Abstract
Experiments show the membrane fusion genes α soluble NSF attachment protein (α-SNAP) and syntaxin 31 (Gm-SYP38) contribute to the ability of Glycine max to defend itself from infection by the plant parasitic nematode Heterodera glycines. Accompanying their expression is the transcriptional activation of the defense genes ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1) and NONEXPRESSOR OF PR1 (NPR1) that function in salicylic acid (SA) signaling. These results implicate the added involvement of the antiapoptotic, environmental response gene LESION SIMULATING DISEASE1 (LSD1) in defense. Roots engineered to overexpress the G. max defense genes Gm-α-SNAP, SYP38, EDS1, NPR1, BOTRYTIS INDUCED KINASE1 (BIK1) and xyloglucan endotransglycosylase/hydrolase (XTH) in the susceptible genotype G. max[Williams 82/PI 518671] have induced Gm-LSD1 (Gm-LSD1–2) transcriptional activity. In reciprocal experiments, roots engineered to overexpress Gm-LSD1–2 in the susceptible genotype G. max[Williams 82/PI 518671] have induced levels of SYP38, EDS1, NPR1, BIK1 and XTH, but not α-SNAP prior to infection. In tests examining the role of Gm-LSD1–2 in defense, its overexpression results in ∼52 to 68% reduction in nematode parasitism. In contrast, RNA interference (RNAi) of Gm-LSD1–2 in the resistant genotype G. max[Peking/PI 548402] results in an 3.24–10.42 fold increased ability of H. glycines to parasitize. The results identify that Gm-LSD1–2 functions in the defense response of G. max to H. glycines parasitism. It is proposed that LSD1, as an antiapoptotic protein, may establish an environment whereby the protected, living plant cell could secrete materials in the vicinity of the parasitizing nematode to disarm it. After the targeted incapacitation of the nematode the parasitized cell succumbs to its targeted demise as the infected root region is becoming fortified.
Abbreviations
| EDS1 | = | enhanced disease susceptibility1 |
| PR1 | = | pathogenesis-related 1 |
| NPR1 | = | nonexpressor of PR1 |
| SA | = | salicylic acid |
| LSD1 | = | lesion simulating disease1 |
| α-SNAP | = | alpha soluble N-ethylmaleimide-sensitive factor attachment protein |
| BIK1 | = | botrytis induced kinase1 |
| XTH | = | xyloglucan endotransglycosylase/hydrolase |
| RNAi | = | RNA interference |
| Sed5p | = | suppressors of the erd2-deletion 5 |
| sec | = | secretion |
| JA | = | jasmonic acid |
| PAD4 | = | phytoalexin deficient 4 |
| SID2 | = | salicylic-acid-induction deficient2 |
| LOL1 | = | LSD1-like |
| qPCR | = | quantitative polymerase chain reaction |
| INA | = | 2,6-dichloroisonicotinic acid |
| SAR | = | systemic acquired resistance |
| O2− | = | superoxide |
| ROI | = | reactive oxygen intermediates |
| CuSOD | = | copper superoxide dismutase |
| SHMT | = | serine hydroxymethyltransferase |
| GOI | = | gene of interest |
| ER | = | endoplasmic reticulum |
| MATE | = | multidrug and toxin extrusion |
| PCD | = | programmed cell death |
Introduction
Knowledge of the ability of biological membranes to fuse, resulting in the delivery of vesicle contents to different cellular destinations, is longstanding.Citation1 Genetic experiments and screens in model organisms have identified the proteins that function in the process and ordered the events that lead to material delivery in the form of secretion.Citation2-4 Subsequent work in other systems has demonstrated that the core protein machinery involved in membrane fusion is highly conserved, found in all eukaryotes (Reviewed in Citation5). The process of membrane fusion requires fidelity and protective measures are taken by the cell to ensure it happens properly.Citation6
Through recent studies, a link between membrane fusion at the cell membrane and also the cis face of the Golgi apparatus with SA signaling has been made in plants.Citation7-9 Genetic work in the plant genetic model, Arabidopsis thaliana has also identified essential roles for proteins involved in membrane fusion.Citation10 The essential nature of these membrane fusion proteins makes them difficult to study since their mutants are lethal or cause highly detrimental developmental anomalies.Citation2,3,10,11 However, it is possible to study these proteins under certain circumstances. For example, a genetic screen employed by Mayer et al.Citation10 has determined the role of vesicles in embryo cytokinesis. This approach has succeeded because the biosynthesis of the phragmoplast which relies on vesicles occurs early during embryo development. Subsequent identification of one of the A. thaliana genes involved in cytokinesis (KNOLLE [At-SYP111]) has determined it to be related to a Saccharomyces cerevisiae membrane associated protein known as suppressors of the erd2-deletion 5 (Sed5p) which is structurally homologous to syntaxin.Citation12-14 Syntaxin is a protein involved in secretion, functioning in the fusion of membranes.Citation12,13 Syntaxins perform membrane fusion through their interaction with a number of other proteins (Reviewed in Citation5). One of these proteins is α-SNAP whose relation to plant defense has been demonstrated.Citation8,12,15,16 Since these discoveries, membrane fusion and vesicle transport have been well documented in plants, with many of the related genes having orthologs in yeast and other systems.Citation14,17,18
The roles that these core membrane fusion proteins perform in eukaryotes is extensive, ranging from signaling, cell growth, mitosis, the endocytic cycle, exocytosis, hormonal release, neurotransmission, fertilization, embryogenesis, development, sporulation and cell death.Citation2,3,Citation13-15,Citation17-33 A variety of studies show membrane fusion to be important to the defense process that plants have toward pathogens as well as different types of defense responses.Citation9,11,Citation34-42 While the list of functions that the membrane fusion and vesicle transport proteins have is large, it is less clear whether the proteins also are engaged in other, but related functions.
Recent experiments in G. max have demonstrated that α-SNAP contributes to the resistance of G. max to the plant parasitic nematode, Heterodera glycines.Citation8,43 The α-SNAP gene was first identified in S. cerevisiae as Sec17p in a genetic screen for temperature sensitive secretion (sec) mutants.Citation3 Subsequent research has demonstrated Sec17p is required for vesicle transport from the endoplasmic reticulum (ER) to the Golgi apparatus as mutants accumulated 50 nm vesicles.Citation4,44 The results presented by Matsye et al.Citation8 identified the existence of a role for α-SNAP that went beyond membrane fusion. Matsye et al.Citation8 examined the effect that the overexpression of an α-SNAP gene had on genes associated with different types of hormonal signaling that have known defense functions. While not comprehensive, these genes included an analysis of the SA-regulated cysteine rich secretory protein gene, pathogenesis-related 1 (PR1).Citation45 Furthermore, the study examined the transcriptional activity of other genes whose protein products are secreted. These genes included the ethylene responsive β-1,3-glucanase, PR2,Citation46 the ethylene and jasmonic acid (JA) responsive chitinase gene, PR3Citation47 and the SA-responsive thaumatin, PR5.Citation48 In those experiments, Matsye et al.Citation8 demonstrated α-SNAP overexpression causes induced expression of PR1, PR2 and PR5. Thus, the induced expression of components of the membrane fusion and vesicular transport machinery (α-SNAP) appears to influence the expression of genes that are vesicle cargo. To expand on this concept further, related experiments have been performed analyzing the effect that the overexpression of the α-SNAP binding partner, syntaxin 31 has on transcription.Citation9 In these experiments, the overexpression of α-SNAP or SYP38 also results in the transcriptional induction of the SA signaling genes EDS1 and NPR1.Citation9 In A. thaliana, SA biosynthesis and signaling occurs through a well-understood pathway including the EDS1 protein binding to the lipase PHYTOALEXIN DEFICIENT 4 (PAD4).Citation49-51 This heterodimer functions upstream of SALICYLIC-ACID-INDUCTION DEFICIENT2 (SID2), a putative chloroplast-localized isochorismate synthase, its allelic EDS16, along with the multidrug and toxin extrusion (MATE) efflux transporter EDS5 to activate SA biosynthesis.Citation52-54 Downstream, a complex composed of SA, the SA hormone receptor protein NPR1, copper ions and the transcription factor TGA2 forms.Citation55,56 The complex binds to a DNA promoter sequence composed of TGACG which results in the induction of PR1 transcription.Citation55,61 Another gene that relates to SA signaling in A. thaliana is LESION SIMULATING DISEASE1 (LSD1).Citation62 In A. thaliana, the LSD1 gene is a negative regulator of programmed cell death (PCD) and its activity is antagonized by a related positive regulator of cell death gene called LSD1-like (LOL1).Citation63-68 Currently, it is unknown whether the G. max LSD1 functions in defense. However, its involvement in establishing a tight boundary between cells targeted and not targeted for apoptosis makes it an intriguing candidate.
