Abstract
Transgenic tomato plants with reduced expression of the sucrose transporter SlSUT2 showed higher efficiency of mycorrhization suggesting a sucrose retrieval function of SlSUT2 from the peri-arbuscular space back into the cell cytoplasm plant cytoplasm thereby limiting mycorrhiza fungal development. Sucrose uptake in colonized root cells requires efficient plasma membrane-targeting of SlSUT2 which is often retained intracellularly in vacuolar vesicles. Protein-protein interaction studies suggested a link between SISUT2 function and components of brassinosteroid biosynthesis and signaling. Indeed, the tomato DWARF mutant dx defective in BR synthesisCitation1 showed significantly reduced mycorrhization parameters.Citation2 The question has been raised whether the impact of brassinosteroids on mycorrhization is a general phenomenon. Here, we include a rice mutant defective in DIM1/DWARF1 involved in BR biosynthesis to investigate the effects on mycorrhization. A model is presented where brassinolides are able to impact mycorrhization by activating SUT2 internalization and inhibiting its role in sucrose retrieval.
Abbreviations
| GO | = | gene ontology |
| SUT | = | sucrose transporter |
| SUC | = | sucrose carrier |
| AM | = | arbuscular mycorrhiza |
| BR | = | brassinosteroids |
| LRR | = | leucine-rich repeat |
| SNARE | = | soluble N-ethylmaleimide-sensitive-factor attachment receptor |
| MSBP | = | membrane steroid binding protein |
| PCR | = | polymerase chain reaction |
| DIM | = | diminuto |
| RNA | = | ribonucleic acid |
| DRM | = | detergent resistant membrane |
So far, our knowledge about the physiological role of the SlSUT2 transport protein is very limited. If expressed in heterologous systems no sucrose transport function has been observed for SlSUT2, which is structurally different from the other sucrose transporters known from tomato plants and which in plants is localized in phloem sieve elementsCitation3 as well as in pollen and pollen tubes.Citation4 Nevertheless, it seems to be physiologically relevant in sucrose uptake since defects in pollen development as well as reduced sucrose uptake in in vitro grown pollen tubes could be observed in tomato with down-regulated SlSUT2 expression.Citation4
Carbohydrate partitioning via the activity of carbohydrate transporters is crucial for the establishment of mutualistic and pathogenic interactions between host plants and fungal partners and the involvement of sugar transporters from both sides the fungal as well as the host plants side was reviewed elsewhere.Citation5 Recently, we obtained information about a role of SlSUT2 in the arbuscular mycorrhizal symbiosis in tomato. SlSUT2-inhibited tomato plants showed significantly increased mycorrhizal parameters suggesting an inhibitory function of the SlSUT2 sucrose transporter by retrieving sucrose from the apoplast back into the plant cell. It is assumed that SlSUT2 affects sucrose retrieval from the periarbuscular space thereby regulating the carbohydrate supply and as a consequence intra- and extraradicular development of the AM fungus.Citation2
Post-Translational Regulation of Sucrose Transporters
The sucrose transporter expression and activity is tightly controlled at the transcriptional, post-transcriptional, translational as well as post-translational level.Citation6 Post-translational regulation involves modulation of sucrose transporter activity via direct protein-protein-interactionCitation7 and/or post-translational protein modification.Citation8,9
When SlSUT2 was used as a bait protein in a split ubiquitin screen for SUT2-interacting proteins, the same protein disulfide isomerase was identified as interaction partner that previously was characterized as SUT1 and SUT4-interacting protein and confirmed by GST pull down assay and Bimolecular Fluorescence Complementation.Citation10 Gene ontology analysis of all SlSUT2-interacting proteins was performed in order to identify interacting candidates which might be overrepresented among the SUT2-interaction partners.Citation11 With respect to the molecular function, protein disulfide isomerases seem to be enriched among the SUT2 interacting proteins, whereas the main cellular component where SUT2 was confined is the cell periphery, the endomembrane system as well as the vacuole in GO-terms (S1). The identification of further SlSUT2-interacting proteins by a split ubiquitin screen presented several interaction partners which are directly or indirectly involved in brassinosteroid synthesis and/or signaling. This includes a SNARE protein involved in subcellular targeting of other proteins, the membrane steroid binding protein MSBP1, a LRR receptor kinase similar to BAK1 as well as the sterol reductase DIMINUTO/DWARF1. DIM/DWARF1 is involved in BR biosynthesisCitation12 and is found in the detergent-resistant membrane (DRM) fraction from plants.Citation13 The question arises whether brassinosteroids per se are able to affect mycorrhizal efficiency or not.
