Abstract
An in vitro method of multiple shoot induction and plant regeneration in Psophocarpus tetragonolobus (L.) DC was developed. Cotyledons, hypocotyls, epicotyls, internodal and young seedling leaves were used as explants. MS media supplemented with various concentrations of either thidiazuron (TDZ) or N6-benzylaminopurine (BAP) along with NAA or IAA combinations were used to determine their influence on multiple shoot induction. MS media supplemented with TDZ induced direct shoot regeneration when epicotyls and internodal segments were used as explants. TDZ at 3 mg L−1 induced highest rate (89.2 ± 3.28%) of regeneration with (13.4 ± 2.04) shoots per explant. MS media supplemented with BAP in combination with NAA or IAA induced callus mediated regeneration when cotyledons and hypocotyls were used as explants. BAP (2.5 mg L−1) and IAA (0.2 mg L−1) induced highest rate (100 ± 2.66%) of regeneration with (23.2 ± 2.66) shoots per explant. Mature plants produced from regenerated shoots were transferred successfully to the greenhouse. In a comparative study, the phenolics contents of various parts of greenhouse-grown plants with that of in vitro-raised plants showed significant variations.
Keywords:
Abbreviations
| BAP | = | 6-benzylaminopurine |
| IAA | = | 3-indoleacetic acid |
| NAA | = | naphthalene-acetic acid |
Introduction
Psophocarpus tetragonolobus (L.) DC. is popularly known as Winged bean or Goa bean. Winged bean is widely distributed and cultivated in tropical and sub-tropical regions of the world.Citation1 Winged bean contains significant amount of protein in all parts of the plant i.e. seeds, pods, leaves and roots or tubers. All these parts are edible. The crude protein and fatty oilCitation2 in the seeds of winged bean is almost comparable with Soybean. Secondly; the crop is an efficient nitrogen-fixer for sustainable agriculture system. Hence, the winged bean is identified as the future soybean, and the crop needs serious attention for its improvement. The delayed and poor germination of seeds is a serious problem experienced by the growers which is attributed due to its hard seed-coat rather than to seed dormancy.Citation3 So, an efficient and reproducible in vitro regeneration system is pre-requisite for using recent advances in biotechnology to improve crop plants. It has been suggested that leguminous species are mostly recalcitrant for inducing shoot regeneration in in vitro culture and highly species-specific methods are required to overcome this difficulty.Citation4 Although, P. tetragonolobus (L.) DC. is considered to be recalcitrant to tissue culture techniques, the first report on regeneration of plants in winged bean was announced by Venketeswaran and Huhtinen (1978).Citation5 In vitro plant regeneration from various explants and callus has been reported in winged bean.Citation6-12 Since then, there is an increasing demand to develop methods which can facilitate high frequency regeneration in winged bean. The present report shows the development of an efficient method of in vitro multiple shoot regeneration and a comparative study of some of the secondary metabolite profiles of greenhouse grown and tissue culture raised plants of P. tetragonolobus.
Materials and Methods
Plant material and media
Seeds of P. tetragonolobus were procured from National Bureau of Plant Genetic Resources (NBPGR), Akola (Maharashtra), India. Mature dry seeds were washed under running tap water for 10 min and were surface sterilized with 0.1% aqueous HgCl2 (w/v) for 7 min followed by rinsing with sterile distilled water for 5 times. The surface sterilized seeds were soaked in sterile distilled water for 16-24 hours. These seeds were incubated in Petri dishes containing sterile and wet filter paper for 24 hours in dark. Then they were inoculated in test tubes containing half strength MS salts,Citation13 3.0% (w/v) sucrose and 0.7% (w/v) agar. All types of media used in this experiment were solidified with 0.7 or 0.8% agar and the pH was adjusted to 5.8 before autoclaving. Cultures were placed under cool-white fluorescent light of 1200 lux light intensity in 16 hours photoperiod at 25–28°C for one to 2 weeks for germination. The germinated seedlings were used in further experiments.
