Abstract
The data presented in this work revealed that in Zea mays the exogenously added auxins indole-3-acetic acid (IAA) and 1-napthaleneacetic acid (NAA), promoted the establishment of subsidiary cell mother cell (SMC) polarity and the subsequent subsidiary cell formation, while treatment with auxin transport inhibitors 2,3,5-triiodobenzoic acid (TIBA) and 1-napthoxyacetic acid (NOA) specifically blocked SMC polarization and asymmetrical division. Furthermore, in young guard cell mother cells (GMCs) the PIN1 auxin efflux carriers were mainly localized in the transverse GMC faces, while in the advanced GMCs they appeared both in the transverse and the lateral ones adjacent to SMCs. Considering that phosphatidyl-inositol-3-kinase (PI3K) is an active component of auxin signal transduction and that phospholipid signaling contributes in the establishment of polarity, treatments with the specific inhibitor of the PI3K LY294002 were carried out. The presence of LY294002 suppressed polarization of SMCs and prevented their asymmetrical division, whereas combined treatment with exogenously added NAA and LY294002 restricted the promotional auxin influence on subsidiary cell formation. These findings support the view that auxin is involved in Z. mays subsidiary cell formation, probably functioning as inducer of the asymmetrical SMC division. Collectively, the results obtained from treatments with auxin transport inhibitors and the appearance of PIN1 proteins in the lateral GMC faces indicate a local transfer of auxin from GMCs to SMCs. Moreover, auxin signal transduction seems to be mediated by the catalytic function of PI3K.
Abbreviations
| AF | = | actin filament |
| DIC | = | differential interference contrast |
| GMC | = | guard cell mother cell |
| IAA | = | indole-3-acetic acid |
| MT | = | microtubule |
| NAA | = | 1-napthaleneacetic acid |
| NOA | = | 1-napthoxyacetic acid |
| PDK | = | 3-phosphoinositide-dependent kinase |
| PI3K | = | phosphatidyl-inositol-3-kinase |
| PLC | = | phospholipase C |
| PLD | = | phospholipase D |
| ROP GTPases | = | Rho-like GTPases of plants |
| SMC | = | subsidiary cell mother cell |
| TIBA | = | 2,3,5-triiodobenzoic acid |
Introduction
Stomatal complexes in Zea mays and generally in Poaceae is the outcome of a definite sequence of three asymmetrical divisions that give rise to the guard cell mother cell (GMC) and two subsidiary cells laterally to it, and a symmetrical one which produces the pair of guard cells ().Citation1-3 Among them, the divisions generating the subsidiary cells have been repeatedly studied, since they constitute a very attractive model to investigate the premitotic cell polarization and the following asymmetrical division. The subsidiary cell mother cell (SMC) asymmetrical division is undoubtedly triggered by a local induction stimulus "emitted" by the GMC (reviews by refs. 2–4; see ).
Figure 1. Diagram illustrating the development of Zea mays stomatal complexes. MT: microtubule; PPB: preprophase band; SMC: subsidiary cell mother cell.
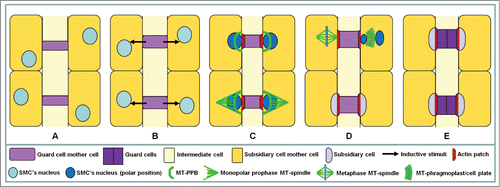
This stimulus triggers a definite sequence of polarization events that precede and accompany the asymmetrical SMC division, which in turn creates a minute subsidiary cell and a large typical epidermal one. Although over the last decades the successive stages of SMC protoplast polarization and the mechanisms that mediate or promote its asymmetrical division have been studied (reviews by refs. 2, 3, 5), the nature of inductive stimulus still remains unknown. This signal might be a chemical substance emitted by the GMCCitation1,2,6-9 or a mechanical stimulus exerted by the GMC on its lateral SMCs.Citation2,10-13 Considering the above, as well as that SMC division is characterized by a shift of the division plane orientation in protoderm from transverse to leaf axis to longitudinal one (review by ref. 2; see also ), it is reasonable to assume that the inductive stimulus might be a hormone-like substance.