Brassinosteroid Biosynthesis Affects Mycorrhization of Rice Plants
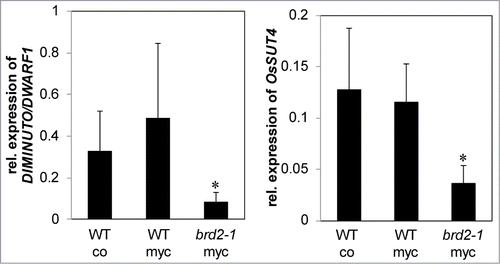
It is striking that several of the SlSUT2-interacting proteins as far as their physiological function was investigated are directly or indirectly involved in brassinosteroid synthesis and/or signaling. In addition, a study in Medicago truncatula indicated that expression of the MSBP1 protein is necessary for a fully established AM symbiosisCitation14 and tomato plants deficient in brassinosteroid biosynthesis showed reduced mycorrhization.Citation2 BR-deficient mutants are described in Pisum sativum Citation15 and rice plants,Citation16 2 model plants which are efficiently colonized by AM fungi. The impact of brassinosteroids on the development of the symbiosis was further investigated in the rice mutant brd2-1. This mutant is defective in the expression of DIM/DWARF1, one of the SUT2 interaction partners in tomato and shows a dwarf phenotype (kindly provided by M. Matsuoka, Japan). In our experiments, the Oryza sativa Nipponbare wild type and brd2-1 rice mutant were inoculated with Rhizophagus irregularis and mycorrhization parameters were estimated 4 weeks after inoculation (). All parameters were significantly reduced in the brassinosteroid mutant suggesting that AM fungal development was severely affected. Further analyses were targeted to the expression of the SlSUT2 homologous gene OsSUT4 (BAC67164,Citation17 ). Quantitative real-time PCR showed no mycorrhizal induction, but confirmed the reduced expression of the DIM/DWARF1-encoding gene and moreover revealed a significant lower RNA accumulation of OsSUT4 in the brd2-1 mutant if compared to Nipponbare wild type plants ().
Figure 1. Mycorrhizal parameters of Oryza sativa Nipponbare wild type rice plants compared to brd2-1 mutant plants defective in BR biosynthesis 6 weeks after inoculation with Rhizophagus irregularis. F%: infection frequency, M%: absolute mycorrhizal colonization, m% relative mycorrhizal colonization, A% absolute arbuscule abundance, a% relative arbuscular abundance. Shown are mean values and standard deviations. Significant differences between WT and brd2-1 mutant plants are indicated by asterisks (Students-t-test, P ≤ 0.05; n = 4-6).
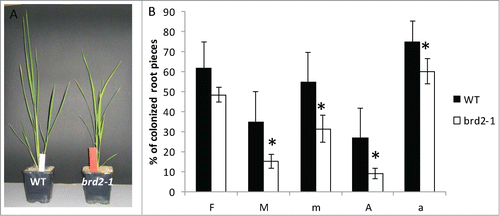
Figure 2. Quantitative real time PCR analysis of transcript levels of the DIM/DWARF1- (Os10g0397400) and OsSUT4-encoding (Q6YK44) genes relative to ubiquitin gene (XR_423446) transcript levels in Nipponbare wild type rice plants compared to the BR-synthesis defective brd2-1 mutant. Wild type plants were non-mycorrhizal (co) or inoculated with the AM fungi R. irregularis (myc). Shown are mean values and standard deviations. Significant differences between WT and brd2-1 mycorrhizal plants are indicated by asterisks (Students-t-test, P ≤ 0.05; n = 3-5).
From the experiments described previously for tomato and in the current paper for rice it became clear that the sucrose transporter SlSUT2 and brassinosteroids are involved in the regulation of the AM symbiosis. Reduced expression of SUT-encoding genes in brassinosteroid mutants and the interaction of SlSUT2 with components of brassinosteroid biosynthesis and signaling imply a concerted action of sucrose transport and functions of the phytohormone.