In-vitro multiple shoot induction and plant regeneration from various explants
Cotyledons from surface sterilized and soaked seeds were cut in to 0.8 cm2 pieces by trimming their edges. Hypocotyl, epicotyl and internodal segments from 2 weeks old seedlings were cut in to 1 cm long. The young leaves from 2 week old seedlings were cut in to ∼1 cm2. All these explants were incubated on media containing MS salts,Citation13 B5 vitamins,Citation14 0.01% myo-inositol and 3.0% sucrose, supplemented with various concentrations i.e., 1.0, 1.5, 2.0, 2.5 and 3.0 mg L−1 of 6-benzylaminopurine (BAP) in combination with various concentrations i.e. at 0.1, 0.15, 0.2, 0.25 and 0.3 mg L−1 of either α-Naphthalene acetic acid (NAA) or 3-Indoleacetic acid (IAA).
For achieving direct shoot induction, various explants were also incubated on MS media supplemented with various concentrations of TDZ (0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 and 4.0 mg L−1). All the media was solidified with 8 g L−1 agar and the pH was adjusted to 5.8 before autoclaving. The cultures were incubated at 25–28°C under cool-white fluorescent light with photosynthetic photon flux (PPF) of about 60 ± 5 μ mol m−2s−1 in 16 hours photoperiod. All the experiments were repeated thrice. In order to prevent tissue browning caused due to exudation of phenolics from the explants, different explants were incubated on media supplemented with polyvinyle pirrilindone-40 (PVP-40) and silver nitrate (AgNO3) at 0.1% to 1.0% concentrations. These explants were subsequently sub-cultured on fresh media at every 2 weeks interval.
Rooting and acclimatization
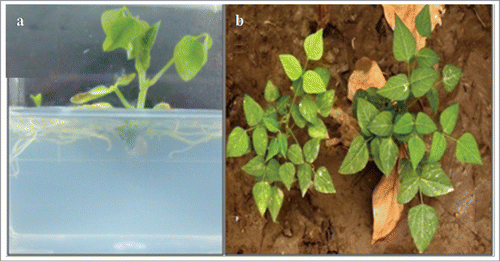
Shoots were isolated carefully from the mass of shoots and transferred to shoot elongation medium containing MS salts, 0.01% myo-inositol, 3.0% sucrose supplemented with 2.0 mg L−1 of zeatin or 2-Isopentyl adenine (2iP), solidified with 0.8% agar. The elongated shoots were transferred to rooting medium containing MS salts, 0.01% myo-inositol and 3.0% sucrose supplemented with no plant growth regulators, and these were solidified with 0.7% agar for rooting. The well rooted plants were transferred to plastic pots containing mixture of soilrite and soil in the ratio of 1:2. Plants were usually covered with polythene bags for 1-2 weeks for acclimatization. Polythene covers were gradually opened before transferring plants to glasshouse.
Estimation of flavonoids in various parts of P. tetragonolobus
For estimation of phenolic and flavonoid contents in various parts i.e., leaf, stem and root of the plant, these tissues were collected from 6 months old plant raised from tissue culture and from greenhouse. Estimation and analysis of polyphenolic compounds by HPLC was followed by Mohanty et al. 2013.Citation15 Briefly, the plant material (1g) was grounded in liquid nitrogen in to fine powder and was added to 3 ml of 80% methanol followed by 10 min sonication. After centrifugation for 5min at 4,000g the extract was adjusted to the final volume of 10 ml with methanol. Estimation of various polyphenolic compounds were performed through HPLC-UV spectrophotometer (Shimadzu LC-10A, Japan) equipped with dual pump LC-10AT binary system, UV detector SPD-10A at 254 nm, rheodyne injection valve furnished with a 20 μl loop on phenomenex Luna RP-C 18 column (4.6 × 250 mm, i.d., 5 μm pore size) preceded with guard column of same chemistry. Commercially available standards Sigma-made were run as controls for estimation of specific polyphenolics. The Data was analyzed by Shimadzu class VP series software. The experiment was repeated thrice. The average data obtained was finally used for further analysis.
