Abstract
During plant growth and development, root tip performs multifarious functions integrating diverse external and internal stimuli to regulate root elongation and architecture. It is believed that a signal originating from root tip inhibits lateral root formation (LRF). The excision of root tip induced LRF in tomato seedlings associated with accumulation of auxin in pericycle founder cells. The excision of cotyledons slightly reduced LRF, whereas severing shoot from root completely abolished LRF. Exogenous ethylene application did not alter LRF. The response was modulated by light with higher LRF in seedlings exposed to light. Our results indicate that light plays a role in LRF in seedlings by likely modulating shoot derived auxin.
Abbreviations
| LRF | = | Lateral root formation |
| ACC | = | 1-Aminocyclopropane-1-carboxylic acid |
| DAG | = | Days after germination |
| LR | = | Lateral root |
During seedling establishment, primary root is the first organ to emerge from seed which anchors the plant to the soil. Root emergence is followed by development of lateral roots (LR) which together with primary root acquire water and minerals for developing seedlings. Subsequent ramification of roots in soil generates a complex root architecture which is influenced by environmental factors such as mechanical impedance, mineral nutrients and endogenous regulators mainly consisting of plant hormones.Citation1 In recent years molecular-genetic examination of root development using mutants and examination of expression of specific genes and their products at the cellular levels have identified the key regulators involved in elongation of primary root and initiation of lateral roots.Citation2 Compared to understanding of molecular basis of lateral root formation (LRF) and elongation, little information is available for possible interaction between the primary root elongation and LRF.
In pea excision of the tip of primary root stimulated formation of LR in apical portion of remaining root base, whereas intact roots formed no LR.Citation3 It was surmised that root tip produced some substance that inhibited LRF and excision relieved this inhibition on LRF. Subsequently it was reported that the root tip may inhibit LRF in a fashion analogous to shoot apical dominance.Citation4 The tip decapitation within 12 h reduced the levels of cytokinin, abscisic acid (ABA) and xanthoxin in the stump region adjacent to the tip. In an alternate approach it was found that shoot also influences LRF, as removal of cotyledons from pea seedlings completely inhibited LRF, while epicotyl removal reduced the magnitude of LRF.Citation5 Since exogenous indoleacetic acid and cytokinin could substitute for cotyledon and root tip respectively, it was proposed that these 2 organs act together to establish auxin and cytokinin concentrations in primary root axis to control LRF.
In recent years evidences have accumulated that the shoot derived auxin is required for LRF formation in Arabidopsis and inhibition of polar auxin transport reduces the LRF.Citation6 Overexpression of bacterial auxin biosynthetic genes in transgenic tobacco increases LRF due to elevation in the endogenous auxin level.Citation7 Conversely, reduced polar auxin transport in Arabidopsis tir3–1 mutant is associated with reduction in formation of lateral roots.Citation8 Likewise evidences indicate that cytokinin acts antagonistically to auxin in LRF.Citation9 Exogenous cytokinin treatment blocks initiation of LRF in pericycle cellsCitation10 and mutants deficient in cytokinin,Citation11 or mutated in cytokinin perception/signaling pathways show increased LRF.Citation12,13
Several other hormones such as brassinosteroid, ABA and ethylene also regulate LRF.Citation14 Brassinosteroid acts as a positive regulator of LR initiation by increasing acropetal auxin transport.Citation15 ABA acts as a negative regulator of LRF and it is proposed that ABA and cytokinin inhibit polar auxin transport and that the resulting depletion in auxin levels reduces LRF.Citation16 Ethylene, a gaseous plant hormone; negatively regulates LRF in various plants. Both ethylene overproducing mutants and constitutive ethylene signaling mutants show decreased LRF.Citation17-19
Compared to hormones, very limited information is available about the role of light on LRF. Most studies only reported the influence of light on root elongation. Light grown tomato seedlings show longer roots than etiolated seedlings.Citation20 In Arabidopsis dark grown seedlings exhibited a reduced LRF compared to light grown seedlings.Citation21 Among plant photoreceptors, it is reported that cryptochromes and phototropin likely play role in root elongation on exposure to blue light.Citation22,23 The total number of LR were higher in the phototropin 1 mutant than in wild-type Arabidopsis.Citation24
To understand the interrelationship between LRF and root tip excision, we examined this response in tomato seedlings. The results obtained in this study demonstrate that root tip excision-mediated LRF in tomato seedlings is modulated by light. In light-grown tomato seedling normally LRF is visually discernible only after 5 day after germination (DAG). To examine the influence of root tip on LRF, the seedlings were grown on vertical agar plates under continuous white light (100 μmoles m− Citation2 s−1) as described in Al-Hammadi et al.Citation20 The root tips were excised at 2 DAG and 4 DAG and the LRF was quantified at 7 DAG. The root tip decapitation significantly enhanced LRF in seedlings () with much higher LR number at 2 DAG than at 4 DAG compared to control seedlings (). This result corroborates earlier reports that decapitation (root tip excision) increases LRF.Citation6,25 The increased LRF indicates that the root tip might be exerting an inhibitory effect on LRF or a shoot derived signal in absence of functional root tip accumulate distal to root tip stimulating LRF.
