Abstract
Transport of proteins via the secretory pathway is controlled by a combination of signal dependent cargo selection as well as unspecific bulk flow of membranes and aqueous lumen. Using the plant vacuolar sorting receptor as model for membrane spanning proteins, we have distinguished bulk flow from signal mediated protein targeting in biosynthetic and endocytic transport routes and investigated the influence of transmembrane domain length. More specifically, long transmembrane domains seem to prevent ER retention, either by stimulating export or preventing recycling from post ER compartments. Long transmembrane domains also seem to prevent endocytic bulk flow from the plasma membrane, but the presence of specific endocytosis signals overrules this in a dominant manner.
When soluble proteins are transported to the apoplast or the vacuole, they effectively leave the secretory pathway. In contrast membrane proteins remain within the pathway, unless they segregate into the intraluminal vesicles of post Golgi organelles to reach either the vacuole lumen for degradationCitation1 or to be secreted as “exosomes.”Citation2-4 While in the pathway, membrane proteins visit and recycle between many compartments, and they thus need a multitude of signals to regulate their steady state levels.
The plant vacuolar sorting receptor (VSR) is a valuable type I membrane spanning protein that completes many transport cycles before it is degraded.Citation5 VSR export from the ER occurs in a COPII dependent manner by bulk flow, without specific signals.Citation6 However, the conserved peptide sequence YMPL in its short cytosolic tail mediates segregation from biosynthetic secretory bulk flow.Citation7 When VSRs reach the prevacuolar compartment (PVC), the YMPL motif also facilitates VSR recycling,Citation8 a rate-limiting transport step that explains why VSRs are best detected at the PVC.Citation9
Interestingly, the VSR tail contains additional contingency signals to deal with mis-sorting. The conserved IM motif prevents plasma membrane accumulation of VSRs when YMPL-mediated sorting to the PVC is impaired.Citation10 To test if the IM motif mediates increased endocytosis or reduced exocytosis, we have used the ligand-binding and release properties of full-length VSRs. By monitoring VSR-mediated vacuolar cargo release at the cell surface, we could show that the IM motif increases endocytic recycling of VSRs rather than preventing plasma membrane arrival (Gershlick et al. 2014, Fig. 9 Citation6). In contrast, the YMPL motif prevents VSRs arrival at the plasma membrane most probably via interactions with AP1 and/or AP4 complexes at the level of TGN or the Golgi apparatus.Citation6,11
These recent results prompted us to ask if endocytosis also has a bulk flow component, similar to biosynthetic bulk flowCitation12 Although it was shown before that long transmembrane domains (TMDs) promote plasma membrane accumulation of membrane proteins,Citation13 it is unknown if this is due to increased anterograde transport or reduced endocytosis. For this reason, we modified the series of C-terminal truncation mutants of VSR () by imposing a long TMD. This would allow us to test the role of the TMD within the context of the presence or absence of biosynthetic and endocytic transport signals in the VSR tail.
Figure 1. Summary of localization of various VSR mutants. (A) A schematic of the various tail deletion mutants described previouslyCitation6 and in this study, together with the corresponding subcellular locations in the presence of a wild type transmembrane domain (wt TMD) or a longer version (Long TMD). (B) Representative confocal laser scanning micrographs of Agrobacterium-infiltrated tobacco leaf epidermis cells expressing either a soluble ER marker (GFP-HDEL) or various mutants of the fluorescent receptor model membrane cargo GFP-VSR2. All VSR variants in this study have been cloned under the control of the weak TR2 promoter.
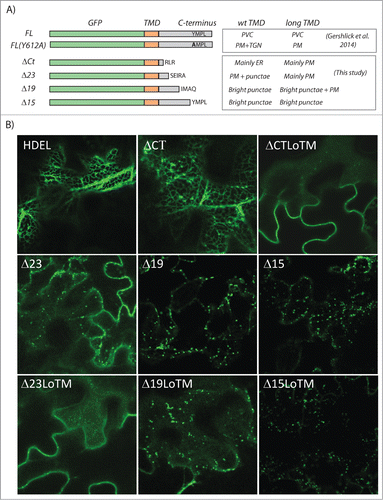
shows that the longest deletion (ΔCT) was mostly ER retained, similar to the standard ER marker GFP-HDEL, except for bright punctae earlier shown to be Golgi and post-Golgi compartments.Citation6 However, introduction of a long TMD led to efficient accumulation at the plasma membrane with only weak punctae detectable (, ΔCTLoTM). This suggests that long TMDs either enhance ER export or inhibit recycling back to the ER. The following deletion Δ23 was detected at the plasma membrane and bright punctate, but showed a significant redistribution to the plasma membrane when a long TMD was introduced (compare Δ23 with Δ23LoTM). While ΔCT and Δ23 with the wild type TMD were targeted very differently, ΔCTLoTM and Δ23LoTM could hardly be distinguished. In sharp contrast, the shorter deletions (Δ19 and Δ15) were hardly affected by the lengthening of the TMD. The Δ19 construct (containing the IM motif) continues to accumulate in bright punctate with only a very weak plasma membrane signal apparent (compare Δ19 with Δ19LoTM). These results suggest that a long TMD may reduce endocytic bulk flow but it cannot prevent signal-mediated endocytosis. Finally, when both IM and YMPL motifs are present (Δ15), PM localization is completely abolished regardless of the presence of a long TMD due to the action of two complementary signals, one to minimize plasma membrane arrival (YMPL-motif) and another to retrieve the few molecules that escaped the YMPL pathway (IM motif).