Results
Control of tissue browning caused by phenolic leaching from explants
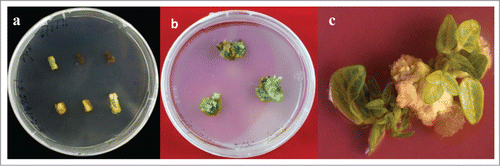
Various explants started turning brown after incubation on media. This is caused by exudation of phenolics from explants. Media supplemented with all concentrations of antioxidants PVP and AgNo3 stopped the tissue browning. Although PVP stopped the tissue browning, explants did not respond to any regeneration process (). AgNo3 at 0.1% in the media successfully prevented the tissue browning without affecting either callus induction or shoot regeneration. Frequent sub-culturing of explants on fresh media for every 2 weeks of intervals also controlled the tissue browning.
Direct shoot regeneration from epicotyle and internodal explants
After 4-5 weeks of culturing of various explants on different media, media supplemented with TDZ induced direct shoot regeneration when epicotyls and internodal segments were used as explants (). Media containing TDZ at 3 mg L−1 induced highest number of (13.4 ± 2.04) shoots per epicotyl explants with highest rate of (89.2 ± 3.28%) explants responded. TDZ at the same concentration (3 mg L−1) in the media gave best results when internodal segments were used as explants, in which (85.3 ± 4.16%) explants were responded and (6.5 ± 23) shoots were produced per explants (). TDZ at either lower (<3 mg L−1) and higher (>3 mg L−1) concentrations in shoot induction media was not much effective (). Multiple shoots were carefully separated from the explants and sub-cultured onto MS medium containing the same hormonal composition for further shoot proliferation. Individual well developed shoots were transferred to elongation media containing zeatin. The elongated shoots further transferred to rooting media.
Table 1. Direct shoot regeneration from epicotyls and internodal segments of P. tetragonolobus. Effect of TDZ concentrations in shoot-induction media on regeneration efficiency
Callus mediated multiple shoot induction from cotyledonery and hypocotyle explants
Media supplemented with BAP in combination with NAA or IAA induced callus mediated regeneration when cotyledons and hypocotyls were used as explants. After (3–4) weeks of sub-culturing various explants started producing callus on media containing different concentrations of BAP and NAA or IAA. (). Callus isolated form explants was sub-cultured on to fresh media containing same growth regulators. After 2 weeks of sub-culturing, multiple shoot buds were produced from the callus (). Media containing BAP 2.0 mgL−1 and IAA 0.2 mgL−1 induced highest regeneration response when both cotyledons (99.66 ± 0.57%) and hypocotyls (100 ± 0.0%) used as explants. Hypocotyls produced (23.2 ± 2.66) shoots per explant; cotyledons produced (18.6 ± 3.45) shoots per explant, in the same media composition (). Media containing either lower concentrations of BAP (<2.0 mg L−1) and NAA or IAA (<0.2 mg L−1), or higher concentrations of the same PGRs (>2.0 mg L−1 and >0.2 mg L−1) reduced the regeneration response of both cotyledon and hypocotyls explants (). Although leaf discs produced callus on media containing all concentration of BAP and NAA or IAA, callus derived from it did not produce any shoots up on further sub-culturing on fresh media ( and ).
Table 2. Callus mediated shoot bud induction from cotyledon, leaf and hypocotyl explants of P. tetragonolobus, effect of BAP in combination with IAA or NAA concentrations on regeneration response
Figure 2. Callus mediated shoot bud induction and shoot regeneration form hypocotyls and cotyledon explants on media containing BAP and NAA or IAA. (A) and (B). Callus induction from hypocotyl explants on media containing various concentrations of plant growth regulators, callus with regeneration potential was indicated with arrow markings; (C) Callus produced from cotyledon explants; (D) Callus induction from leaf discs incubated BAP and NAA containing medium. (E) Shoot buds and shoots produced from the callus which was obtained from hypocotyls explants; (F) Incubation of shoots for further proliferation on shoot induction medium.
