Abstract
Aerobic methane (CH4) emission from plant vegetative parts has been confirmed by many studies. However, the origin of aerobic CH4 from plants and its emission from reproductive parts have not been well documented. We determined the effects of developmental stages (early, mid, late) and incubation conditions (darkness, dim light, bright light) on CH4 emissions from stinkweed (Thlaspi arvense) capsules. We found that CH4 emissions from capsules varied with developmental stage and incubation light. Methane emission was highest for the late harvested capsules and for those incubated under lower (dim) light condition. Our results also showed a significant negative correlation between CH4 emission and capsule moisture content. We conclude that CH4 emissions vary with capsule age and diurnal light environment.
Abbreviations
| AWOVA | = | analysis of variance |
| dw | = | dry weight |
| LSD | = | least significant difference |
| PPFD | = | photosynthetically active photon flux density |
Methane is the second most important greenhouse gas, producing an enhanced ‘greenhouse effect’ and contributing to global warming. It is approximately 25 times more potent than CO2 in trapping heat.Citation10 Atmospheric concentrations of CH4 have more than doubled since pre-industrial times.Citation1 Its natural and anthropogenic sources have contributed to increased air temperature in recent years.Citation2,17 Keppler et al.Citation11 have shown that, besides anaerobic sources, plants can also emit CH4 under aerobic conditions and this new source may have implications to global methane budget. There has been discussion on the methods of estimation,Citation6,8,9 emission rates,Citation7,13,21 and origin of aerobic CH4 from plants.Citation4,5,12,14 In these studies, CH4 emissions were measured mostly from plant vegetative parts,Citation5 but rarely from reproductive parts.Citation3 It has been shown that stressed plants emit more methane than non-stressed ones,Citation5, Citation18-21 and emission rates vary with plant species. The objective of this study was to measure CH4 emissions from the stinkweed capsules at 3 developmental stages that were incubated under 3 light conditions. We hypothesized that aerobic CH4 emissions increase with capsule maturity, which can be influenced by light conditions and capsule moisture content.
We used stinkweed (Thlaspi arvense) to measure CH4 emissions from its capsules. Stinkweed was selected because of its prolific nature of seed production, its potential for aggressively competing with crops under future climate conditions, its distinct and spread out seed pods along the stem, and its wide distribution in Canada. In September 2007, mature seeds of stinkweed were collected from a population located near the fence at the corner of Shaganappi Trail NW and 40 Avenue NW in Calgary, Canada. Plants were grown from seeds in 1 L pots, containing a mixture of peat moss, Perlite, Vermiculite and Terragreen (2:1:1:0.5, by volume) and a few pellets of slow-releasing fertilizer (N-P-K, 14–14–14) in a controlled-environment walk-in growth chamber (model GRW36, Econair Technologies, Inc.., Winnipeg, MB, Canada) under a temperature regime of 22/18°C (16 h day/8 h night) and a relative humidity of 60%. Light was provided by a mix of cool white fluorescent tubes (24 of 2.44 m Philips F96T12/T12/CW/VHO 1500, 6 of 1.22 m Philips F48T12/CW/VHO 1500, Philips Lighting Company, Somerset, NJ) and incandescent lamps (36 of Philips 60 W, Philips Electronics Ltd., Markham, ON, Canada) on a 16-h photoperiod. The photosynthetically active photon flux density (PPFD), measured with a quantum LI-185B radiometer/photometer (LI-COR, Inc.., Lincoln, NE), was 600 μmol m−2 s−1 at the shoot apex (). Plants were watered daily to field capacity until the conclusion of the experiment – seed maturity. We measured CH4 emission from capsules that were harvested at 3 developmental stages (early, mid, late; ) and incubated in clear glass vials, sealed with gas sampling septa, under 3 light conditions (PPFD of 0, 5 and 160 μmol m−2 s−1) at room temperature (∼22°C) for 2 hours. A gas chromatograph with a flame ionization detector was used to measure CH4 emission ratesCitation17 (ng g dw−1 h−1) from freshly detached capsules. Moisture content of capsules was also measured, as described previously.16 Data were analyzed by a 2-way analysis of variance (ANOVA) and differences among treatments were determined by Fisher's least significant difference (LSD) test at the 5% level.Citation15 Pearson's correlation analysis was used to show the relationship between each of the parameters.Citation15
