Annu Rev Genet 1989; 23:637-61; PMID:2694946; http://dx.doi.org/10.1146/annurev.ge.23.120189.003225
- Ikemura T, Wada K, Aota S. Giant G+C% mosaic structures of the human genome found by arrangement of GeneBank human DNA sequences according to genetic positions. Genomics 1990; 8:207-16; PMID:2249845; http://dx.doi.org/10.1016/0888-7543(90)90273-W
- Hiratani I, Gilbert DM. Replication timing as an epigenetic mark. Epigenetics 2009; 4:93-7; PMID:19242104; http://dx.doi.org/10.4161/epi.4.2.7772
- Saccone S, Federico C, Solovei I, Croquette MF, Valle GD, Bernardi G. Identification of the gene-richest bands in human prometaphase chromosomes. Chromosome Res 1999; 7:379-86; PMID:10515213; http://dx.doi.org/10.1023/A:1009220131225
- Bernardi G. Isochores and the evolutionary genomics of vertebrates: organization of the human genome. Gene 2000; 241:3-17; PMID:10607893; http://dx.doi.org/10.1016/S0378-1119(99)00485-0
- Watanabe Y, Shibata K, Sugimura H, Maekawa M. p53-dependent change in replication timing of the human genome. Biochem Biophys Res Comm 2007; 364:289-93; PMID:17949684; http://dx.doi.org/10.1016/j.bbrc.2007.09.136
- Weddington N, Stuy A, Hiratani I, Ryba T, Yokochi T, Gilbert DM. ReplicationDomain: a visualization tool and comparative database for genome-wide replication timing data. BMC Bioinformatics 2008; 9:530; PMID:19077204; http://dx.doi.org/10.1186/1471-2105-9-530
- Watanabe Y, Tenzen T, Nagasaka Y, Inoko H, Ikemura T. Replication timing of the human X-inactivation center (XIC) region: correlation with chromosome bands. Gene 2000; 252:163-72; PMID:10903448; http://dx.doi.org/10.1016/S0378-1119(00)00208-0
- Fukagawa T, Sugaya K, Matumoto K, Okumura K, Ando A, Inoko H, Ikemura T. A boundary of long-range G+C% mosaic domains in the human MHC locus: pseudoautosomal boundary-like sequence exists near the boundary. Genomics 1995; 25:184-91; PMID:7774916; http://dx.doi.org/10.1016/0888-7543(95)80124-5
- Tenzen T, Yamagata T, Fukagawa T, Sugaya K, Ando A, Inoko H, Gojobori T, Fujiyama A, Okumura K, Ikemura T. Precise switching of DNA replication timing in the GC content transition area in the human major histocompatibility complex. Mol Cell Biol 1997; 17:4043-50; PMID:9199339
- Watanabe Y, Abe T, Ikemura T, Maekawa M. Relationships between replication timing and GC content of cancer-related genes on human chromosomes 11q and 21q. Gene 2009; 433:26-31; PMID:19124063; http://dx.doi.org/10.1016/j.gene.2008.12.004
- Watanabe Y, Kazuki Y, Oshimura M, Ikemura T, Maekawa M. Replication timing in a single human chromosome 11 transferred into the Chinese hamster ovary (CHO) cell line. Gene 2012; 510:1-6; PMID:22964274; http://dx.doi.org/10.1016/j.gene.2012.08.045
- Kimura A, Umehara T, Horikoshi M. Chromosomal gradient of histone acetylation established by Sas2p and Sir2p functions as a shield against gene silencing. Nat Genet 2002; 32:370-77; PMID:12410229; http://dx.doi.org/10.1038/ng993
- Goldman MA, Holmquist GP, Gray MC, Caston LA, Nag A. Replication timing of genes and middle repetitive sequences. Science 1984; 224:686-92; PMID:6719109; http://dx.doi.org/10.1126/science.6719109
- Yamazaki S, Ishii A, Kanoh Y, Oda M, Nishito Y, Masai H. Rif1 regulates the replication timing domains on the human genome. EMBO J 2012; 31:3667-77; PMID:22850674; http://dx.doi.org/10.1038/emboj.2012.180
- Yamazaki S, Hayano M, Masai H. Replication timing regulation of eukaryotic replicons: Rif1 as a global regulator of replication timing. Trends Genet 2013; 29:449-60; PMID:23809990; http://dx.doi.org/10.1016/j.tig.2013.05.001
- Gerhardt J, Tomishima MJ, Zaninovic N, Colak D, Yan Z, Zhan Q, Rosenwaks Z, Jaffrey SR, Schildkraut CL. The DNA replication program is altered at the FMR1 locus in fragile X embryonic stem cells. Mol Cell 2014; 53:19-31; PMID:24289922; http://dx.doi.org/10.1016/j.molcel.2013.10.029