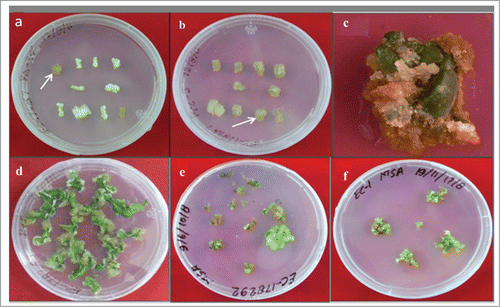
The shoot buds produced from callus was separated carefully and transferred to fresh media for further shoot proliferation (). It was observed that the morphology of the callus associated with different shoot bud induction potential, changed with concentrations of BAP and NAA or IAA used in the media (). Media supplemented with BAP (2.5 mg L−1) and IAA (0.2 mg L−1) induced callus with green color and very loose texture, with highest regeneration efficiency (100 ± 0.0).This callus, on sub-culturing on shoot induction media produced highest number of shoots (23.2 ± 2.66) per explant ( and ; ). Media containing other concentrations of BAP and NAA or IAA, produced the callus with various other morphologies which produced less or no shoot buds (). BAP and IAA at 2.5 mg L−1 and 0.2 mg L−1 and 2.5 mg L−1 and 0.25 mg L−1 concentrations in the media supported the growth rate of callus. This was evaluated in terms of the calculated dry weight of callus at periodic intervals (). Individual shoots were carefully separated and transferred on to MS media containing Zeatin for elongation. The elongated shoots were produced roots when transferred to rooting media (). These mature plants after hardening process were transferred successfully to glasshouse ().
Table 3. Effect of BAP, NAA and IAA concentrations on morphology of the callus associated with regeneration response. Observations were made when hypocotyl segments were used as explants
Comparative study of flavanoids in tissue culture raised and greenhouse grown P. tetragonolobus plants
Major flavonoids present in various parts i.e. stem, leaf and root of the P. tetragonolobus plant such as phenolic acids: gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid and ferulic acid, and flavonols : rutin, quercetin and kaempferol were quantified. In the comparative analysis it was observed that the contents of these secondary metabolites in various parts of green house grown plants varied significantly with that of tissue cultured raised plants. Contents of flavonoids such as gallic acid, chlorogenic acid, rutin and ferulic acid in the stems of green house grown plants showed significant variation with that of tissue culture raised plants (). However, the difference in levels of protocatechueic acid, quercetin, caffeic acid and kaempferol were not much significant (). Contents of flavonoids such as gallic acid, rutin, ferulic acid and kaempferol in the leaves of greenhouse grown plants showed significant variation with that of tissue culture raised plants. However, contents of protocatechuic acid, chlorogenic acid, quercetin and caffeic acid in leaves of greenhouse grown plants did not show much variation when compared with that of tissue culture raised plants (). In case of roots, green house grown plants showed the significant difference in levels of flavonoids such as gallic acid, rutin, ferulic acid quercetin and caffeic acid with that of tissue culture raised plants. Contents of ferulic acid and caffeic acid were in trace amounts in roots of tissue culture grown plants. Whereas, these were present at (12.5 ± 2.0) and (11.66 ± 3.1) μg/g tissue in roots of greenhouse grown plants. Contents of protocatechueic acid and kaempferol in roots of both types of plants did not show significant variation ().
Table 4. Contents of secondary metabolites in different parts of P. tetragonolobus plants from green house and tissue culture raised plants
Figure 4. Comparative analysis of polyphenolic compounds present in stem, leaf and roots of green house grown (labeled as green) and tissue culture raised (labeled as red) P. tetragonolobus plants; (A) Graph showing contents of poyphenolic compounds in Stems of both type of plants; (B) Graph showing contents of poyphenolic compounds in Leaves of both type of plants; (C) Graph showing contents of poyphenolic compounds in Roots of both type of plants. Graphs were plotted with concentrations of polyphenolic compounds (mg/g) on x-axis and various types of polyphenolic compounds on y-axis.