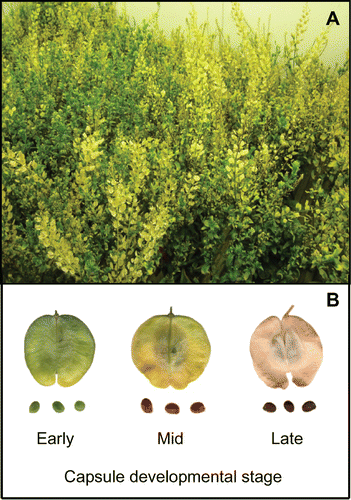
Figure 1. Mature plants of stinkweed (Thlaspi arvense) and their capsules that were used to measure methane emissions. (A) Plants that were grown in a walk-in growth chamber under a temperature regime of 22/18°C (16 h day/8 h night), and (B) capsules and seeds harvested from plants at 3 developmental stages – early, mid and late.
Overall, we found significantly higher CH4 emissions from those capsules that were incubated under dim light than those incubated under bright light. Methane emission was 1.22 times lower in the latter incubation conditions than that in the earlier ones (). Capsules that were incubated in darkness did not differ in CH4 emission than those incubated under dim or bright light (). In general, there were no differences in moisture content within each category of capsules that were incubated under 3 light conditions (). Thus, differences in CH4 emissions among incubation conditions were strongly related to incubation light than to capsule moisture. In contrast, Keppler et al.Citation11 have reported that the CH4 emission rate was higher from tissues exposed to sunlight than those measured in darkness. However, they did not discuss the age of plant tissues, which also play a role in the processes, as discussed below.
Table 1. Methane emissions and moisture contents from stinkweed (Thlaspi arvense) capsules harvested at 3 developmental stages and incubated under 3 light conditions for 2 hours
Our study revealed that capsules at late developmental stage emit 1.23 times more CH4 than those at mid developmental stage (). However, capsules from the early developmental stage did not have significant difference in CH4 emission than those from the other 2 developmental stages (). Capsules from the late developmental stage had significantly less moisture content (3.10 and 2.65 times, respectively) than those from the early and mid developmental stages (). A significant negative correlation (r = –0.6, P < 0.001) was found between CH4 emission and the moisture content of capsule (see ). We previously have shown that water-stressed plants emit more CH4 than well-watered plants.Citation17,18
We also analyzed data on CH4 emissions from capsules at 3 developmental stages for each incubation condition separately (). In darkness, capsules from the late developmental stage had significantly higher CH4 emission than capsules from the mid developmental stage (P < 0.001), but they did not differ than those from the early stage (). Capsules from the late developmental stage had significantly lower moisture than those from the other 2 stages (P < 0.001, ). At dim light, capsules from the late developmental stage had significantly higher CH4 emission, but lower moisture, than capsules from the early or mid developmental stage (P < 0.001, ). At bright light, capsules from the early developmental stage emitted more CH4 than those from the later stages and there were no differences in moisture content among them (P < 0.001, ). These findings indicate the importance of incubation conditions on methane emission from plants, in general, and plant parts, in particular. The results suggest that CH4 emissions vary with capsule age and light condition.
Figure 2. Methane emissions and moisture contents from stinkweed (Thlaspi arvense) capsules harvested at 3 developmental stages and incubated under 3 light conditions for 2 hours. Capsules incubated under darkness (0 μmol m−2 s−1), dim light (5 μmol m−2 s−1), and bright light (160 μmol m−2 s−1). Letters that follow the symbols (mean ± SE) indicate capsule developmental stages (E, early; M, mid; L, late).