In the analysis presented here, the relationship between the G. max α-SNAP, Gm-SYP38 and SA signaling is examined further, adding to information generated in prior experiments.Citation9 Gene expression experiments have identified induced levels of Gm-LSD1 (Gm-LSD1–2) in roots engineered to overexpress α-SNAP or SYP38. These results further strengthen a link between vesicle transport and SA signaling. Genetic engineering experiments reveal that the overexpression of Gm-LSD1–2 results in engineered resistance. In contrast, RNAi of Gm-LSD1–2 in a G. max genotype that is normally resistant to H. glycines infection results in roots that permit parasitism at a higher frequency. It is shown the Gm-LSD1–2 overexpression positively influences the transcriptional activity of G. max SYP38, EDS1, NPR1 and BIK1. Furthermore, the overexpression of Gm-LSD1–2 also results in the induction of the expression of the hemicellulose-modifying, vesicle-cargo gene XTH43. In contrast, their expression is suppressed in roots expressing an LSD1–2 RNAi construct. The experiments presented here identify an antiapoptotic aspect of defense in the G. max-H. glycines pathosystem.
Results
Gm-LSD1 is expressed in roots overexpressing α-SNAP, SYP38 and genes relating to SA signaling
Deep sequencing experiments show that the overexpression of the G. max Gm-SYP38, results in the induction of 5 α-SNAP paralogs, including the rhg1 component Glyma18g02590 and Glyma11g35820 (). This result strengthened prior observations of the importance of α-SNAP to the process of defense.Citation8 Furthermore, Pant et al.Citation9 has demonstrated that along with the involvement of Gm-SYP38 during the defense of G. max to H. glycines, its overexpression also results in induced levels of the SA signaling gene EDS1. The demonstration that SA signaling genes function in the defense of G. max to H. glycines has led to an analysis showing that Gm-LSD1 (Gm-LSD1–2) is induced in roots overexpressing Gm-SYP38 (). During parasitism, a well demarcated boundary is established between parasitized and nonparasitized cells in the G. max-H. glycines pathosystem (). To understand the nature of Gm-LSD1–2 in relation to resistance (), qPCR experiments have been performed using cDNA template from genetically engineered G. max roots that acquired the ability to defend itself from H. glycines parasitism. Roots genetically engineered to overexpress G. max α-SNAP, SYP38, NPR1, EDS1, BIK1 or XTH43 exhibit induced levels of LSD1–2 (). The association of Gm-LSD1–2 expression in roots undergoing defense indicates that it may be performing an important role in the process. To test this hypothesis, the susceptible G. max[Williams 82/PI 518671] has been engineered to overexpress Gm-LSD1–2 (). No statistically significant effect is observed in root growth (Fig. S1). In experiments presented here, the overexpression of the Gm-LSD1–2 results in a significant reduction in parasitism (). To examine the specificity of the overexpression experiments, the expression of an RNAi cassette for Gm-LSD1–2 in the normally resistant genotype G. max[Peking/PI 548402] was done (). No statistically significant effect is observed in root growth (Fig. S1). The expression of an RNAi cassette for Gm-LSD1–2 in the normally resistant genotype G. max[Peking/PtdIns 548402] results in an increased capability of H. glycines to parasitize the resistant G. max[Peking/PI 548402] ().
Table 1. Deep sequencing of mRNA isolated from uninfected Gm-SYP38 overexpressing roots reveals altered transcriptional activity of the rhg1 resistance gene, α-SNAP (Glyma18g02590) and paralogs of α-SNAP
Table 2. qPCR of G. max roots overexpressing defense-related genes. qPCR was performed using primers designed specifically against LSD1–2 The experiments used the ribosomal S21 gene8 as a control to standardize the experiments
Figure 1. A 3 dpi image of H. glycines successfully parasitizing a root of G. max[Williams 82/PI 518671]. Black arrow, nematode; red arrows, boundary of the nurse cell (syncytium). Bar = 100 μm.
![Figure 1. A 3 dpi image of H. glycines successfully parasitizing a root of G. max[Williams 82/PI 518671]. Black arrow, nematode; red arrows, boundary of the nurse cell (syncytium). Bar = 100 μm.](/cms/asset/dde7cc96-fed7-47c8-9ad8-4d088fcb6c09/kpsb_a_977737_f0001_c.gif)
Figure 2. The Golgi apparatus serves a central role in resistance as a defense engine, processing proteins for their eventual transport. The overexpression of α-SNAP resulted in engineered resistance.Citation8 Furthermore, α-SNAP overexpression results in the induction of Gm-SYP38 transcription.Citation9 In reciprocal experiments, Gm-SYP38 overexpression results in the transcriptional activation of α-SNAP and its paralogs (). The overexpression of Gm-SYP38 results in the transcriptional activation of EDS1 which functions upstream of SA biosynthesis (dashed lines). The overexpression of Gm-SYP38 also results in the transcriptional activation of the SA receptor, NPR1, the DNA binding β-ZIP transcription factor TGA2 and the GATA-like transcription factor LSD1. The binding of SA to NPR1 results in its translocation to the nucleus. NPR1 and TGA2 are directly involved in the transcriptional activation of PR1 and PR5. For presentation purposes, on the right side of the Golgi apparatus are shown vesicles undergoing anterograde transport while those on the left are undergoing retrograde transport. Vesicles are shown released from the trans-Golgi network, moving toward the endosome. Ultimately, secretory vesicles fuse with the plasma membrane to deliver receptor components and secrete contents into the apoplast. Some of these secreted contents, like Gm-XTH43, play important roles in defense.Citation9 In contrast, vesicles emerge from the plasma membrane and fuse with the endosome, recycling contents. Not shown, Gm-SYP38 and α-SNAP overexpression results in induced expression of the cytoplasmic receptor-like kinase BIK1 that is important for defense Citation9
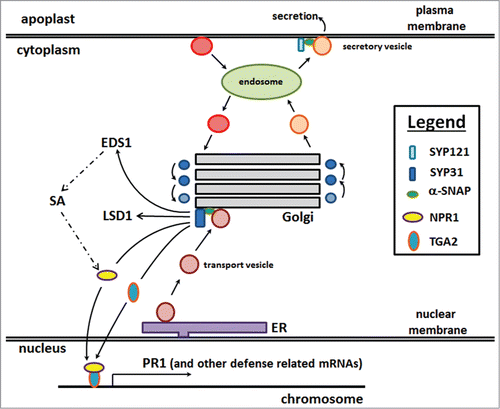
Figure 3. Representative control and transgenic LSD1–2 overexpressing and LSD1–2 RNAi G. max plants. (A) Control susceptible G. max[Williams 82/PtdIns 518671] plant. (B) Genetically engineered G. max[Williams 82/PI 518671] overexpressing Gm-LSD1–2. (C) Control resistant G. max[Peking/PtdIns 548402] plant. (D) A resistant G. max[Peking/PI 548402] plant genetically engineered to express an LSD1–2 RNAi construct. Scale provided on left of each image.
![Figure 3. Representative control and transgenic LSD1–2 overexpressing and LSD1–2 RNAi G. max plants. (A) Control susceptible G. max[Williams 82/PtdIns 518671] plant. (B) Genetically engineered G. max[Williams 82/PI 518671] overexpressing Gm-LSD1–2. (C) Control resistant G. max[Peking/PtdIns 548402] plant. (D) A resistant G. max[Peking/PI 548402] plant genetically engineered to express an LSD1–2 RNAi construct. Scale provided on left of each image.](/cms/asset/cfdcd5b5-8fc4-4af2-af0c-f8118d6ee80e/kpsb_a_977737_f0003_c.gif)
Figure 4. The female index for transgenic G. max plants genetically engineered to overexpress Gm-LSD1–2 and infected with H. glycines. Replicate 1 (R1) control plants had 28.39 cysts per gram (12 plants); LSD1–2-R1-overexpressing plants (LSD1–2-R1: oe) had 13.66 cysts per gram (12 plants). The FI = 47.92; P-value = 0.0216541 which is statistically significant (P < 0.05). R2 control plants (replicate 2) had 30.40 cysts per gram (16 plants); LSD1–2-R2-overexpressing plants (LSD1–2-R2: oe) had 9.85 cysts per gram (12 plants). The FI = 32.4; P-value = 0.000059234 which is statistically significant (P < 0.05). R3 control plants had 32.98 cysts per gram (20 plants); LSD1–2-R3 overexpressing plants (LSD1–2-R3: oe) had 14.07 cysts per gram (18 plants). The FI = 42.662; P-value = 3.36219e-06 which is statistically significant (P < 0.05).