Expression analyses on RNA level () and on protein levelCitation2 suggest a regulation of SUT2 expression by brassinosteroids. This is in agreement with the recent publication of the BR-regulated transcriptional network showing that AtSUT2/SUC3 from Arabidopsis is among the BR-upregulated genes.Citation18 Further confirmation of BR-induced up-regulation of SUT2 expression comes from the Arabidopsis Co-response database (http://csbdb.mpimp-golm.mpg.de/csbdb/dbcor/ath/ath_txp.html) where AtSUT2 (At2g02860) expression increases after application of epi-brassinolide, as well as the BR-precursor molecule castasterone.
In rice plants, a member of the Rac/Rop GTPase family was shown to play an important role in innate immunity and to be shifted to the detergent resistant membrane (DRM) fraction of the plasma membrane by elicitor treatment.Citation19 Interestingly, the authors identified in the DRM fraction of rice not only 6 different SNARE proteins, but also the DIMINUTO protein, a BRI1-associated LRR receptor kinase and many other proteins previously identified in DRMs of other plant species and identified as SUT2-interacting proteins.
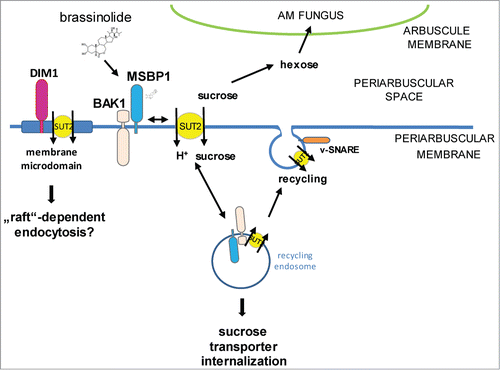
One might speculate that SlSUT2-interacting proteins are involved in the recruitment of SlSUT2 to the plasma membrane. This is summarized in the model shown in . Increasing numbers of studies deal with the involvement of membrane trafficking not only in plant-pathogen interactions, but in plant-microbe interactions in general including symbiotic interactions.Citation20 Recent elucidation of the membrane-linked interactome from Arabidopsis revealed direct interaction between the BR-receptor BRI1 with the SNARE proteins VAMP727 and SYP22,Citation21 thereby affecting brassinosteroid signaling either through modulation of the amount of BRI1 at the plasma membrane or in endosomes or by affecting BRI1 trafficking to the vacuole for degradation.
Figure 3. Hypothetical model describing the involvement of SlSUT2-interacting proteins in the subcellular localization of the sucrose transporter. SlSUT2 interacts with MSBP1, the LRR-receptor kinase BAK1-like and DIM1 that is associated to membrane microdomains. Endocytosis inhibits SlSUT2 transport activity, whereas recycling to the plasma membrane potentially via SNARE proteins is required for activation and sucrose retrieval from the periarbuscular space away from the AM fungus via SlSUT2. Brassinolide is assumed to affect mycorrhization via enabling MSBP1-SUT2 interaction for internalisation of SUT2 and inactivation its sucrose retrieval function.
Further experiments are needed to show, if the impact of BR in the mycorrhizal symbiosis involves the regulation of defense responses against the AM fungus, the localization of the sucrose transporter and/or further until now unknown interactions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figure_S1.pptx
Download MS Power Point (437.4 KB)References
- Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, et al. The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc Natl Acad Sci U S A 1999; 96:1761-6; PMID:9990098; http://dx.doi.org/10.1073/pnas.96.4.1761.
- Bitterlich M, Krügel U, Boldt-Burisch K, Franken P, Kühn C. The sucrose transporter SlSUT2 from tomato interacts with brassinosteroid functioning and affects arbuscular mycorrhiza formation. Plant J 2014; PMID:24654931.
- Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, et al. SUT2, a putative sucrose sensor in sieve elements. Plant Cell 2000; 12:1153-64; PMID:10899981; http://dx.doi.org/10.1105/tpc.12.7.1153.
- Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kühn C. Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J 2006; 45:180-92; PMID:16367963; http://dx.doi.org/10.1111/j.1365-313X.2005.02572.x.
- Doidy J, Grace E, Kühn C, Simon-Plas F, Casieri L, Wipf D. Sugar transporters in plants and in their interactions with fungi. Trends Plant Sci 2012; 17:413-22; PMID:22513109; http://dx.doi.org/10.1016/j.tplants.2012.03.009.