Figure 1. Excision induced lateral root formation in tomato. (A) The root tips were excised from the seedlings at 4 DAG and seedlings were photographed at 7 DAG. (B) The root tips were excised either at 2 DAG or 4 DAG and number of lateral LRF was counted at 7 DAG. The control refers to intact seedlings. (n ≥ 10 ± SE) (C) The root tips were excised at 2 DAG from DR5::GUS transgenic seedlings. The roots were stained for GUS activity 24 h post-excision. (i) Intact roots at 2 DAG (ii) Root tip-excised roots after 24 h (iii) Intact seedling root at 3 DAG.
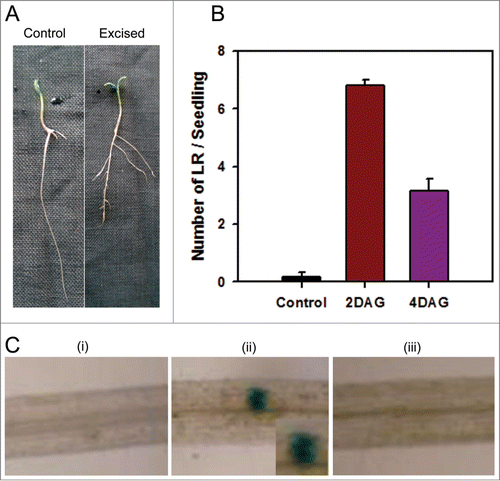
While the nature of root tip derived signal is not known, it is known that auxin application to growing plants stimulates LRFCitation26 and auxin deficient mutant shows reduced LRF.Citation27 Most importantly auxin is required to initiate LR primordia, to establish a population of rapidly dividing cells in the pericycle. Taking in account the relationship between auxin and LRF, we examined the accumulation of auxin in LR primordia of intact and decapitated roots using an auxin-response reporter DR5::GUS expression. Several studies have indicated that activity of the synthetic consensus auxin reporter, DR5::GUS, closely reflects the auxin induced gene expression in plant tissues.Citation28,29 The root tips were excised at 4 DAG from DR5::GUS transgenic seedlings and roots were stained for GUS activity 24 h after excision, using a protocol described in Santisree et al.Citation30 In root tip decapitated seedlings, DR5::GUS activity was localized in the pericycle region associated with the primordia of emerging lateral roots (). On the contrary similar localization of DR5::GUS activity in the pericycle regions was not discernible in the intact root. The above observation indicated that decapitation induced LRF by localized increase in the auxin level in root pericycle initiating LR primordia.
Considering that gaseous plant hormone ethylene negatively regulates LRF,Citation17-19 we examined the effect of ethylene precursor 1-aminocyclopropane carboxylic acid (ACC) on LRF. The root tips were excised at 2 DAG and at the same time 20 μM ACC was supplemented to the agar plates.Citation19 Our results show that LR induction on root tip excision is not related to ethylene (). The addition of ACC had no effect on LRF both in excised and control seedlings.