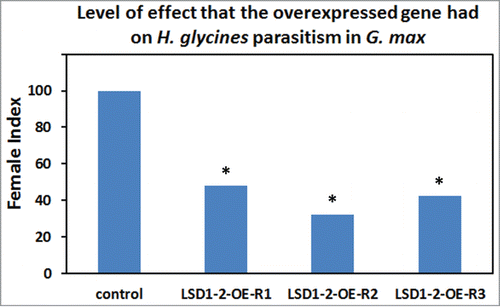
Figure 5. G. max plants genetically engineered for RNAi of Gm-LSD1–2 and infected with H. glycines have an increased capability, shown as fold change, for parasitism. Replicate 1 (R1) control plants (resistant G. max[Peking/PtdIns 548402]) had 1.98 cysts per gram (10 plants). LSD1–2-RNAi-R1 (LSD1–2-R1: RNAi) in resistant G. max[Peking/PI 548402]) had 6.41 cysts per gram (11 plants). The results were statistically significant (p = 0.00255251). Replicate 2 (R2) control plants (resistant G. max[Peking/PtdIns 548402]) had 0.79 cysts per gram (12 plants). LSD1–2-RNAi-R2 (LSD1–2-R2: RNAi) in resistant G. max[Peking/PI 548402]) had 8.63 cysts per gram (5 plants). The results were statistically significant (p = 0.0117053). Replicate 3 (R3) control plants (resistant G. max[Peking/PtdIns 548402]) had 2.51 cysts per gram (10 plants). LSD1–2-RNAi-R3 (LSD1–2-R3: RNAi) in resistant G. max[Peking/PI 548402]) had 11.7 cysts per gram (7 plants). The results were statistically significant (p = 0.0120138).
![Figure 5. G. max plants genetically engineered for RNAi of Gm-LSD1–2 and infected with H. glycines have an increased capability, shown as fold change, for parasitism. Replicate 1 (R1) control plants (resistant G. max[Peking/PtdIns 548402]) had 1.98 cysts per gram (10 plants). LSD1–2-RNAi-R1 (LSD1–2-R1: RNAi) in resistant G. max[Peking/PI 548402]) had 6.41 cysts per gram (11 plants). The results were statistically significant (p = 0.00255251). Replicate 2 (R2) control plants (resistant G. max[Peking/PtdIns 548402]) had 0.79 cysts per gram (12 plants). LSD1–2-RNAi-R2 (LSD1–2-R2: RNAi) in resistant G. max[Peking/PI 548402]) had 8.63 cysts per gram (5 plants). The results were statistically significant (p = 0.0117053). Replicate 3 (R3) control plants (resistant G. max[Peking/PtdIns 548402]) had 2.51 cysts per gram (10 plants). LSD1–2-RNAi-R3 (LSD1–2-R3: RNAi) in resistant G. max[Peking/PI 548402]) had 11.7 cysts per gram (7 plants). The results were statistically significant (p = 0.0120138).](/cms/asset/382bcd9f-4684-43ba-a70b-0f2ff0ec05a3/kpsb_a_977737_f0005_c.gif)
Gm-LSD1–2 overexpression induces the expression of genes relating to membrane fusion and SA signaling
To understand the relationship between Gm-LSD1–2 and resistance, a series of qPCR analyses have been performed using cDNA synthesized from RNA isolated from roots overexpressing Gm-LSD1–2 (). The gene expression analysis demonstrates that Gm-LSD1–2 overexpression results in induced mRNA levels of LSD1–2 as well as EDS1–2, NPR1–2, BIK1–6, XTH43 and SYP38. In contrast, Gm-LSD1–2 overexpression results in suppressed levels of α-SNAP prior to infection. This result is not surprising since recent experiments have shown that α-SNAP becomes highly induced later during the resistant reaction.Citation9 In reciprocal experiments, the expression of an RNAi cassette for Gm-LSD1–2 in the normally resistant genotype G. max[Peking/PtdIns 548402] results in suppressed transcriptional activity for LSD1–2 as well as EDS1–2, NPR1–2, BIK1–6, XTH43 and SYP38 (). Expression of α-SNAP was not detected under the experimental conditions. The results confirm and provide further context for the existence of a link between the membrane fusion gene SYP38 and SA signaling.
Table 3. qPCR of G. max roots either overexpressing LSD1-2 or genetically engineered with a RNAi construct targeting LSD1–2 The experiments used the ribosomal S21 gene as a control to standardize the experiments. *expression not detected
Discussion
LSD1 was first discovered in A. thaliana in a forward genetic screen designed to identify spontaneous lesion simulating mutants.Citation62 The 5 identified lsd mutants have been divided into 2 classes. One class forms spontaneous necrotic lesions that are determinate in nature.Citation62 In this class, the expansion of necrosis into adjacent tissue is limited.Citation62 Furthermore, lesion formation is not influenced by pathogens or chemicals such as SA and the non SA-inducing 2,6-dichloroisonicotinic acid (INA) that induce the onset of systemic acquired resistance (SAR).Citation62,70 The second class of lsd mutants, defined by LSD1, is described as a feedback or propagation mutant.Citation62 The lsd1 mutant forms spontaneous lesions under long day growth conditions.Citation62 In contrast, lesion formation is suppressed under short days.Citation62 These characteristics indicate that light influences the process at some level. The lsd1 mutant is characterized by indeterminate lesions that eventually consume the whole leaf or plant.Citation62 Another characteristic of lsd1 mutants is that plants grown under permissive short day conditions develop lesions that eventually consume the whole plant when switched to long day.Citation62 Furthermore, the lsd1 mutant initiates lesion formation by fungal or bacterial pathogens and inducers of SAR, including SA and INA.Citation62 Related experiments using lsd1 mutants demonstrate that superoxide (O2−) accumulates in the cells adjacent to the cells undergoing cell death.Citation63 This result demonstrates that O2− is both necessary and sufficient to initiate lesion formation and promote its spreading into adjacent cells.Citation63 This result also identifies a link between photorespiration and lesion development.