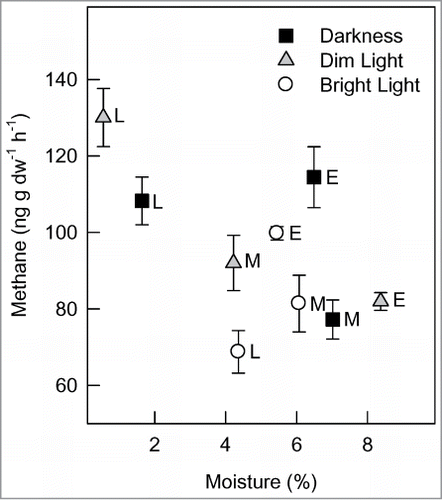
Not only did incubation condition and capsule developmental stage individually affect CH4 emissions, but their interactions also significantly (P < 0.001) changed emission rates. Lower (dim) light intensity led to the highest rate of CH4 emission from capsules at the late developmental stage (130.06 ± 7.61 SE), but higher (bright) light intensity caused the lowest emission rate from capsules at the same developmental stage (68.75 ± 5.56 SE) (see ).
In summary, CH4 emission increases with capsule age, the lower incubation light condition accelerates methane emission, and an inverse relationship between methane emission and capsule moisture content exists.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We appreciate useful comments on the manuscript from 2 anonymous referees.
Funding
We thank the Natural Sciences and Engineering Research Council (NSERC) of Canada for financial support through Discovery grants to MM Qaderi and DM Reid. The financial support from Mount Saint Vincent University through an Internal Research grant to MMQ is greatly appreciated.
References
- Bousquet P, Ciais P, Miller JB, Dlugokencky EJ, Hauglustaine DA, Prigent C, Van der Werf GR, Peylin P, Brunke E-G, Carouge C, et al. Contribution of anthropogenic and natural sources to atmospheric methane variability. Nature 2006; 443:439-43; PMID:17006511; http://dx.doi.org/10.1038/nature05132
- Brüggemann N, Meier R, Steigner D, Zimmer I, Louis S, Schnitzler J-P. Nonmicrobial aerobic methane emission from poplar shoot cultures under low-light conditions. New Phytol 2009; 182:912-8; PMID:19281477; http://dx.doi.org/10.1111/j.1469-8137.2009.02797.x
- Bruhn D, Mikkelsen TN, Øbro J, Willats WGT, Ambus P. Effects of temperature, ultraviolet radiation and pectin methyl esterase on aerobic methane release from plant material. Plant Biol 2009; 11(Suppl. One):43-8; PMID:19778367; http://dx.doi.org/10.1111/j.1438-8677.2009.00202.x
- Bruhn D, Mikkelsen TN, Rolsted MMM, Egsgaard H, Ambus P. Leaf surface wax is a source of plant methane formation under UV radiation and in the presence of oxygen. Plant Biol 2014; 16:512-6; PMID:24400835; http://dx.doi.org/10.1111/plb.12137
- Bruhn D, Møller IM, Mikkelsen TN, Ambus P. Terrestrial plant methane production and emission. Physiol Plant 2012; 144:201-9; PMID:22136562; http://dx.doi.org/10.1111/j.1399-3054.2011.01551.x
- Butenhoff CL, Khalil MAK. Global methane emissions from terrestrial plants. Environ Sci Technol 2007; 41:4032-4037; PMID:17612186; http://dx.doi.org/10.1021/es062404i
- Dueck TA, de Visser R, Poorter H, Persijn S, Gorissen A, de Visser W, Schapendonk A, Verhagen J, Snel J, Harren FJM, et al. No evidence for substantial aerobic methane emission by terrestrial plants: a 13C-labelling approach. New Phytol 2007; 175:29-35; PMID:17547664; http://dx.doi.org/10.1111/j.1469-8137.2007.02103.x
- Ferretti DF, Miller JB, White JWC, Lassey KR, Lowe DC, Etheridge DM. Stable isotopes provide revised global limits of aerobic methane emissions from plants. Atmos Chem Phys 2007; 7:237-41; http://dx.doi.org/10.5194/acp-7-237-2007
- Houweling S, Röckmann T, Aben I, Keppler F, Krol M, Meirink JF, Dlugokencky E, Frankenberg C. Atmospheric constraints on global emissions of methane from plants. Geophys Res Lett 2006; 33:L15821; PMID:19122778; http://dx.doi.org/10.1029/2006GL026162
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds TF Stocker, D Qin, G-K Plattner, M Tignor, SK Allen, J Boschung, A Nauels, Y Xia, V Bex, PM Midgley). Cambridge University Press, Cambridge, UK & New York, NY, USA, 2013; 1535.