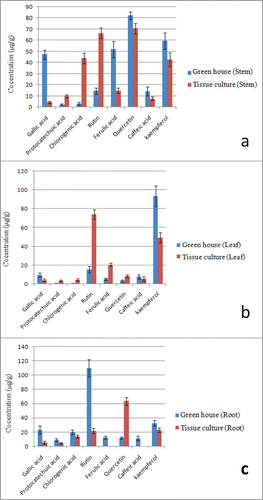
Gallic acid, Ferulic acid, Quercetin and Kaempferol were found to be in major quantities in stems of green house grown plants, where as chlorogenic acid, rutin, quercetin and kaempferol were in major quantities in stems of tissue culture raised plants ( and ). Roots of green house grown plants contained rutin only in major quantity, while the roots of tissue culture raised plants contained only quercetin in major quantity ( and ). Only kaempferol was in major quantity in leaves of green house grown plants, but leaves of tissue culture raised plants contained rutin and kaempferol in major quantity ( and ).
Discussion
Winged bean is an underutilized legume crop and needs much attention because of its utility value. Our earlier report showed characterization of winged bean (Psophocarpus tetragonolobus (L.) DC.) based on molecular, chemical and physiological parameters, and has provided significant insights for further improvement of winged bean crop for its qualitative and quantitative traits.Citation15 Crop improvement through biotechnological approach mainly requires efficient method of tissue culture and genetic transformation. Leguminous plants are generally recalcitrant to tissue culture and are highly genotype-specific.Citation16 We have developed an efficient method of both direct shoot regeneration and callus mediated shoot regeneration by using various explants of P. tetragonolobus. The major problem encountered in tissue culture of leguminous species is tissue browning caused by phenolic exudation from explants. Since the members of the family leguminoseae contain high levels of phenolics, plant recalcitrance caused by the oxidation of explants is highly prevalent.Citation17 Antioxidants such as PVP, ascorbic acid, cysteine and AgNO3 are used to prevent tissue browning problem in tissue culture of various plants.Citation18 Quick transfer of explants at short intervals on a fresh media is the simplest and effective method to protect explants from detrimental effects of oxidative browning. In the present study, addition of AgNO3 in media prevented tissue browning in winged bean explants. The tissue browning problem also stopped by frequent sub-culturing of explants on a fresh media for every 2 weeks interval. Hence, a combination of both methods i.e., addition of AgNO3 in the media and quick sub-culturing of explants was adopted in our experiment to prevent tissue browning.
The choice of initial explant is a critical factor for shoot regeneration. Regeneration from different explants such as shoot-tip,Citation19 leaf segments,Citation20 cotyledonary nodes,Citation21 apical meristem,Citation22 root,Citation23 petioleCitation24 and hypocotylCitation25 has been reported in many plant species. In this report we have tried various explants such as cotyledons, hypocotyls, epicotyles, internodes and young leaf for in vitro regeneration. Except leaf explants, all the other explants produced in vitro regeneration against various plant growth regulators used in the media. Our report shows 2 types of in vitro regenerations: (a) Direct shoot regeneration from explants such as epicotyl and intermodal segments when TDZ supplemented in media () and (b) Callus mediated shoot regeneration from cotyledons and hypocotyls when BAP and either NAA or IAA supplemented in the media ().