It is clear from these studies that lsd1 mutants are impaired in their ability to establish a boundary beyond which the neighboring cells are not consumed in the wave of cell death. Sequence analysis of LSD1 demonstrates it to be a novel zinc finger, GATA-type transcription factor.Citation64 In this regard, the data presented here provides an example of a GATA-type transcription factor involved in G. max defense against H. glycines. From observations made in A. thaliana it has been hypothesized that LSD1 is responsible either to negatively regulate a pro-death pathway or activate a repressor of cell death.Citation64 As a regulator, LSD1 would function very early in the process. In A. thaliana, LSD1 has since been shown to function in relation to genes composing the SA signaling pathway, including EDS1, PAD4 and NPR1 as well as the signaling molecule SA.Citation65,71,72 Notably, LSD1 as an antiapoptotic gene, functions in the cells adjacent to the infected cell that is undergoing cell death.Citation65,71,72 Experiments have shown that runaway cell death was dependent on SA and NPR1 in lsd1 mutants.Citation72 In contrast, LSD1 has been shown to negatively regulate SA and NPR1-independent basal disease resistance.Citation72 From these studies, it has been proposed that SA and NPR1 function in runaway cell death in the lsd1 mutant through their participation in a signal amplification loop that promotes apoptosis.Citation71,72 It has been shown that an important component of runaway cell death is the generation of reactive oxygen intermediates (ROI) such as O2−.Citation63,65,71,72 Additional studies further link the lsd1 mutant to impaired photorespiration, leading to the accumulation of excess excitation energy and subsequent cell death.Citation73 In contrast, cell death is prevented in the lsd1 mutants by impeding conditions that lead to photorespiration.Citation73 These results explain the link between the lsd1 and photo-oxidative damage. Thus, it has been proposed that the LSD1 protein functions like a rheostat whereby above a ROI threshold, the cell would undergo cell death.Citation63,64,65,71,72,73 In contrast, below a certain threshold, the cell would survive. From this work, a signal potentiation loop has been coined to describe how in the absence of LSD1 protein, the accumulation of signaling components leads to runaway apoptosis.Citation72
These experiments focused in on the above ground portions of A. thaliana. Subsequently, a number of experiments examining LSD1 have studied specific aspects of root biology. Under certain adaptive environmental circumstances (i.e. water saturated conditions and low oxygen [hypoxia]), root cells become targeted for apoptosis through a process called lysigeny. As a consequence of this process, the roots develop aerenchyma which increases the ability of roots to maintain higher O2 levels. Experiments in A. thaliana have shown that lysigeny is under the control of LSD1.Citation74 Under conditions of hypoxia, LSD1, EDS1 and PAD4 function upstream of H2O2 production and ethylene signaling events that lead to lysigeny.Citation74 Under normal conditions in A. thaliana, LSD1 functions as a negative regulator of the apoptosis-promoting EDS1 and PAD4. In contrast, under hypoxia, LSD1 is negatively regulated, permitting EDS1 and PAD4 to promote cell death in A. thaliana.Citation74 To understand how H2O2 production could be regulated in the roots, earlier experiments performed on aerial portions of A. thaliana demonstrated that LSD1 controls H2O2 production through SA-regulated transcription of CuSOD.Citation65 This is an important finding since plants can produce the highly toxic O2− during plant defense by the activities of NADPH oxidase.Citation75 Recent findings performed in A. thaliana have shown a direct link between NADPH oxidase and BIK1.Citation76 In those experiments, BIK1 directly phosphorylates NADPH oxidase to produce O2− and activate defense pathways. Plants then detoxify O2− to H2O2 through major antioxidant enzymes like CuSOD. Thus, certain aspects of LSD1 function in A. thaliana are similar between the shoot and root. Furthermore, recent findings in A. thaliana have also revealed LSD1 has many functions with regard to basic aspects of plant growth, development and its ability to function under different environmental conditions and stresses.Citation77 These observations place some context into the observation that Gm-BIK1 functions in defense in the G. max-H. glycines pathosystem.Citation9
LSD1 transcription is induced in G. max roots overexpressing the membrane fusion gene α-SNAP
Two major H. glycines resistance loci have been identified from screening ecological collections of G. max.Citation78,79 These loci, the recessive rhg1 and the dominant Rhg4, have been mapped and cloned through traditional means and aided further by transcriptomics and candidate gene approaches.Citation43,78,79,Citation80-83 Genetic crosses of rhg1 and Rhg4-containing genotypes leads to progeny with further-enhanced, nearly full resistance. The additive effect that these loci have, regarding H. glycines resistance, indicate that the genes function in different genetic pathways that converge on the same outcome (resistance). The rhg1 locus, depending on the resistant genotype examined, is composed of multiple tandem repeated copies of 3 or 4 genes. These genes include an amino acid transporter, α-SNAP, a wound inducible protein and in some genotypes, a gene known as placenta-specific gene 8 protein (PLAC8).Citation43,81,82,84 Among these genes, the overexpression of α-SNAP has been shown to yield a resistant reaction when overexpressed on its own. As part of the secretory pathway, α-SNAP would function in many essential cellular processes.Citation3 The other resistance gene, Rhg4, gene is a SHMT which plays a role in photorespiration. In overexpression studies, SHMT suppresses the ability of H. glycines to parasitize G. max.Citation69,83
The overexpression of α-SNAP leads to an increase in expression of its binding partner, syntaxin 31 (Gm-SYP38). Syntaxin 31 functions at the cis face of the Golgi apparatus to facilitate the fusion of transport vesicles transported from the endoplasmic reticulum.Citation3,4,44,Citation85-88 In G. max, the overexpression of α-SNAP and Gm-SYP38 results in induced levels of the SA signaling genes EDS1, NPR1 and PR1.Citation9 While the observation of an influence of vesicle transport on SA signaling is not a new concept,Citation7 the results of Pant et al.Citation9 indicates that SA signaling may be important to the process of defense in the G. max-H. glycines pathosystem. To test this hypothesis, the overexpression of Gm-EDS1 and NPR1 has been shown to lead to resistance.Citation9 In related experiments, the overexpression of EDS1 and NPR1 in G. max leads to induced levels of SHMT prior to infection.Citation9 Furthermore, the overexpression of G. max syntaxin 31 leads to slightly induced levels of EDS1 and SHMT during infection.Citation9 While these experiments were not comprehensive, they indicate that genes composing the rhg1 locus can influence the expression of Rhg4.
The observation in G. max that EDS1 and NPR1 function in resistance to H. glycines indicated other genes relating to them may also function in the process. An obvious candidate is Gm-LSD1. In qPCR experiments examining G. max roots overexpressing α-SNAP, it is shown that Gm-LSD1–2 transcription is induced. Complimentary experiments presented here show that Gm-LSD1–2 is also induced in roots engineered to overexpress Gm-SYP38. Furthermore, Gm-LSD1–2 transcription is also induced in roots overexpressing BIK1, EDS1, NPR1 or XTH. The strong association of Gm-LSD1–2 with engineered forms of resistance led to the idea that it may perform a direct role in the process. Since A. thaliana LSD1 is known to play roles in establishing and maintaining a tight boundary around the cells and tissues involved in pathogen infection, it is possible that the expression of Gm-LSD1–2 could be performing an important role in regulating the expansion and/or initial survival of parasitized cells. The H. glycines-parasitized root cells undergo a slow process taking days to conclude that ultimately leads to resistance.Citation89 During this time, the parasitized root cell would have time to synthesize and secrete molecules in the vicinity of the nematode to neutralize its activities while fortifying the parasitized area. One such enzyme is Gm-XTH43. Notably, XTH contains a signal peptide and is transported through the vesicle transport machinery to the apoplast where it modifies hemicellulose.Citation9,90 Furthermore, the parasitized cell may produce O2− whose subsequent metabolism to H2O2 has been shown in A. thaliana to be under regulation by LSD1.Citation63,65,Citation70-74 In the analysis presented here, the overexpression of Gm-LSD1–2 in G. max[Williams 82/PI 518671] roots that are otherwise susceptible to H. glycines parasitism, resulted in ∼52 to 68% reduction in nematode parasitism. Roots overexpressing Gm-LSD1–2, when tested for the expression of markers of resistance (i.e., XTH43, SYP38, NPR1, EDS1 and BIK1) show that each is induced in its expression prior to H. glycines infection. In examining molecular markers of different signaling processes, highly induced levels of PR2 were observed in Gm-LSD1–2 overexpressing roots prior to their infection by H. glycines. The induction of PR2 transcription indicates ethylene may also be a component of in Gm-LSD1–2-mediated resistance. The contribution of PR2 to resistance has been demonstrated, linking ethylene to the process.Citation69 In contrast, RNAi of Gm-LSD1–2 in the resistant genotype G. max[Peking/PI 548402] demonstrates specificity. In these experiments, the normally resistant G. max[Peking/PI 548402] roots engineered with the Gm-LSD1–2 RNAi cassette lacked the induction of LSD1–2 expression and exhibited an increase in parasitism capability. These results provide direct evidence that Gm-LSD1–2 plays an important role in the ability of G. max to prevent parasitism by H. glycines, contrasting with recent heterologous expression studies.Citation91 In examining this discrepancy between the heterologous expression of A. thaliana LSD1 and Gm-LSD1–2 further, the conceptually translated At-LSD1 gene studied in Matthews et al.Citation91 is 66.5% identical to the tested G. max LSD1–2 protein (Glyma08g13630) presented here. Thus, part of the difference observed between the capability of At-LSD1 and Gm-LSD1–2 proteins to function in G. max may arise from gene sequence variation. To reinforce our observation that Gm-LSD1–2 functioned in resistance, we present through a double-blind analysis experimental and biological replicates in both the Gm-LSD1–2 overexpression and RNAi experiments.