- Mirkin EV, Mirkin SM. To switch or not to switch: at the origin of repeat expansion disease. Mol Cell 2014: 53:1-3; PMID:24411078; http://dx.doi.org/10.1016/j.molcel.2013.12.021
- Oda M, Kanoh Y, Watanabe Y, Masai H. Regulation of DNA replication timing on human chromosome by a cell-type specific DNA binding protein SATB1. PLoS One 2012; 7:e42375; PMID:22879953; http://dx.doi.org/10.1371/journal.pone.0042375
- Ermakova OV, Nguyen LH, Little RD, Chevillard C, Riblet R, Ashouian N, Birshtein BK, Schildkraut CL. Evidence that a single replication fork proceeds from early to late replicating domains in the Igh locus in a non-B cell line. Mol Cell 1999; 3:321-30; PMID:10198634; http://dx.doi.org/10.1016/S1097-2765(00)80459-1
- Letessier A, Millot GA, Koundrioukoff S, Lachagès AM, Vogt N, Hansen RS, Malfoy B, Brison O, Debatisse M. Cell-type-specific replication initiation programs set fragility of the FRA3B fragile site. Nature 2011; 470:120-3; PMID:21258320; http://dx.doi.org/10.1038/nature09745
- Debatisse M, Le Tallec B, Letessier A, Dutrillaux B, Brison O. Common fragile sites: mechanisms of instability revisited. Trends Genet 2012; 28:22-32; PMID:22094264; http://dx.doi.org/10.1016/j.tig.2011.10.003
- Bierne H, Michel B. When replication forks stop. Mol. Microbiol. 1994; 13:17-23; PMID:7984091; http://dx.doi.org/10.1111/j.1365-2958.1994.tb00398.x
- Verbovaia LV, Razin SV. Mapping of replication origins and termination sites in the Duchenne muscular dystrophy gene. Genomics 1997; 45:24-30; PMID:9339357; http://dx.doi.org/10.1006/geno.1997.4875
- Rothstein R, Michel B, Gangloff S. Replication fork pausing and recombination or ‘gimme a break’. Genes Dev 2000; 14:1-14; PMID:10640269
- Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer 1980; 26:171-6; PMID:6970727; http://dx.doi.org/10.1002/ijc.2910260208
- Mitalipova M, J Calhoun, S Shin, D Wininger, T Schulz, S, Noggle, A Venable, Lyons I, Robins A, Stice S. Human embryonic stem cell lines derived from discarded embryos. Stem Cells 2003; 21:521-6; PMID:12968106; http://dx.doi.org/10.1634/stemcells.21-5-521
- Zhang SC, Wernig M, Duncan ID, Brustle O, and Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nature Biotechnol 2001; 19:1129-33; http://dx.doi.org/10.1038/nbt1201-1129
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell 2002; 110:385-97; PMID:12176325; http://dx.doi.org/10.1016/S0092-8674(02)00835-8
- Hansen RS, Canfield TK, Lamb MM, Gartler SM, Laird CD. Association of fragile X syndrome with delayed replication of the FMR1 gene. Cell 1993; 73:1403-9; PMID:8324827; http://dx.doi.org/10.1016/0092-8674(93)90365-W
- Hansen RS, Canfield TK, Fjeld AD, Mumm S, Laird CD, Gartler SM. A variable domain of delayed replication in FRAXA fragile X chromosomes: X inactivation-like spread of late replication. Proc Natl Acad Sci USA 1997; 94:4587-92; PMID:9114034; http://dx.doi.org/10.1073/pnas.94.9.4587
- Luo W, Karpf AR, Deeb KK, Muindi JR, Morrison CD, Johnson CS, Trump DL. Epigenetic regulation of vitamin D 24-hydroxylaseCYP24A1 in human prostate cancer. Cancer Res 2010; 70:5953-62; PMID:20587525; http://dx.doi.org/10.1158/0008-5472.CAN-10-0617
- Li Y, Tollefsbol TO. DNA methylation detection: bisulfite genomic sequencing analysis. Methods Mol Biol 2011; 791:11-21; PMID:21913068; http://dx.doi.org/10.1007/978-1-61779-316-5_2
- Kanemoto M, Shirahata M, Nakauma A, Nakanishi K, Taniguchi K, Kukita Y, Arakawa Y, Miyamoto S, Kato K. Prognostic prediction of glioblastoma by quantitative assessment of the methylation status of the entire MGMT promoter region. BMC Cancer 2014; 14:641; PMID:25175833; http://dx.doi.org/10.1186/1471-2407-14-641