TDZ has been used in various leguminous species which are known to be recalcitrant for regeneration to induce direct shoot regeneration and direct somatic embryogenesis.Citation26 It was observed that TDZ was much effective when used at lower concentrations in the regeneration media. TDZ at lower concentrations i.e. 0.01 mg L−1 in the regeneration media was shown to be the best for direct shoot regeneration in Abelmoschus moschatus.Citation27 The protocol that we have developed for direct shoot regeneration in winged bean plant will be useful for its genetic improvement. For Agrobacterium-mediated genetic transformation direct shoot regeneration from the explants is more desirable than going through an intermediate callus phase.Citation28-31 Further, the callus mediated shoot regeneration from cotyledon, hypocotyl explants of winged bean when BAP and either NAA or IAA used in the regeneration media ().BAP in presence of both NAA and IAA induced callus mediated shoot bud induction. Best results were obtained when BAP and IAA or NAA used in the media at 2.0 mg L−1 and 0.2 mg L−1 respectively. Hypocotyl explants were shown to give highest number of shoots per explant i.e., (23.2 ± 2.66). At either decreasing or increasing concentrations of these Cytokinin and Auxin in the media gradually reduced the regeneration efficiency (). Previous reports on leguminous species have showed maximum in vitro regeneration in auxin, cytokinin supplemented media. BAP along with IAA/NAA/IBA was found to give maximum regeneration when cotyledons were used as explants in winged bean.Citation32 The beneficial effects of BAP on adventitious shoot induction were also observed in other legumes such as Vigna angularis Citation33 and V. mungo. Citation34
In this study, effect of cytokinin and auxin concentration on morphology of the callus was further related to shoot regeneration efficiency. This was observed in case of both cotyledons and hypocotyles used as explants, the data was recorded and represented of the hypocotyl explants (; and ). The multiple shoot induction rate and morphogenetic response significantly varied according to the explant type and concentrations of plant growth regulators.Citation35,36
This study showed a comparative polyphenolics profiles in greenhouse grown plant with that of tissue culture raised plants. Significant variations were observed in the contents of various polyphenolic compounds among various tissues. The content of the total flavonoids in various parts of greenhouse grown plants were observed as in the following order: Stem> roots>leaf, and in the tissue cultured raised plants the order was: Stem> leaf >root (). The observation made in our studies also supports earlier reports where it was reported that flavonoid biosynthesis is highly regulated by developmental and environmental factors.Citation37-39 In case of Withania somnifera, it has been observed that the contents of withanolides, phenolics, flavonoids and polysaccharides in various parts of in vitro grown plants greatly varied with that of greenhouse grown plants.Citation40
Conclusion
In the present study we are reporting efficient method of in vitro regeneration in P. tetragonolobus plant. We have established both direct and callus mediated shoot regeneration. Media supplemented with TDZ induced direct shoot induction and media supplemented with BAP in combination with NAA or IAA induced callus mediated shoot regeneration. These in vitro regeneration methods are useful in micro-propagation and genetic transformation of winged bean plant. This report also shows the comparative analysis of phenolics in plants raised in 2 different growth conditions, which will provide an insight for further improvement of the crop.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
This work was supported under EMPOWER program of Council of Scientific and Industrial Research (CSIR), India. Director CSIR-NBRI is duly acknowledged for providing basic infrastructure facilities to carry out this research work.
References
- Anon. The winged bean. A high protein crop for the tropics. National Academy Press, Washington D.C. 1981; 46(2).
- Mohanty CS, Pradhan RC, Singh V, Singh N, Pattanayak R, Prakash O, Chanotiya CS, Rout PK. Physicochemical analysis of Psophocarpus tetragonolobus (L.) DC seed with fatty acid and total lipid composition. J Food Sci Technol 2014; http://dx.doi.org/10.1007/s13197-014-1436-1
- Solanki SS, Joshi RP. Methods of increasing seed germination of winged bean. In: Prog Hort 1983;15:210-212.
- Atkins CA, Smith PMC. Genetic transformation and regeneration of legumes. In: Legocki H, Bothe H, Puhler A (eds) Biological fixation of nitrogen For ecology and sustainable agriculture, vol.39 Springer, Berlin Heidelberg New York 1997;283-304.
- Venketeswaran S, Huhtinen O. In vitro root and shoot differential form callus cultures of a legume, the winged bean,(psophocarpous tetragonolobus). In vitro (Abstr.) 1978.