Spatial and temporal aspects regarding LSD1
The demonstration that Gm-LSD1–2 is important to the defense process clarifies the paradox that parasitized G. max root cells tolerate the establishment and maintenance of the attacked cell early during H. glycines parasitism prior to the commitment of the parasitized cell for demise. The association of LSD1 with the antiapoptotic activities of photorespiration in A. thaliana links its function to G. max Rhg4-mediated defense.Citation63,65,Citation70-73 The demonstration that induced levels of Gm-LSD1–2 transcription in roots overexpressing the rhg1 gene α-SNAP and SYP38 links LSD1 to the process of vesicle transport at some level. At this point, many details remain concerning the genetic program responsible for the establishment and maintenance parasitized cell and surrounding root cells. From these observations, it is plausible that Gm-LSD1–2 functions initially in both the parasitized cell and surrounding cells to prevent cell death and establish a boundary. The demonstration that Gm-BIK1 is important to resistance implicates NADPH oxidase performing a role in the process.Citation9,76 NADPH oxidase would provide the O2− that could antagonize H. glycines. During this time, as the cell is protected from apoptosis, the vesicle transport machinery including the rhg1 gene α-SNAP would function to deliver antimicrobials, cell wall modifying enzymes and other substances to the site of parasitism. However, the process of resistance is not limited to this framework.
Methods
Gene cloning
The candidate gene overexpression study presented here has been done according to our published procedures using the pRAP15 and pRAP17 vectors.Citation8,9 The primers used to clone Gm-LSD1–2 (Glyma08g13630) are provided (Table S1). The nature of the hairy root system is that each transgenic root system functions as an independent transformant line.Citation9,92 Amplicons, representing the gene of interest (GOI) generated by PCR have been gel purified in 1.0% agarose using the Qiagen® gel purification kit, ligated into the directional pENTR/D-TOPO® vector and transformed into chemically competent E. coli strain One Shot TOP10. Chemical selection has been done on LB-kanamycin (50 μg/ml) according to protocol (Invitrogen®). Amplicons have been confirmed by sequencing and comparing the sequence to its original Genbank accession. The G. max amplicon has been shuttled into the pRAP15 or pRAP17 destination vector using LR clonase (Invitrogen®). The engineered pRAP15 or pRAP17 vector have been transformed into chemically competent A. rhizogenes strain K599 (K599)Citation93 using the freeze-thaw methodCitation94 on LB-tetracycline (5 μg/ml).
The infection of G. max by H. glycines
Genetic transformation overexpression experiments have been performed according to Pant et al.Citation9 in the functionally hypomorphic rhg1−/− genetic background of G. max[Williams 82/PtdIns 518671], lacking a defense response to H. glycines parasitism. In contrast, RNAi studies have been performed in the rhg1+/+ genetic background of G. max[Peking/PI 548402] according to Pant et al.Citation9 Female H. glycines[NL1-Rhg/HG-type 7/race 3] have been purified by sucrose flotation.Citation95,96 Each root has been inoculated with one ml of nematodes at a concentration of 2,000 second stage juveniles (J2s)/ml per root system (per plant), infected for 30 d and confirmed by acid fuchsin staining.Citation97 At the end of the experiment, the cysts (fully matured females) have been collected over nested 20 and 100-mesh sieves.Citation9 Furthermore, the soil has been washed several times and the rinse water sieved to assure collection of all cysts.Citation9 The accepted assay to accurately reflect if a condition exerts an influence on H. glycines development is the female index (FI).Citation98 The FI has been calculated in a double blind analysis as FI = (Nx/Ns) X 100, where Nx is the average number of females on the test cultivar and Ns is the average number of females on the standard susceptible cultivar.Citation98 Nx is the pRAP15-transformed line that had the engineered GOI. Ns is the pRAP15 control in their G. max[Williams 82/PtdIns 518671]. The effect of the overexpressed gene on parasitism has been tested statistically using the Mann–Whitney–Wilcoxon (MWW) Rank-Sum Test, P < 0.05.Citation9
RNA-seq
Exon sequencing (RNA seq) has been performed according to our original published work with modifications.Citation81 RNA has been extracted from G. max roots using the UltraClean® Plant RNA Isolation Kit (Mo Bio Laboratories®, Inc.; Carlsbad, CA) and treated with DNase I to remove genomic DNA.Citation8,9 RNA-seq analyses have been performed using the Illumina® HighSeq 2500® platform (Eurofins MWG Operon; Huntsville, Alabama). The RNAseq procedures that identified transcript (tag) counts and chromosomal coordinates of the G. max genomeCitation84 along with the associated gene ontology (GO) annotationsCitation99 are outlined here, subsequently. The qualities of raw reads have been checked using program FASTQC. The updated genome sequence and annotation of G. maxCitation84 have been obtained from Phytozome v9.0 (dated: Nov 27, 2011). The abundance of transcripts across all samples has been measured and comparedCitation100 and default setting of the programs used unless specified. Briefly, the raw reads for each sample have been mapped on G. max genome using TopHat v2.0.6.Citation101 Then, Cufflinks v2.0.2Citation102 program have been used to assemble the mapped reads into transcripts. The FPKM values were calculated for all genes in all samples and their differential transcript expression (log base 2) computed using program Cuffdiff.Citation102
Quantitative real-time PCR (qPCR)
The qPCR experiments examining LSD-1–2 overexpression have been performed according to Pant et al.Citation9 The same root mRNA used in Pant et al.Citation9 has been used here for the qPCR analyses of roots overexpressing G. max SYP38, α-SNAP, EDS1–2, NPR1–2, XTH43, BIK1–6. Primers used in qPCR gene expression experiments are provided (Table S2). The experiments, presented as log base 2, use the ribosomal protein gene S21 as a control.Citation8,9 Gene expression has been tested in relation to several different classes of pathogenesis related (PR) genes, and defense genes (Table S1). The qPCR experiments have used Taqman® 6-carboxyfluorescein (6-FAM) probes and Black Hole Quencher (BHQ1) (MWG Operon; Birmingham, AL). The qPCR differential expression tests have been performed according to Livak and Schmittgen.Citation103 The qPCR reaction conditions have been prepared according to Pant et al.Citation9 and includes a 20 μl Taqman Gene Expression Master Mix (Applied Biosystems; Foster City, CA), 0.9 μl of μM forward primer, 0.9 μl of 100 μM reverse primer, 2 μl of 2.5 μM 6-FAM (MWG Operon®) probe and 9.0 μl of template DNA. The qPCR reactions have been executed on an ABI 7300 (Applied Biosystems®). The qPCR conditions include a preincubation of 50° C for 2 min, followed by 95°C for 10 min. This step has been followed by alternating 95°C for 15 sec followed by 60°C for 1 min for 40 cycles.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental_Figure_1.tiff
Download TIFF Image (3 MB)Supplemental_Table_1.xlsx
Download MS Excel (11.7 KB)Acknowledgments
The authors thank George Hopper, Reuben Moore and Wes Burger (MAFES) whose support has made the research possible. Greenhouse space to support the research has been provided by the Department of Biochemistry, Molecular Biology, Entomology and Plant Pathology (BMEP) and the Department of Plant and Soil Sciences (PSS) at Mississippi State University. The authors thank Dr. Giselle Thibaudeau and Amanda Lawrence, Institute for Imaging and Analytical Technologies, Mississippi State University for imaging expertise and technical suggestions during the course of the research. Postdoctoral research support has been provided to Aparna Krishnavajhala through a competitive Special Research Initiative grant awarded by MAFES. Yixiu Pinnix (BMEP) has provided technical support. The efforts of Keshav Sharma, Jian Jiang, Prakash Nirula and Jillian Harris are acknowledged. Much of the research was supported by undergraduate students including Ashley Dowdy, Nishi Sunthwal, John Clune, Hannah Burson, Chase Robinson, Meghan Calhoun and Austin Martindale and Annedrea McMillan (DBS) and by James McKibben, Cody Roman and Micah Schneider (BMEP). The Shackouls Honors College is acknowledged. VPK acknowledges Dr. Ben Matthews and Dr. Perry Cregan at the USDA-ARS (Beltsville, MD) for support throughout the process.