Auxin might be an ideal inducer of polarity, since it directs cellular patterning by controlling division plane orientation.Citation14-16 This hormone plays a key role in plant development regulating a remarkably wide range of developmental processes.Citation17 In Arabidopsis, auxin participates in leaf development, where among others, seems to coordinate asymmetrical cell differentiation and division.Citation18,19 The unique morphogenetic auxin properties could be, at least in part, attributed to its ability to become asymmetrically and polarly distributed.Citation17 In Zea mays, it is polarly transported along the main leaf axis.Citation20,21 Auxin transportation is mediated by several families of auxin transporters, which participate in intracellular and intercellular auxin influx and efflux.Citation22 Transporters that provide directionality to the intercellular auxin flow are, among others, the PIN-FORMED (PIN) efflux carriers and the AUXIN INSENSITIVE1 (AUX1) influx carriers. Citation17,22-24
In developing stomatal complexes of grasses, like those of Z. mays, the shift in division plane orientation, as well as SMC polarization, might be induced by a local change in the direction of auxin flow in growing epidermis. To investigate this hypothesis the effects of indole-3-acetic acid (IAA), its synthetic analog 1-napthaleneacetic acid (NAA), and those of the specific auxin transport inhibitors 2,3,5-triiodobenzoic acid (TIBA)Citation25 and 1-napthoxyacetic acid (NOA)Citation25 on the asymmetrical divisions generating subsidiary cells in Z. mays, were monitored. TIBA disrupts the cycling of PIN1 carriers and affects auxin transport by interfering with membrane trafficking processes.Citation26 NOA is considered as an inhibitor of auxin influx since it has been shown to affect cellular auxin uptake facilitated by AUX1 transporters.Citation25 In addition, taking into consideration that polar PIN localization is indicative of the auxin flow direction,Citation27 the localization of PIN1, one of the first identified PIN efflux carriers,Citation22 in the developing Z. mays stomatal complexes was also examined.
Interestingly, phospholipid signaling is required for PIN localization and cooperates with auxin in establishing cell polarity.Citation28 In addition, phospholipases C and D (PLC/PLD) signal transduction pathways seem to promote the induction or perception of the stimuli emitted by the GMC, controlling the SMC asymmetrical division.Citation12 On the other hand, phosphatidyl-inositol-3-kinase (PI3K) modulates phospholipid turnover catalyzing the phosphorylation of phosphoinositides and importantly, its function assists auxin signaling.Citation29 Moreover, PI3K catalytic activity is necessary for generation of cell polarity in migrating monospores of the red alga Porphyra yezoensis.Citation30 Thus, the role of PI3K in the asymmetrical division of SMCs and its potential implication in mediating signal transduction of auxin was evaluated, using the specific PI3K inhibitor LY294002.Citation31 As far as we know, this is the first study involving auxin signaling in polarization/asymmetrical division of SMCs in grasses.
Results
Subsidiary cell formation in control seedlings
General remarks
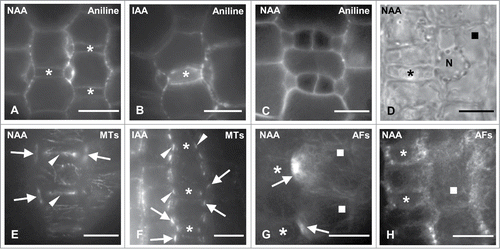
In Z. mays, cells of particular protodermal rows, the stomatal ones, undergo asymmetrical divisions close to basal leaf meristem producing the GMCs (). Initially, GMCs appear rectangular in paradermal view (), however, as they develop, GMCs elongate along the stomatal row axis and before their division they tend to assume an almost square shape in paradermal view (). The assembly of an interphase microtubule (MT)-ring lining the mid-region of lateral and periclinal GMC cell walls (), which is followed by deposition of a cellulose microfibril ring at these regions, plays a critical role in GMC morphogenesis.Citation2,32 The cellulose microfibril ring allows the elongation of the lateral cell walls and simultaneously prevents the expansion of the transverse ones (). The anticlinal cell walls oriented parallel to the leaf axis are defined as lateral walls, while those oriented transversely to leaf axis are the transverse cell walls. The periclinal ones are those exhibiting a parallel to leaf surface orientation.
Figure 2. (A–D) Areas of Z. mays stomatal rows, as observed with DIC optics, displaying young GMCs (A), GMCs in an intermediate developmental stage (B), advanced GMCs (C) and young stomatal complexes (D). The double arrow in (A) shows the longitudinal leaf axis. The asterisks mark the GMCs and the squares the SMCs. In the SMC shown in (C) the nucleus (N) has occupied its polar position. The arrows point to the daughter cell walls of the asymmetrical divisions that create the subsidiary cells. The arrows in (D) show subsidiary cells and the arrowheads young guard cells. EC: epidermal cell; ICSR: intervening cell of the stomatal row. (E and F) GMCs (asterisks) and SMCs (squares) after tubulin immunolabeling in external (E) and median (F) optical planes. The arrows point to preprophase MT- band profiles of a SMC and the arrowheads to those of an interphase MT-ring in GMCs. (G) Tubulin immunolabeling in a prophase SMC (square). The arrows indicate the preprophase MT- band, the arrowheads the half prophase MT-spindle, while the asterisk marks the inducing GMC. N: nucleus. (H) AF-patch (arrow) in SMC (square), which bulges toward the inducing GMC (asterisk). Scale bars: 10 μm.