- Ahmed R, Dutta Gupta S, De DN. Somatic embryogenesis and plant regeneration from leaf derived callus of winged bean Psophocarpus tetragonolobus(L.) DC). Plant Cell Rep 1996; 15:531-535; PMID:24178467; http://dx.doi.org/10.1007/BF00232988
- Bottino PJ, Maire CE, Goff LM. Tissue culture and Organogenesis in the winged bean. Can J bot 1979; 57:1773-6; http://dx.doi.org/10.1139/b79-219
- Dutta Gupta S, Ahmed R, De DN. Direct somatic embryogenesis and plantlet regeration from seedling leaves of winged bean. Plant Cell Rep 1997; 16:628-31; http://dx.doi.org/10.1007/BF01275504
- Gregory HM, Haq N, Evans PK. Regeneration of plantlet from leaf callus of Winged bean (Phophocarpus tetragonolobus L) DE. Plant Sci Lett 1980; 18:395-400; http://dx.doi.org/10.1016/0304-4211(80)90104-2
- Mehta U, Mohan Ram HY. Tissue culture and whole plant regeneration in the Winged bean (Phophocarpus tetragonolobus (L) DC.) Ann Bot 1981; 47:163-6.
- Trinh TH, Lie-Schricke H, Tran Thanh Van K. Formation direct de bourgeon a partirdes fragments et des couches cellulaires minces de difference organs chez le ((Psophocarpus tetragonolobus). Zeitshrschrift fur pflanzenphysiologie 1981;102:127-39; http://dx.doi.org/10.1016/S0044-328X(81)80191-2
- Venkatswaran S, Dias MADL, Weyers UV. Organogenisis and somatic embrogenisis from callus of winged bean.(Psophocarpus tetragonolobus L. DC) Acta. Hortic 1992; 280:202-6.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962; 15:473-97.
- Gamborg OL, Miller RA, Ojima O. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res 1968; 50:151-8; http://dx.doi.org/10.1016/0014-4827(68)90403-5
- Mohanty CS, Verma S, Singh V, Khan S, Gaur P, Gupta P, Nizar MA, Dikshit N, Pattanayak R, Shukla A, et al. Characterization of winged bean (Psophocarpus tetragonolobus (L.) DC.) based on molecular, chemical and physiological parameters. AJMB 2013; 3, 187-197; http://dx.doi.org/10.4236/ajmb.2013.34025
- Somers DA, Samac DA, Olhoft PM. Recent advances in legume transformation. Plant Physiol 2003; 131: 892-9; PMID:12644642; http://dx.doi.org/10.1104/pp.102.017681
- Anthony JM, Senaratna T, Dixon KW, Sivasithamparam K , Bunn E. In vitro regeneration of recalcitrant Australian plants. In vitro Cell Dev Biol 1999; 4:35-53.
- Ishtiaq A, Tanveer H, Irfan A, Muhammad N, Marvam Muhammad R, Muhammad I. Lethal effects of secondary metabolites on plant tissue culture. Americaln-Eurosian. J Agric & Environ Sci 2013;13(4): 539-47.
- Gulati A, Jaiwal PK. Culture conditions effecting plant regeneration from cotyledon of Vigna radiata. Plant Cell Tiss Org 1990; 23:1-7; http://dx.doi.org/10.1007/BF00116082
- Meijer EGM, Brown DCW. Screening of diploid Medicago sativa germplasm for somatic embryogenesis. Plant Cell Rep 1985; 4:285-288; PMID:24253990; http://dx.doi.org/10.1007/BF00269379
- Wright E, Dixon RA, Wang ZY. Medicago truncatula transformation using cotyledon explants. Methods Mol Biol 2006; 343: 129-36; PMID:16988339
- Agarwal DC, Benerjee AK, Kolala RR, Dhage AB, Kulkarni AV, Nalawade SM, Haxra S, Krishnamurthy KV. In vitro induction of multiple shoots and plant regeneration in cotton (Gossypium hirsutum L.). Plant Cell Rep 1997; 16:647-52; http://dx.doi.org/10.1007/BF01275508
- Valvekens D, Montagu MV, Lijsebettens MV. Agrobacterium tumefaciens mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA 1988; 85: 5536-40; PMID:16593964; http://dx.doi.org/10.1073/pnas.85.15.5536
- Svetoslavova G, Vlahova M, Iantcheva A, Atanassov A. High frequency plant regeneration of diploid Medicago caerulea through somatic embryogenesis. Biotechnol Biotech Eq 2005; 19: 2-7; http://dx.doi.org/10.1080/13102818.2005.10817191
- Isabelle RD, Fredric SS, Rajbir and Brigitte SS. In plants 2, 3, 5 triiodobenzoic acid treatment promotes high frequency and routine in vitro regeneration of sugar beet plants. Plant Cell Rep 1996; 16:142-6; PMID:24177540; http://dx.doi.org/10.1007/BF01890855
- Cruz DE, Carvalho MH, Van LEB, Zuilyfodil Y, Phamthi AT, Tran Thanh Van K. Efficient whole plant regeneration of common bean(Phaseolus vulgaris L.) using thin-cell-layer culture and silver nitrate. Plant Science 2000; vol. 159 2:223-232; http://dx.doi.org/10.1016/S0168-9452(00)00346-0
- Sharma R, Shahzad A. Thidiazuran (TDZ) Induced Regeneration from Cotyledonary Node Explant of Abelmoschus moschatus Medik. L., (A Valuable Medicinal Plant). World J Agr Sci 2008;4 (4):449-52.