The results obtained are consistent with the following model for membrane protein transport. Short or medium length TMDs are compatible with biosynthetic bulk flow of membrane proteins to the plasma membrane, but they can also undergo endocytic bulk flow, leading to degradation in the vacuole. This explains why the complete deletion of the VSR tail does not totally prevent vacuolar arrival of the truncated receptor.Citation7 Our model also explains why deletion of the di-lysine motif of p24 proteins causes the truncated protein to be mis-targeted to the plasma membrane, multivesicular bodies (PVCs and LPVCs) and the vacuole.Citation14 When active sorting signals are lacking, long TMDs promote plasma membrane accumulation by inhibiting endocytic bulk flow, as suggested for mammalian cells.Citation15 However, active endocytosis signals such as the IM motif are dominant signals that take precedence (, Δ19, Δ19LoTM, Δ15 and Δ15LoTM). Other dominant internalization signals may be constituted by AP2-interacting tyrosine motifsCitation16,17 or ubiquitination.Citation18,19
Besides its influence during endocytosis, long TMDs may also affect the early secretory pathway. Even though the shortest deletion (ΔCT) was shown to cycle via the plasma membraneCitation6 the dramatic difference between ΔCT and ΔCTLoTM cannot be explained by an inhibition of endocytosis alone as it would not explain the redistribution of the ER-retained portion. Long TMDs could either promote incorporation into COPII coated ER export carriers or be poor cargo for COPI-mediated recycling. Further work will be required to distinguish between these two possibilities.
We can now ask if plasma membrane transport of different types of membrane proteins occurs via the same pathway as constitutive secretion of soluble cargo. The established VSR constructs with specific deletions and point mutations provides an ideal toolbox to test single parameters that influence membrane protein sorting and with the help of Rab6 and Rab11 mutants we may be able to dissect the role of the Golgi and TGN in biosynthetic as well as endocytic transport to and from the plasma membrane.Citation20-24
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Reyes FC, Buono R, Otegui MS. Plant endosomal trafficking pathways. Curr Opin Plant Biol 2011; 14:666-73; PMID:21821464; http://dx.doi.org/10.1016/j.pbi.2011.07.009
- An Q, Hückelhoven R, Kogel KH, van Bel AJ. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol 2006; 8:1009-19; PMID:16681841
- Regente M, Pinedo M, Elizalde M, de la Canal L. Apoplastic exosome-like vesicles: a new way of protein secretion in plants? Plant Signal Behav 2012; 7:544-6; PMID:22516827; http://dx.doi.org/10.4161/psb.19675
- Drakakaki G, Dandekar A. Protein secretion: how many secretory routes does a plant cell have? Plant Sci 2013; 203-4:74-8; PMID:23415330; http://dx.doi.org/10.1016/j.plantsci.2012.12.017
- De Marcos Lousa C, Gershlick DC, Denecke J. Mechanisms and concepts paving the way towards a complete transport cycle of plant vacuolar sorting receptors. Plant Cell 2012; 24:1714-32; PMID:22570446; http://dx.doi.org/10.1105/tpc.112.095679
- Gershlick DC, de Marcos Lousa C., Foresti O, Lee AJ, Pereira EA, daSilva LL, Bottanelli F, Denecke J. Golgi-dependent transport of vacuolar sorting receptors is regulated by COPII, AP1, and AP4 protein complexes in tobacco. Plant Cell 2014; 26:1308-29; PMID:24642936; http://dx.doi.org/10.1105/tpc.113.122226
- daSilva LL, Foresti O, Denecke J. Targeting of the plant vacuolar sorting receptor BP80 is dependent on multiple sorting signals in the cytosolic tail. Plant Cell 2006; 18:1477-97; PMID:16714388; http://dx.doi.org/10.1105/tpc.105.040394
- Foresti O, Gershlick DC, Bottanelli F, Hummel E, Hawes C, Denecke J. A recycling-defective vacuolar sorting receptor reveals an intermediate compartment situated between prevacuoles and vacuoles in tobacco. Plant Cell 2010; 22:3992-4008; PMID:21177482; http://dx.doi.org/10.1105/tpc.110