- Liesche J, Krügel U, He H, Chincinska I, Hackel A, Kühn C. Sucrose transporter regulation at the transcriptional, post-transcriptional and post-translational level. J Plant Physiol 2011; 168:1426-33; PMID:21444123; http://dx.doi.org/10.1016/j.jplph.2011.02.005.
- Fan RC, Peng CC, Xu YH, Wang XF, Li Y, Shang Y, et al. Apple sucrose transporter SUT1 and sorbitol transporter SOT6 interact with cytochrome b5 to regulate their affinity for substrate sugars. Plant Physiol 2009; 150:1880-901; PMID:19502355; http://dx.doi.org/10.1104/pp.109.141374.
- Niittylä T, Fuglsang AT, Palmgren MG, Frommer WB, Schulze WX. Temporal analysis of sucrose-induced phosphorylation changes in plasma membrane proteins of Arabidopsis. Mol Cell Proteomics 2007; 6:1711-26; http://dx.doi.org/10.1074/mcp.M700164-MCP200.
- Roblin G, Sakr S, Bonmort J, Delrot S. Regulation of a plant plasma membrane sucrose transporter by phosphorylation. FEBS Lett 1998; 424:165-8; PMID:9539143; http://dx.doi.org/10.1016/S0014-5793(98)00165-3.
- Krügel U, He HX, Gier K, Reins J, Chincinska I, Grimm B, et al. The potato sucrose transporter StSUT1 interacts with a DRM-associated protein disulfide isomerase. Mol Plant 2012; 5:43-62; PMID:21746698; http://dx.doi.org/10.1093/mp/ssr048.
- Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S, et al. AmiGO: online access to ontology and annotation data. Bioinformatics 2009; 25:288-9; PMID:19033274; http://dx.doi.org/10.1093/bioinformatics/btn615.
- Hossain Z, McGarvey B, Amyot L, Gruber M, Jung J, Hannoufa A. DIMINUTO 1 affects the lignin profile and secondary cell wall formation in Arabidopsis. Planta 2012; 235:485-98; PMID:21947665; http://dx.doi.org/10.1007/s00425-011-1519-4.
- Mongrand S, Morel J, Laroche J, Claverol S, Carde JP, Hartmann MA, et al. Lipid rafts in higher plant cells: purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J Biol Chem 2004; 279:36277-86; PMID:15190066; http://dx.doi.org/10.1074/jbc.M403440200.
- Kuhn H, Kuster H, Requena N. Membrane steroid-binding protein 1 induced by a diffusible fungal signal is critical for mycorrhization in Medicago truncatula. New Phytol 2010; 185:716-33; PMID:20003073; http://dx.doi.org/10.1111/j.1469-8137.2009.03116.x.
- Ferguson BJ, Ross JJ, Reid JB. Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiol 2005; 138:2396-405; PMID:16055684; http://dx.doi.org/10.1104/pp.105.062414.
- Hong Z, Ueguchi-Tanaka M, Fujioka S, Takatsuto S, Yoshida S, Hasegawa Y, et al. The Rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell 2005; 17:2243-54; PMID:15994910; http://dx.doi.org/10.1105/tpc.105.030973.
- Aoki N, Hirose T, Scofield GN, Whitfeld PR, Furbank RT. The sucrose transporter gene family in rice. Plant Cell Physiol 2003; 44:223-32; PMID:12668768; http://dx.doi.org/10.1093/pcp/pcg030.
- Yu X, Li L, Zola J, Aluru M, Ye H, Foudree A, et al. A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J 2011; 65:634-46; PMID:21214652; http://dx.doi.org/10.1111/j.1365-313X.2010.04449.x.
- Fujiwara M, Hamada S, Hiratsuka M, Fukao Y, Kawasaki T, Shimamoto K. Proteome analysis of detergent-resistant membranes (DRMs) associated with OsRac1-mediated innate immunity in rice. Plant Cell Physiol 2009; 50:1191-200; PMID:19502382; http://dx.doi.org/10.1093/pcp/pcp077.
- Inada N, Ueda T. Membrane Trafficking Pathways and their Roles in Plant-Microbe Interactions. Plant Cell Physiol 2014; 55:672-86; PMID:24616268; http://dx.doi.org/10.1093/pcp/pcu046.
- Jones AM, Xuan Y, Xu M, Wang RS, Ho CH, Lalonde S, et al. Border control–a membrane-linked interactome of Arabidopsis. Science 2014; 344:711-6; PMID:24833385; http://dx.doi.org/10.1126/science.1251358.