Funding
The research has been supported by the start-up package provided by Mississippi State University and the Department of Biological Sciences (DBS). The authors thank the Mississippi Soybean Promotion Board for support. The research is supported jointly between the College of Arts and Sciences (DBS) and the Mississippi Agricultural and Forestry Experimental Station (MAFES). The authors acknowledge the Office of the Graduate School at Mississippi State University for providing competitive Summer Research Program for Undergraduate Students research award to Tineka Burkhead.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Palade GE. Intracellular aspects of protein secretion. Science 1975; 189:347-58; PMID:1096303; http://dx.doi.org/10.1126/science.1096303
- Novick P, Schekman R. Secretion and cell surface growth are blocked in a temperature sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 1979; 76:1858-62; http://dx.doi.org/10.1073/pnas.76.4.1858
- Novick P, Field C, Schekman R. The identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 1980; 21:205-15; PMID:6996832; http://dx.doi.org/10.1016/0092-8674(80)90128-2
- Novick P, Ferro S, Schekman R. Order of events in the yeast secretory pathway. Cell 1981; 25:461-9; PMID:7026045; http://dx.doi.org/10.1016/0092-8674(81)90064-7
- Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature 2012; 490:201-7; PMID:23060190; http://dx.doi.org/10.1038/nature11320
- Lobingier BT, Nickerson DP, Lo S-Y, Merz AJ. SM proteins Sly1 and Vps33 co-assemble with Sec17 and SNARE complexes to oppose SNARE disassembly by Sec18. ELife 2014; PMID:24837546; http://dx.doi.org/10.7554/eLife.02272
- Zhang Z, Feechan A, Pedersen C, Newman MA, Qiu JL, Olesen KL, Thordal-Christensen H. A SNARE-protein has opposing functions in penetration resistance and defence signalling pathways. Plant J 2007; 49:302-12; PMID:17241452; http://dx.doi.org/10.1111/j.1365-313X.2006.02961.x
- Matsye PD, Lawrence GW, Youssef RM, Kim K-H, Matthews BF, Lawrence KS, Klink VP. The expression of a naturally occurring, truncated allele of an a-SNAP gene suppresses plant parasitic nematode infection. Plant Mol Biol 2012; 80:131-55; PMID:22689004; http://dx.doi.org/10.1007/s11103-012-9932-z
- Pant SR, Matsye PD, McNeece BT, Sharma K, Krishnavajhala A, Lawrence GW, Klink VP. Syntaxin 31 functions in Glycine max resistance to the plant parasitic nematode Heterodera glycines Plant Mol Biol 2014; 85:107-21; PMID:24452833; http://dx.doi.org/10.1007/s11103-014-0172-2
- Mayer U, Torres Ruiz RA, Berleth T, Mise´ra S, Ju¨rgens G. Mutations affecting body organization in the Arabidopsis embryo. Nature 1991; 353:402-7; http://dx.doi.org/10.1038/353402a0
- Kwon C, Neu C, Pajonk S, Yun HS, Lipka U, Humphry M, Bau S, Straus M, Kwaaitaal M, Rampelt H, et al. Co-option of a default secretory pathway for plant immune responses. Nature 2008; 451:835-40; PMID:18273019; http://dx.doi.org/10.1038/nature06545
- Hardwick KG, Pelham HR. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol 1992; 119:513-21; PMID:1400588; http://dx.doi.org/10.1083/jcb.119.3.513
- Lukowitz W, Mayer U, Jürgens G. Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 1996; 84:61-71; PMID:8548827; http://dx.doi.org/10.1016/S0092-8674(00)80993-9
- Sanderfoot AA, Farhah F, Assaad FF, Natasha V, Raikhel NV. The Arabidopsis genome. An abundance of soluble N-ethylmaleimide-sensitive factor adaptor protein receptors. Plant Physiol 2001a; 124:1558-156; http://dx.doi.org/10.1104/pp.124.4.1558
- Clary DO, Griff IC, Rothman JE. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell 1990; 61:709-21; PMID:2111733; http://dx.doi.org/10.1016/0092-8674(90)90482-T
- Lupashin VV, Pokrovskaya ID, McNew JA, Waters MG. Characterization of a novel yeast SNARE protein implicated in Golgi retrograde traffic. Mol Biol Cell 1997; 8:2659-76; PMID:9398683; http://dx.doi.org/10.1091/mbc.8.12.2659
- Sanderfoot AA, Pilgrim M, Adam L, Raikhel NV. Disruption of individual members of Arabidopsis syntaxin gene families indicates each has essential functions. Plant Cell 2001b; 13:659-66; http://dx.doi.org/10.1105/tpc.13.3.659
- Sanderfoot AA, Kovaleva V, Bassham DC, Raikhel NV. Interactions between Syntaxins Identify at Least five SNARE complexes within the Golgi/Prevacuolar system of the Arabidopsis cell. Mol Biol Cell 2001c; 12:3733-43; http://dx.doi.org/10.1091/mbc.12.12.3733
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 1992; 257:255-9; PMID:1321498; http://dx.doi.org/10.1126/science.1321498
- Boyd RS, Duggan MJ, Shone CC, Foster KA. The effect of botulinum neurotoxins on the release of insulin from the insulinoma cell lines HIT-15 and RINm5F J Biol Chem 1995; 270:18216-8; PMID:7629139; http://dx.doi.org/10.1074/jbc.270.31.18216
- Vroemen CW, Langeveld S, Mayer U, Ripper G, Jurgens G, Van Kammen A, De Vries SC. Pattern formation in the Arabidopsis embryo revealed by position-specific lipid transfer protein gene expression. Plant Cell 1996; 8:783-91; PMID:12239400; http://dx.doi.org/10.1105/tpc.8.5.783
- Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, Lukowitz W, Jürgens G. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol 1997; 139:1485-93; PMID:9396754; http://dx.doi.org/10.1083/jcb.139.6.1485
- Burgess RW, Deitcher DL, Schwarz TL. The synaptic protein syntaxin1 is required for cellularization of Drosophila embryos. J Cell Biol 1997; 138:861-75; PMID:9265652; http://dx.doi.org/10.1083/jcb.138.4.861
- Schulz JR, Wessel GM, Vacquier VD. The exocytosis regulatory proteins syntaxin and VAMP are shed from sea urchin sperm during the acrosome reaction. Dev Biol 1997; 191:80-7; PMID:9356173; http://dx.doi.org/10.1006/dbio.1997.8712
- Neiman AM. Prospore membrane formation defines a developmentally regulated branch of the secretory pathway in yeast. J Cell Biol 1998; 140:29-37; PMID:9425151; http://dx.doi.org/10.1083/jcb.140.1.29
- Peter F, Wong SH, Subramaniam VN, Tang BL, Hong W. Alpha-SNAP but not gamma-SNAP is required for ER-Golgi transport after vesicle budding and the Rab1-requiring step but before the EGTA-sensitive step. J Cell Sci 1998; 111:2625-33; PMID:9701561
- Ramalho-Santos J, Moreno RD, Sutovsky P, Chan AW, Hewitson L, Wessel GM, Simerly CR, Schatten G. SNAREs in mammalian sperm: possible implications for fertilization. Dev Biol 2000; 223:54-69; PMID:10864460; http://dx.doi.org/10.1006/dbio.2000.9745
- Waizenegger I, Lukowitz W, Assaad F, Schwarz H, Jürgens G, Mayer U. The Arabidopsis KNOLLE and KEULE genes interact to promote vesicle fusion during cytokinesis. Curr Biol 2000; 2:1371-4; http://dx.doi.org/10.1016/S0960-9822(00)00775-2
- Babcock M, Macleod GT, Leither J, Pallanck L. Genetic analysis of soluble N ethylmaleimide-sensitive factor attachment protein function in Drosophila reveals positive and negative secretory roles. J Neurosci 2004; 24:3964-73; PMID:15102912; http://dx.doi.org/10.1523/JNEUROSCI.5259-03.2004
- Hong K–K, Chakravarti A, Takahashi JS. The gene for soluble N-ethylmaleimide sensitive factor attachment protein a is mutated in hydrocephaly with hop gait (hyh) mice. Proc Natl Acad Sci USA 2004; 101:1748-53; PMID:14755058; http://dx.doi.org/10.1073/pnas.0308268100
- Perrotta C, Bizzozero L, Cazzato D, Morlacchi S, Assi E, Simbari F, Zhang Y, Gulbins E, Bassi MT, Rosa P, et al. Syntaxin 4 is required for acid sphingomyelinase activity and apoptotic function. J Biol Chem 2010; 285: 40240-51; PMID:20956541; http://dx.doi.org/10.1074/jbc.M110.139287