Figure 2. Effect of ethylene and light on LRF. (A) The root tips were excised at 2 DAG and at the same time 20 μM ACC was supplemented to the germination boxes. The LRF was counted at 7 DAG. (n ≥ 10 ± SE) (B) The cotyledons or whole shoot was excised from seedlings at 2 DAG either with or without root tip excision. The LRF was counted at 7 DAG. The seedling with intact roots served as a control. (n ≥ 10 ± SE) (C) Effect of red (R), or far red (FR), and white light (WL) was examined after excision of root tips at 2 DAG or 4 DAG. The LRF was counted at 7 DAG. (n ≥ 10 ± SE).
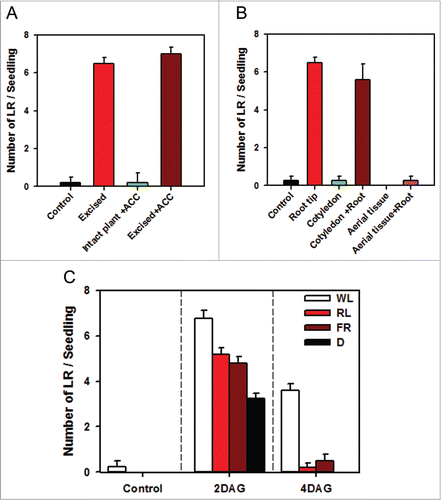
In root the auxin is acropetally transported in the pericycle cells and basipetally transported in the subepidermal cells.Citation31,32 It is possible that root tip excision reduced the basipetal auxin transport emanating from root tip and at the same time decapitation abolished acropetal transport of auxin by eliminating sink at the root tip. This may have probably increased the accumulation of auxin in pericycle cells leading to LRF. To examine that a shoot derived signal induced LRF, the shoot tissue was excised along with the root tip at 2 DAG. If the shoot derived auxin regulates the emergence of LR, then the removal of shoot should abolish this process. We performed 2 types of excision, either removing the whole shoot by severing it at hypocotyl/root junction or severing only cotyledons. The removal of shoot tissue had an inhibitory effect on LRF (). In seedlings the removal of cotyledon alone did not influence LRF, but cotyledon excision in conjunction with root tip excision slightly reduced LRF. The above results indicated that root tip has primary influence on LRF and the shoot derived signal modulates the above response. Since excision of whole shoot totally abolished LRF, it indicated 2 possibilities. First, shoot removal deprived root of the auxin source required for the LRF initiation, thus in absence of auxin there were no LRF. Secondly, the loss of LRF may also be related to carbohydrate starvation as developing root depends on shoot for energy. However these 2 possibilities are not mutually exclusive and together may have contributed to loss of LRF. While most studies suggest that the shoot derived auxin is necessary for excision induced LRF,Citation6,21 the possibility of carbohydrate starvation has not been examined in detail.
To examine whether LRF as a result of excision also would require a specific light/ photoreceptor, an experiment was done in which seedlings were grown under red light (5 μmol m−2s−1), far-red light (3 μmol m−2s−1), white light (100 μmol m−2s−1) and darkness and subsequently their root tips were removed at 2 DAG. Though LRF was observed in seedlings irrespective of the exposure to different lights, the LRF was higher in seedlings grown in white light, compared to dark-grown seedlings. In contrast, seedlings exposed to red and far-red light showed intermediate LRF. The intermediate effect of red and far-red light indicates likely participation of phytochrome in LRF. Considering that higher LRF is seen in white light, it is likely that in addition to phytochrome other photoreceptors may be involved (). There is already evidence that phototropin, the blue absorbing photoreceptors regulate LRF.Citation24 Our experiments indicate that this process may also be aided by phytochrome. Several lines of evidences indicate that light quality received by shoot also affects root function and morphology. It is observed that the shift in R:FR ratio affects allocation of biomass to roots and symbiotic interaction between roots and neighboring microbes.Citation33 It is interesting to speculate the likely role of these photoreceptors in natural conditions, as the roots grows in soil and have little chance to encounter light. Since LRF formation is always localized close to the surface of soil, it may be an adaptive mechanism to form LR when the primary root is in proximity of soil surface.