- Karp A, Nelson RS, Thomas E, Bright SW. Chromosome variation in protoplast-derived potato plants. Theor Appl Genet 1982; 63:265-72; http://dx.doi.org/10.1007/BF00304006
- Kovacs EI. Regulation of karyotype stability in tobacco tissue cultures of normal and tumorous genotypes. Theor Appl Genet 1985; 70:548-54; http://dx.doi.org/10.1007/BF00305989
- Larkin PJ, Scowcroft WR. Somaclonal variation-a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 1981; 60:197-214; http://dx.doi.org/10.1007/BF02342540
- Stiekema WJ, Heidekamp F, Louwerse JD, Verhoeven HA, Dijkhuis P. Introduction of foreign genes into potato cultivars bintje and desiree using an agrobacterium tumefaciens binary vector. Plant cell Rep 1988; 7: 47-50; PMID:24241414; http://dx.doi.org/10.1007/BF00272976
- Koshy EP, Alex BK, John P. Regeneration potential of right and left cotyledons in winged bean (psophocarpus tetragonolobus (L.) DC). IJANS 2013; 2319-4014; 2 (3):41-4.
- Avenido RA, Hattori K. Benzyladenine-induced adventitious shoot regeneration from hypocotyls of adzukibean (Vigna angularis (Willd) Ohwi & Ohashi). Plant Growth Regulation 2000; vol. 31 3:147-53; http://dx.doi.org/10.1023/A:1006302816178
- Saini R, Jaiwal PK. Age: Position in mother seedling, orientation and polarity of the epicotyls segments of blackgram (Vigna mungo L. Hepper) determines its morphogenic response. Plant Sci 2002; vol.163 1:101-9; http://dx.doi.org/10.1016/S0168-9452(02)00062-6
- Ozgen M, Ozcan S, Sevimay CS, Sancak C, Yıldız M. High frequency adventitious shoot regeneration in sainfoin. Plant Cell,Tissue Organ Cult 1998; 52:205-8.
- Uranbey S. Thidiazuron induced adventitious shoot regeneration in henbane (Hyoscyamus niger L.), Biol. Plant. 2005; 49(3):427-30; http://dx.doi.org/10.1007/s10535-005-0021-x
- Feinbaum RL, Ausubel FM. Transcriptional regulation of the Arabidopsis thaliana chalcone synthase gene. Mol Cell Biol 1988; 8(5):1985-92; PMID:3386631
- Kubasek WL, Shirley BW, McKillop A, Goodman HM, Briggs W, Ausubel FM. Regulation of flavonoid biosynthetic genes in germinating Arabidopsis seedlings. Plant Cell 1992; 4:1229-36; PMID:12297632; http://dx.doi.org/10.1105/tpc.4.10.1229
- Winkel, Shirley B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol 2002; 5:218-23
- Dewir YH, Chakrabarty D, Lee SH, Hahn EJ, Paek KY. Indirect regeneration of Withania somnifera and comparative analysis of withanolides in in vitro and greenhouse grown plants. Biol Plant 2010; 54:357-60; http://dx.doi.org/10.1007/s10535-010-0063-6