- Cotrufo T, Pérez-Brangulí F, Muhaisen A, Ros O, Andrés R, Baeriswyl T, Fuschini G, Tarrago T, Pascual M, Ureña J, et al. A signaling mechanism coupling netrin-1/deleted in colorectal cancer chemoattraction to SNARE-mediated exocytosis in axonal growth cones. J Neurosci 2011; 31:14463-80; PMID:21994363; http://dx.doi.org/10.1523/JNEUROSCI.3018-11.2011
- Rodrıguez F, Bustos MA, Zanetti MN, Ruete MC, Mayorga LS, Tomes CN. a-SNAP prevents docking of the acrosome during sperm exocytosis because it sequesters monomeric syntaxin. PLoS One 2011; 6:e21925; PMID:21789195; http://dx.doi.org/10.1371/journal.pone.0021925
- Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, Hückelhoven R, Stein M, Freialdenhoven A, Somerville SC, et al. SNARE-protein mediated disease resistance at the plant cell wall. Nature 2003; 425:973-7; PMID:14586469; http://dx.doi.org/10.1038/nature02076
- Assaad FF, Qiu JL, Youngs H, Ehrhardt D, Zimmerli L, Kalde M, Wanner G, Peck SC, Edwards H, Ramonell K, et al. The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell 2004; 15:5118-29; PMID:15342780; http://dx.doi.org/10.1091/mbc.E04-02-0140
- An Q, Ehlers K, Kogel KH, van Bel AJ, Hückelhoven R. Multivesicular compartments proliferate in susceptible and resistant MLA12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol 2006a; 172:563-57; http://dx.doi.org/10.1111/j.1469-8137.2006.01844.x
- An Q, Hückelhoven R, Kogel KH, van Bel AJ. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol 2006b; 8:1009-19; http://dx.doi.org/10.1111/j.1462-5822.2006.00683.x
- Kalde M, Nühse TS, Findlay K, Peck SC. The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc Natl Acad Sci U S A 2007; 104:11850-5; PMID:17592123; http://dx.doi.org/10.1073/pnas.0701083104
- Patel S, Dinesh-Kumar SP. Arabidopsis ATG6 is required to limit the pathogen-associated cell death response. Autophagy 2008; 4:20-7; PMID:17932459; http://dx.doi.org/10.4161/auto.5056
- Hofius D, Schultz-Larsen T, Joensen J, Tsitsigiannis DI, Petersen NH, Mattsson O, Jørgensen LB, Jones JD, Mundy J, Petersen M. Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell 2009; 137:773-83; PMID:19450522; http://dx.doi.org/10.1016/j.cell.2009.02.036
- Lenz HD, Haller E, Melzer E, Kober K, Wurster K, Stahl M, Bassham DC, Vierstra RD, Parker JE, Bautor J, et al. Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J 2011; 66:818-30; PMID:21332848; http://dx.doi.org/10.1111/j.1365-313X.2011.04546.x
- Lai Z, Wang F, Zheng Z, Fan B, Chen Z. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J 2011; 66:953-68; PMID:21395886; http://dx.doi.org/10.1111/j.1365-313X.2011.04553.x
- Cook DE, Lee TG, Guo X, Melito S, Wang K, Bayless A, Wang J, Hughes TJ, Willis DK, Clemente T, et al. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science 2012; 338:1206-9; PMID:23065905; http://dx.doi.org/10.1126/science.1228746
- Esmon B, Novick P, Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell 1981; 25:451-60; PMID:7026044; http://dx.doi.org/10.1016/0092-8674(81)90063-5
- Antoniw JF, Pierpoint WS. The purification and properties of one of the 'b" proteins from virus-infected tobacco plants. J Gen Virol 1978; 39:343-50; http://dx.doi.org/10.1099/0022-1317-39-2-343
- Kauffmann S, Legrand M, Geoffroy P, Fritig B. Biological function of 'pathogenesis-related" proteins: four PR proteins of tobacco have 1,3-b-glucanase activity. EMBO J 1987; 6:3209-12; PMID:16453802
- Legrand M, Kauffman S, Geoffroy P, Fritig B. Biological function of pathogenesis-related proteins: four tobacco pathogenesis related proteins are chitinases. Proc Natl Acad Sci USA 1987; 84: 6750-4; PMID:16578819; http://dx.doi.org/10.1073/pnas.84.19.6750
- Kauffmann S, Legrand M, Fritig B. Isolation and characterization of six pathogenesis-related (PR) proteins of Samsun NN tobacco. Plant Mol Biol 1990; 14:381-90; PMID:2102821; http://dx.doi.org/10.1007/BF00028774
- Falk A, Feys BJ, Frost LN, Jones JDG, Daniels MJ, Parker JE. EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc Natl Acad Sci U S A 1999; 96:3292-7
- Feys BJ, Moisan LJ, Newman MA, Parker JE. Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J 2001; 20:5400-11; PMID:11574472; http://dx.doi.org/10.1093/emboj/20.19.5400
- Zhou N, Tootle TL, Tsui F, Klessig DF, Glazebrook J. PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 1998; 10:1021-30; PMID:9634589; http://dx.doi.org/10.1105/tpc.10.6.1021
- Nawrath C, Me´traux JP. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 1999; 11:1393-404; PMID:10449575
- Nawrath C, Heck S, Parinthawong N, Me´traux J-P. EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 2002; 14:275-86; PMID:11826312; http://dx.doi.org/10.1105/tpc.010376
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defense. Nature 2001; 414:562-5; PMID:11734859; http://dx.doi.org/10.1038/35107108
- Niggeweg R, Thurow C, Kegler C, Gatz C. Tobacco transcription factor TGA2.2 is the main component of as-1-binding factor ASF1 and is involved in salicylic acid- and auxin-inducible expression of as-1-containing target promoters. J Biol Chem 2000; 275:19897-905; PMID:10751419; http://dx.doi.org/10.1074/jbc.M909267199
- Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Despre C. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep 2012; 1:639-47; PMID:22813739; http://dx.doi.org/10.1016/j.celrep.2012.05.008
- Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 1994; 6:1583-92; PMID:12244227; http://dx.doi.org/10.1105/tpc.6.11.1583
- Delaney TP, Friedrich L, Ryals JA. Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA 1995; 92:6602-6; PMID:11607555; http://dx.doi.org/10.1073/pnas.92.14.6602
- Glazebrook J, Rogers EE, Ausubel FM. Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 1996; 143:973-98; PMID:8725243
- Shah J, Tsui F, Klessig DF. Characterization of a salicylic acid insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol Plant Microbe Interact 1997; 10:69-78; PMID:9002272; http://dx.doi.org/10.1094/MPMI.1997.10.1.69
- Pieterse CMJ, Van Loon LC. NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 2004; 7:456-64; PMID:15231270; http://dx.doi.org/10.1016/j.pbi.2004.05.006
- Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL. Arabidopsis mutants simulating disease resistance response. Cell 1994; 77:565-77; PMID:8187176; http://dx.doi.org/10.1016/0092-8674(94)90218-6
- Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 1996; 273:1853-6; PMID:8791589; http://dx.doi.org/10.1126/science.273.5283.1853
- Dietrich RA, Richberg MH, Schmidt R, Dean C, Dangl JL. A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell 1997; 88:685-94; PMID:9054508; http://dx.doi.org/10.1016/S0092-8674(00)81911-X
- Kliebenstein DJ, Dietrich RA, Martin AC, Last RL, Dangl JL. LSD1 regulates salicylic acid induction of copper zinc superoxide dismutase in Arabidopsis thaliana. Mol Plant Microbe Interact 1999; 12:1022-6; PMID:10550898; http://dx.doi.org/10.1094/MPMI.1999.12.11.1022
- Epple P, Mack AA, Morris VR, Dangl JL. Antagonistic control of oxidative stress-induced cell death in Arabidopsis by two related, plant-specific zinc finger proteins. Proc Natl Acad Sci U S A 2001; 100:6831-6; http://dx.doi.org/10.1073/pnas.1130421100
- Wituszynska W, Slesak I, Vanderauwera S, Szechynska-Hebda M, Kornas A, Van Der Kelen K, Mühlenbock P, Karpinska B, Mackowski S, Van Breusegem F, et al. Lesion simulating disease1, enhanced disease susceptibility1, and phytoalexin deficient4 conditionally regulate cellular signaling homeostasis, photosynthesis, water use efficiency, and seed yield in Arabidopsis. Plant Physiol 2013; 161:1795-805; PMID:23400705; http://dx.doi.org/10.1104/pp.112.208116