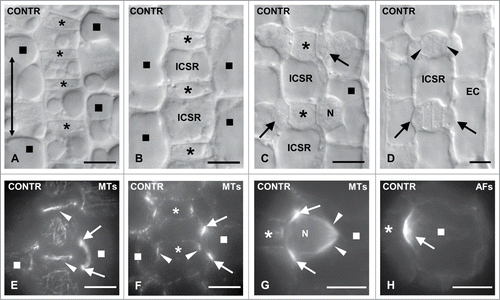
Before division, the length of GMCs, which represents the dimension parallel to the stomatal row axis, increases about 125.00%. Simultaneously, its width, the dimension vertical to the same axis, appears 34.60% decreased.Citation13 As a result, SMCs bulge locally toward their adjacent GMCs (). The latter cells emit a stimulus that induces asymmetrical division of the SMCs yielding a minute lens-shaped subsidiary cell, adjacent to the inducing GMC (). The local bulging of Z. mays SMCs toward the inducing GMCs is also facilitated by the patterned expansion of intervening cells of the stomatal row (see also ).Citation13
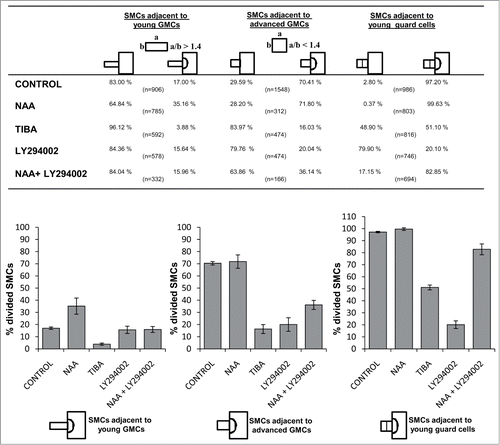
It has been estimated that in Z. mays seedlings grown in dark at 25 ± 1°C, the GMCs exhibiting a width/length ratio between 0.8 and 1.4 are in their majority capable of emanating inductive stimulus toward the adjacent SMCs.Citation12 GMCs aspect ratio was assessed in a central optical cell plane using a light microscope equipped with a micrometer scale. In the current study, according to their aspect ratio, the GMCs were classified in two subgroups: (a) GMCs with aspect ratio more than 1.4 which are present close to meristem leaf region, from now on called “young” GMCs and (b) GMCs with respective ratio less than 1.4, which are located at protodermal regions where the subsidiary cell formation occurs, from now on called “advanced” GMCs. Based on the above consideration, each SMC was classified according to the particular category of its neighbor GMC and its status (divided or not). Laterally to advanced GMCs 70.41% of SMCs (1090 out of 1548 SMCs measured) have been divided asymmetrically (), whereas the percentage of divided SMCs adjacent to young GMCs is 17.00% (154 out of 906 SMCs measured; ). Besides, 97.20% of SMCs adjacent to young guard cells () have been divided (958 out of 986 SMCs; ).
SMC polarization
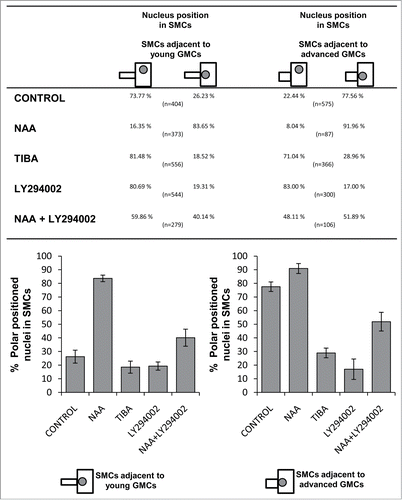
The primary protoplasmic events of SMCs' polarization preceding asymmetrical division are: (a) The migration of nucleus toward the inducing GMC (). It has been calculated that in 77.56% of SMCs (446 out of 575 measured) adjacent to advanced GMCs the nucleus occupies its polar position (). The percentage of SMCs adjacent to young GMCs possessing a properly positioned nucleus is 26.23% (106 out of 404 SMCs; ). (b) The cortical cytoplasm lining the SMC cell wall region shared with the GMC lacks MTs ().Citation33,34 (c) The assembly of an unusually shaped preprophase MT-band at the polar end of SMC (reviews by refs. 2, 3, 5; see also ). (d) The organization of a monopolar prophase MT half-spindle, which is connected with the preprophase MT-band and seems to anchor the nucleus to its polar position ().Citation33 (e) The organization of an actin filament (AF)-patch (for literature see refs. 2, 3; see also ) and an endoplasmic reticulum-patchCitation13 at the polar end of SMC.