- Keppler F, Hamilton JTG, Braß M, Röckmann T. Methane emissions from terrestrial plants under aerobic conditions. Nature 2006; 439:187-91; PMID:16407949; http://dx.doi.org/10.1038/nature04420
- Keppler F, Hamilton JTG, McRoberts WC, Vigano I, Brass M, Röckmann T. Methoxyl groups of plant pectin as a precursor of atmospheric methane: evidence from deuterium labelling studies. New Phytol 2008; 178:808-14; PMID:18346110; http://dx.doi.org/10.1111/j.1469-8137.2008.02411.x
- Kirschbaum MUF, Walcroft A. No detectable aerobic methane efflux from plant material, nor from adsorptiondesorption processes. Biogeosci Discuss 2008; 5:2773-94; http://dx.doi.org/10.5194/bgd-5-2773-2008
- McLeod AR, Fry SC, Loake GJ, Messenger DJ, Reay DS, Smith KA, Yun BW. Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol 2008; 180:124-32; PMID:18657215; http://dx.doi.org/10.1111/j.1469-8137.2008.02571.x
- Minitab Inc. Minitab® Release 16.2.4.4, Statistical Software for Windows®. Minitab Inc., State College, Pennsylvania, USA 2013. www.minitab.com/en-us/
- Qaderi, MM, Cavers PB, Hamill AS, Bernards MA. Effects of collection time and after-ripening on chemical constituents and germinability of Scotch thistle (Onopordum acanthium) cypselas. Botany 2012; 90:755-62; http://dx.doi.org/10.1139/b2012-035
- Qaderi MM, Reid DM. Methane emissions from six crop species exposed to three components of global climate change: temperature, ultraviolet-B radiation and water stress. Physiol Plant 2009; 137:139-47; PMID:19678898; http://dx.doi.org/10.1111/j.1399-3054.2009.01268.x
- Qaderi MM, Reid DM. Stressed crops emit more methane despite the mitigating effects of elevated carbon dioxide. Func Plant Biol 2011; 38:97-05; http://dx.doi.org/10.1071/FP10119
- Vigano I, van Weelden H, Holzinger R, Keppler F, Röckmann T. Effect of UV radiation and temperature on the emission of methane from plant biomass and structural components. Biogeosci Discuss 2008; 5:243-70; http://dx.doi.org/10.5194/bgd-5-243-2008
- Wang Z-P, Chang SX, Chen H, Han X-G. Widespread non-microbial methane production by organic compounds and the impact of environmental stresses. Earth-Sci Rev 2013; 127:193-202; http://dx.doi.org/10.1016/j.earscirev.2013.10.001
- Wang Z-P, Han X-G, Wang GG, Song Y, Gulledge J. Aerobic methane emission from plants in the Inner Mongolia Steppe. Environ Sci Technol 2008; 42:62-8; PMID:18350876; http://dx.doi.org/10.1021/es071224l