- daSilva LL, Taylor JP, Hadlington JL, Hanton SL, Snowden CJ, Fox SJ, Foresti O, Brandizzi F, Denecke J. Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 2005; 17:132-48; PMID:15632053; http://dx.doi.org/10.1105/tpc.104.026351
- Saint-Jean B, Seveno-Carpentier E, Alcon C, Neuhaus JM, Paris N. The cytosolic tail dipeptide Ile-Met of the pea receptor BP80 is required for recycling from the prevacuole and for endocytosis. Plant Cell 2010; 22:2825-37; PMID:20807880; http://dx.doi.org/10.1105/tpc.109.072215
- Park M, Song K, Reichardt I, Kim H, Mayer U, Stierhof YD, Hwang I, Jürgens G. Arabidopsis μ-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth. Proc Natl Acad Sci USA 2013; 110:10318-23; PMID:23733933; http://dx.doi.org/10.1073/pnas.1300460110
- Denecke J, Botterman J, Deblaere R. Protein secretion in plant cells can occur via a default pathway. Plant Cell 1990; 2:51-9; PMID:1967050; http://dx.doi.org/10.1105/tpc.2.1.51
- Brandizzi F, Frangne N, Marc-Martin S, Hawes C, Neuhaus JM, Paris N. The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell 2002; 14:1077-92; PMID:12034898; http://dx.doi.org/10.1105/tpc.000620
- Langhans M, Marcote MJ, Pimpl P, Virgili-López G, Robinson DG, Aniento F. In vivo trafficking and localization of p24 proteins in plant cells. Traffic 2008; 9:770-85; PMID:18266912; http://dx.doi.org/10.1111/j.1600-0854.2008.00719.x
- Mercanti V, Marchetti A, Lelong E, Perez F, Orci L, Cosson P. Transmembrane domains control exclusion of membrane proteins from clathrin-coated pits. J Cell Sci 2010; 123:3329-35; PMID:20826467; http://dx.doi.org/10.1242/jcs.073031
- Bar M, Avni A. EHD2 inhibits ligand-induced endocytosis and signaling of the leucine-rich repeat receptor-like protein LeEix2. Plant J 2009; 59:600-11; PMID:19392695; http://dx.doi.org/10.1111/j.1365-313X.2009.03897.x
- Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, Onouchi H, Naito S, Fujiwara T. Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci USA 2010; 107:5220-5; PMID:20194745; http://dx.doi.org/10.1073/pnas.0910744107
- Scheuring D, Künzl F, Viotti C, Yan MS, Jiang L, Schellmann S, Robinson DG, Pimpl P. Ubiquitin initiates sorting of Golgi and plasma membrane proteins into the vacuolar degradation pathway. BMC Plant Biol 2012; 12:164; PMID:22970698; http://dx.doi.org/10.1186/1471-2229-12-164
- Katsiarimpa A, Muñoz A, Kalinowska K, Uemura T, Rojo E, Isono E. The ESCRT-III-interacting deubiquitinating enzyme AMSH3 is essential for degradation of ubiquitinated membrane proteins in Arabidopsis thaliana. Plant Cell Physiol 2014; 55:727-36; PMID:24486765; http://dx.doi.org/10.1093/pcp/pcu019
- Bednarek SY, Reynolds TL, Schroeder M, Grabowski R, Hengst L, Gallwitz D, Raikhel NV. A small GTP-binding protein from Arabidopsis thaliana functionally complements the yeast YPT6 null mutant. Plant Physiol 1994; 104:591-6; PMID:8159788; http://dx.doi.org/10.1104/pp.104.2.591
- Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, Akhmanova A. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell 2007; 13:305-14; PMID:17681140; http://dx.doi.org/10.1016/j.devcel.2007.06.010
- Chow CM, Neto H, Foucart C, Moore I. Rab-A2 and Rab-A3 GTPases define a trans-golgi endosomal membrane domain in Arabidopsis that contributes substantially to the cell plate. Plant Cell 2008; 20:101-23; PMID:18239134; http://dx.doi.org/10.1105/tpc.107.052001
- Feraru E, Feraru MI, Asaoka R, Paciorek T, De Rycke R, Tanaka H, Nakano A, Friml J. BEX5/RabA1b regulates trans-Golgi network-to-plasma membrane protein trafficking in Arabidopsis. Plant Cell 2012; 24:3074-86; PMID:22773752; http://dx.doi.org/10.1105/tpc.112.098152
- Choi SW, Tamaki T, Ebine K, Uemura T, Ueda T, Nakano A. RABA members act in distinct steps of subcellular trafficking of the FLAGELLIN SENSING2 receptor. Plant Cell 2013; 25:1174-87; PMID:23532067; http://dx.doi.org/10.1105/tpc.112.108803