It is believed that LR initiation is regulated by auxin likely redirected or originated from the root tip.Citation34,35 However, since the root tip excision induced LRF in tomato, it is unlikely that the tip derived signal could be auxin. Our results rather indicate a negative influence of root tip on LRF. On the contrary, the LR emergence depends on auxin derived from the shoot.Citation21 The increased induction of LRF in light may be related to light mediated modulation of transport of shoot derived auxin in root. In summary, root tip excision initiates a signal transduction chain that stimulates LRF in tomato seedlings. The loss of LRF on shoot removal suggests that shoot likely contributes to LRF by providing necessary auxin and/or nutrition. The light has a modulating role in regulating LRF and possibly phytochrome also plays a role in this response. The usage of different photoreceptor mutants in future may help to decipher the role of light in regulation of LRF in tomato.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
S.T is thankful to University Grants Commission, New Delhi for the financial support in the form of research fellowship. Y.S and R.S thank Department of Biotechnology, New Delhi for the financial assistance.
References
- Jones B, Ljung K. Subterranean space exploration: the development of root system architecture. Curr Opin Plant Biol 2012; 15:97-102; PMID:22037466; http://dx.doi.org/10.1016/j.pbi.2011.10.003
- Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L. Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 2013; 18:450-8; PMID:23701908; http://dx.doi.org/10.1016/j.tplants.2013.04.006
- Torrey JG. The induction of lateral roots by indole acetic acid and root decapitation. Am J Bot 1950; 37:257-64; http://dx.doi.org/10.2307/2437843
- Böttger M. Apical dominance in roots of Pisum sativum L. Planta 1974; 121:253-61; PMID:24442804; http://dx.doi.org/10.1007/BF00389325
- Hinchee MAW, Rost TL. The control of lateral root development in cultured pea seedlings. I. The role of seedling organs and plant growth regulators. Bot Gaz 1986; 147:137-47; http://dx.doi.org/10.1086/337579
- Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 1998; 118:1369-78; PMID:9847111; http://dx.doi.org/10.1104/pp.118.4.1369
- Sitbon FB, Hennion S, Sundberg B, Little CHA, Olsson O, Sand berg G. Transgenic tobacco plants coexpressing the Agrobacterium tumefaciens iaaM and iaaH genes display altered growth and indoleacetic acid metabolism. Plant Physiol 1992; 99:1062-9; PMID:16668972; http://dx.doi.org/10.1104/pp.99.3.1062
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 1997; 9:745-57; PMID:9165751; http://dx.doi.org/10.1105/tpc.9.5.745
- Aloni R, Aloni E, Langhans M, Ullrich CI. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot 2006; 97:883-93; PMID:16473866; http://dx.doi.org/10.1093/aob/mcl027
- Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, Swarup R, Weijers D, Calvo V, Parizot B, Begon M. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 2007; 19:3889-900; PMID:18065686; http://dx.doi.org/10.1105/tpc.107.055863
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003; 15:2532-50; PMID:14555694; http://dx.doi.org/10.1105/tpc.014928
- Mason MG, Mathews DE, Argyros DA, Maxwell BB, Kieber JJ, Alonso JM, Ecker JR, Schaller GE. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 2005; 17:3007-18; PMID:16227453; http://dx.doi.org/10.1105/tpc.105.035451
- Riefler M, Novak O, Strnad M, Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 2006; 18:40-54; PMID:16361392; http://dx.doi.org/10.1105/tpc.105.037796
- Fukaki H, Tasaka M. Hormone interactions during lateral root formation. Plant Mol Biol 2009; 69:437-49; PMID:18982413; http://dx.doi.org/10.1007/s11103-008-9417-2
- Bao F, Shen J, Brady SR, Muday GK, Asami T, Yang Z. Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol 2004; 134:1624-31; PMID:15047895; http://dx.doi.org/10.1104/pp.103.036897