- Liu S, Kandoth PK, Warren SD, Yeckel G, Heinz R, Alden J, Yang C, Jamai A, El-Mellouki T, Juvale PS, et al. A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature 2012; 492:256-60; PMID:23235880; http://dx.doi.org/10.1038/nature11651
- Matthews BF, Beard H, MacDonald MH, Kabir S, Youssef RM, Hosseini P, Brewer E. Engineered resistance and hypersusceptibility through functional metabolic studies of 100 genes in soybean to its major pathogen, the soybean cyst nematode. Planta 2013; 237:1337-57; PMID:23389673; http://dx.doi.org/10.1007/s00425-013-1840-1
- Vernooij, B, Friedrich, L, Ahl Goy, P, Staub, T, Kessmann, H, Ryals, J. 2,6-Dichloroisonicotinic acid-induced resistance to pathogens without the accumulation of salicylic acid. Mol Plant-Microbe Interact 1995; 8:228-34
- Rusterucci C, Aviv DH, Holt BF 3rd, Dangl JL, Parker JE. The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. Plant Cell 2001; 13:2211-24; PMID:11595797; http://dx.doi.org/10.1105/tpc.13.10.2211
- Aviv DH, Rusterucci C, Holt III BF, Dietrich RA, Parker JE, Jeffery L. Dangl JL. Runaway cell death, but not basal disease resistance, in lsd1 is SA- and NIM1/NPR1-dependent. Plant J 2002; 29: 381-91; PMID:11844114; http://dx.doi.org/10.1046/j.0960-7412.2001.01225.x
- Mateo A, Muhlenbock P, Rusterucci C, Chang CC-C, Miszalski Z, Karpinska B, Parker JE, Mullineaux PM, Karpinski S. Lesion simulating disease 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiol 2004; 136: 2818-30; PMID:15347794; http://dx.doi.org/10.1104/pp.104.043646
- Muhlenbock P, Plaszczyca M, Plaszczyca M, Mellerowicz E Karpinski S. Lysigenous aerenchyma formation in arabidopsis is controlled by lesion simulating disease1. Plant Cell 2007; 19:3819-30; PMID:18055613; http://dx.doi.org/10.1105/tpc.106.048843
- Desikan R, Hancock JT, Coffey MJ, Neill SJ. Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett 1996; 382(1-2):213-7; PMID:8612756; http://dx.doi.org/10.1016/0014-5793(96)00177-9
- Kadota Y, Sklenal J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JDG, Shirasu K, Menke F, Jones A, et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell 2014; 54:43-55; PMID:24630626; http://dx.doi.org/10.1016/j.molcel.2014.02.021
- Wituszynska W, Slesak I, Vanderauwera S, Szechynska-Hebda M, Kornas A, Van Der Kelen K, Mühlenbock P, Karpinska B, Mackowski S, Van Breusegem F, et al. Lesion simulating disease1, enhanced disease susceptibility1, and phytoalexin deficient4 conditionally regulate cellular signaling homeostasis, photosynthesis, water use efficiency, and seed yield in Arabidopsis. Plant Physiol 2013; 161: 1795-1805; PMID:23400705
- Caldwell BE, Brim CA, Ross JP. Inheritance of resistance of soybeans to the soybean cyst nematode, Heterodera glycines. Agron J 1960; 52:635-6; http://dx.doi.org/10.2134/agronj1960.00021962005200110007x
- Matson AL, Williams LF. Evidence of a fourth gene for resistance to the soybean cyst nematode. Crop Sci 1965; 5:477; http://dx.doi.org/10.2135/cropsci1965.0011183X000500050032x
- Kim M, Hyten DL, Bent AF, Diers BW. Fine mapping of the SCN resistance locus rhg1-b from PI 88788. Plant Genome 2010; 3:81-9; http://dx.doi.org/10.3835/plantgenome2010.02.0001
- Matsye PD, Kumar R, Hosseini P, Jones CM, Tremblay A, Alkharouf NW, Matthews BF, Klink VP. Mapping cell fate decisions that occur during soybean defense responses. Plant Mol Biol 2011; 77:513-28; PMID:21986905; http://dx.doi.org/10.1007/s11103-011-9828-3
- Cook DE, Bayless AM, Wang K, Guo X, Song Q Jiang, J, Bent AF. 2014. Distinct copy number, coding sequence and locus methylation patterns underlie Rhg1-mediated soybean resistance to soybean cyst nematode. Plant Physiol; 165:630-647.
- Liu S, Kandoth PK, Warren SD, Yeckel G, Heinz R, Alden J, Yang C, Jamai A, El-Mellouki T, Juvale PS, et al. A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature 2012; 492:256-60; PMID:23235880; http://dx.doi.org/10.1038/nature11651
- Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, et al. Genome sequence of the palaeopolyploid soybean. Nature 2010; 463:178-83; PMID:20075913; http://dx.doi.org/10.1038/nature08670
- Banfield DK, Lewis MJ, Pelham HR. A SNARE-like protein required for traffic through the Golgi complex. Nature 1995; 375:806-9; PMID:7596416; http://dx.doi.org/10.1038/375806a0
- Bubeck J, Scheuring D, Hummel E, Langhans M, Viotti C, Foresti O, Denecke J, Banfield DK, Robinson DG. The syntaxins SYP31 and SYP81 control ER-Golgi trafficking in the plant secretory pathway. Traffic 2008; 9:1629-52; PMID:18764818; http://dx.doi.org/10.1111/j.1600-0854.2008.00803.x
- Melser S, Wattelet-Boyer V, Brandizzi F, Moreau P. Blocking ER export of the Golgi SNARE SYP31 affects plant growth. Plant Signal Behav 2009; 4:962-4; PMID:19826222; http://dx.doi.org/10.4161/psb.4.10.9643
- Chatre L, Wattelet-Boyer V, Melser S, Maneta-Peyret L, Brandizzi F, Moreau P. A novel di-acidic motif facilitates ER export of the syntaxin SYP31. J Exp Bot 2009; 60:3157-65; PMID:19516076; http://dx.doi.org/10.1093/jxb/erp155
- Endo BY. Histological responses of resistant and susceptible soybean varieties, and backcross progeny to entry development of Heterodera glycines. Phytopathology 1965; 55:375-81
- Yokoyama R, Nishitani K. Endoxyloglucan transferase is localized both in the cell plate and in the secretory pathway destined for the apoplast in tobacco cells. Plant Cell Physiol 2001; 42: 292-300; PMID:11266580; http://dx.doi.org/10.1093/pcp/pce034
- Matthews BF, Beard H, Brewer E, Kabir S, MacDonald MH, Youssef RM. Arabidopsis genes, AtNPR1, AtTGA2 and AtPR-5, confer partial resistance to soybean cyst nematode (Heterodera glycines) when overexpressed in transgenic soybean roots. BMC Plant Biol 2014; 14:96; PMID:24739302; http://dx.doi.org/10.1186/1471-2229-14-96
- Tepfer D. Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell 1984; 37:959-67; PMID:6744417; http://dx.doi.org/10.1016/0092-8674(84)90430-6
- Haas JH, Moore LW, Ream W, Manulis S. Universal PCR primers for detection of phytopathogenic Agrobacterium strains. Appl Environ Microbiol 1995; 61:2879-84; PMID:7487020
- Hofgen R, Willmitzer L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res 1988; 16:9877; PMID:3186459; http://dx.doi.org/10.1093/nar/16.20.9877
- Jenkins WR. A rapid centrifugal flotation technique for separating nematodes from soil. Plant Dis Rep 1964; 48:692
- Matthews B, MacDonald MH, Thai VK, Tucker ML. Molecular characterization of argenine kinase in the soybean cyst nematode (Heterodera glycines). J Nematol 2003; 35:252-8; PMID:19262758
- Byrd DW Jr, Kirkpatrick T, Barker KR. An improved technique for clearing and staining plant tissue for detection of nematodes. J Nematol 1983; 15:142-3; PMID:19295781
- Golden AM, Epps JM, Riggs RD, Duclos LA, Fox JA, Bernard RL. Terminology and identity of infraspecific forms of the soybean cyst nematode (Heterodera glycines). Plant Dis Rep 1970; 54:544-6
- Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, Foulger R, Eilbeck K, Lewis S, Marshall B, Mungall C, Richter J, et al. The gene ontology (GO) database and informatics resource. Nucleic Acids Res 2004; 32: D 258-61
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 2012; 7:562-78; PMID:22383036; http://dx.doi.org/10.1038/nprot.2012.016
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009; 25:1105-11; PMID:19289445; http://dx.doi.org/10.1093/bioinformatics/btp120
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 2010; 28:511-5; PMID:20436464; http://dx.doi.org/10.1038/nbt.1621
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25:402-8; http://dx.doi.org/10.1006/meth.2001.1262