- Shkolnik-Inbar D, Bar-Zvi D. ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 2010; 22:3560-73; PMID:21097710; http://dx.doi.org/10.1105/tpc.110.074641
- Ivanchenko MG, Muday GK, Dubrovsky JG. Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 2008; 55:335-47; PMID:18435826; http://dx.doi.org/10.1111/j.1365-313X.2008.03528.x
- Negi S, Ivanchenko MG, Muday GK. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J 2008; 55:175-87; PMID:18363780; http://dx.doi.org/10.1111/j.1365-313X.2008.03495.x
- Negi S, Sukumar P, Liu X, Cohen JD, Muday GK. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J 2010; 61:3-15; PMID:19793078; http://dx.doi.org/10.1111/j.1365-313X.2009.04027.x
- Al-Hammadi AS, Sreelakshmi Y, Negi S, Siddiqi I, Sharma R. The polycotyledon mutant of tomato shows enhanced polar auxin transport. Plant Physiol 2003; 133:113-25; PMID:12970479; http://dx.doi.org/10.1104/pp.103.025478
- Bhalerao RP, Eklof J, Ljung K, Marchant A, Bennett M, Sandberg G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 2002; 29:325-32; PMID:11844109; http://dx.doi.org/10.1046/j.0960-7412.2001.01217.x
- Canamero RC, Bakrim N, Bouly JP, Garay A, Dudkin EE, Habricot Y, Ahmad M. Cryptochrome photoreceptors cry1 and cry2 antagonistically regulate primary root elongation in Arabidopsis thaliana. Planta 2006; 224:995-1003; PMID:16703358; http://dx.doi.org/10.1007/s00425-006-0280-6
- Galen C, Rabenold JJ, Liscum E. Light-sensing in roots. Plant Signal Behav 2007; 2:106-8; PMID:19704750; http://dx.doi.org/10.4161/psb.2.2.3638
- Moni A, Lee AY, Briggs WR, Han IS. The blue light receptor Phototropin 1 suppresses lateral root growth by controlling cell elongation. Plant Biol 2014; PMID:24803136; DOI 10.1111/plb.12187
- Wightman F, Thimann KV. Hormonal factors controlling the initiation and development of lateral roots. I. Sources of primordia-inducing substances in the primary root of pea seedlings. Physiol Plant 1980; 49:13-20; http://dx.doi.org/10.1111/j.1399-3054.1980.tb08640.x
- Muday GK, Haworth P. Tomato root growth, gravitropism, and lateral development: correlation with auxin transport. Plant Physiol Biochem 1994; 33:193-203; PMID:11540612
- Celenza JL, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 1995; 9:2131-42; PMID:7657165; http://dx.doi.org/10.1101/gad.9.17.2131
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 1997; 9:1963-71; PMID:9401121; http://dx.doi.org/10.1105/tpc.9.11.1963
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 1999; 99:463-72; PMID:10589675; http://dx.doi.org/10.1016/S0092-8674(00)81535-4
- Santisree P, Nongmaithem S, Vasuki H, Sreelakshmi Y, Ivanchenko MG, Sharma R. Tomato root penetration in soil requires a coaction between ethylene and auxin signaling. Plant Physiol 2011; 156:1424-38; PMID:21571667; http://dx.doi.org/10.1104/pp.111.177014
- Mitchell EK, Davies PJ. Evidence for three different systems of movement of indoleacetic acid in intact roots of Phaseolus coccineus. Physiol Plant 1975; 33:290-4; http://dx.doi.org/10.1111/j.1399-3054.1975.tb03171.x
- Tsurumi S, Ohwaki Y. Transport of 14C-labeled indoleacetic acid in Vicia root segments. Plant Cell Physiol 1978; 19:1195-206
- Gundel PE, Pierik R, Mommer L, Ballaré CL. Competing neighbors: light perception and root function. Oecologia 2014; 176:1-10;PMID:24894371; http://dx.doi.org/10.1007/s00442-014-2983-x
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al. Auxin transport promotesArabidopsis lateral root initiation. Plant Cell 2001; 13:843-52; PMID:11283340; http://dx.doi.org/10.1105/tpc.13.4.843
- De Smet I, Tetsumura T, De Rybel B, Frei dit Frey N, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, Inzé D, et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 2007; 134:681-90; PMID:17215297; http://dx.doi.org/10.1242/dev.02753