PIN1 localization in GMCs and SMCs
Immunolabeling revealed that PIN1 auxin efflux carriers in Z. mays protodermal cells were polarly localized. In regions close to meristem, where newly formed GMCs are found, PIN1 fluorescence signal was mainly emitted by the transverse cell faces, including those of GMCs and only a weak signal emitted by the lateral ones (). During GMC growth, the intensity of PIN1 fluorescence in the latter regions was increased and eventually in advanced GMCs, PIN1 seemed to accumulate in both transverse and lateral GMC faces (; cf. ). In some advanced GMCs the fluorescence of PIN1 carriers appeared even more intense along the lateral GMC faces than along the transverse ones (). Therefore, the lateral faces of many GMCs, which are close to SMCs, are enriched in PIN1 carriers ().
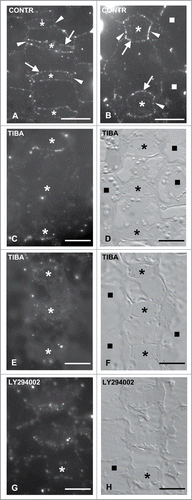
Figure 5. Immunolocalization of PIN1 carriers in control (A, B) and treated (C–H) GMCs (asterisks) and SMCs (squares). The arrows point to transverse and the arrowheads to lateral cell walls. (A) Young GMCs. (B) Advanced GMCs. (C–F) TIBA-treated GMCs (asterisks) as they appear after PIN1 carrier immunolabeling (C and E) and using DIC optics (D and F). (G and H) Immunolabeling of PIN1 carriers in a LY294002-treated GMC (asterisk) (G) and the respective DIC image (H). Treatments: (C–F) TIBA 300 μM, 48 h; (G and H): LY294002 50 μM, 48 h. Scale bars: 10 μm.
Exogenous addition of auxins promotes subsidiary cell formation
Although IAA or NAA, at durations applied in this work, did not detectably affect seedling growth, they led to a remarkable increase in SMC polarization and promoted its asymmetrical division, behaving similarly. In protodermal areas displaying advanced GMCs, the amount of divided SMCs was comparable to that of the controls. Out of 312 NAA-treated SMCs measured, 71.80% have been successfully divided (). However, in regions exhibiting young GMCs the percentage of divided SMCs was 35.16% (276 out of 785 SMCs measured), considerably increased compared to that of the control leaves (; see also ). Moreover, in leaves treated with auxins all young stomatal complexes possessed two subsidiary cells (). Even in controls, a small amount of undivided SMCs adjacent to young guard cells exists (2.80%; ). These data suggest that the addition of exogenous NAA and IAA favors, to some extent, the asymmetrical division of SMCs and interestingly, promotes the initiation of SMC division in protodermal regions close to leaf meristem. Besides, in treated seedlings, many GMCs divided symmetrically before acquiring their almost square, in paradermal view, shape (; cf. ). This observation suggests that the exogenously provided auxins promote both asymmetrical and symmetrical stomatal cell divisions and consequently, the stomatal complex development was attained more closely to the leaf base than in the control leaves (; cf. ).
Figure 6. (A–C) Protodermal areas near the leaf base of NAA- (A and C) and IAA-treated (B) seedlings as viewed after aniline blue staining. Bilaterally to the young GMCs (asterisks in (A) and (B)) subsidiary cells have been formed (cf. ). The stomatal complexes shown in (C) have been formed before the GMCs acquire a square shape (compare ). (D) DIC optical view of polarized NAA-treated SMC (square) in contact with a young GMC (asterisk) (cf. ). N: nucleus. (E and F) NAA- (E) and IAA- (F) treated young GMCs (asterisks) and their neighbor SMCs after tubulin immunolabeling in external (E) and median (F) optical plane. The arrows point to profiles of preprophase MT-band in SMCs and the arrowheads to the interphase MT-ring in GMCs. (G) AF-organization in NAA-treated SMCs (squares) in contact with advanced GMCs (asterisks).The arrows show AF-patches. (H) AF-organization in young NAA-treated GMCs (asterisks) and their adjacent SMCs (square). Treatments: NAA 100 μM, 48 h; IAA 200 μM, 48 h. Scale bars: 10 μm.
Premitotic polarization of the auxin-treated SMCs was also favored. The amount of SMCs possessing the nucleus at its polar position was considerably increased. This was very intense in protodermal regions close to leaf meristem, where the polarized SMCs adjacent to young GMCs was 83.65% (312 out of 373 SMCs; see also ), nearly 3-fold higher compared to the controls (). Similarly, the respective amount of polarized SMCs adjacent to advanced GMCs was increased (). Besides, the interphase GMC MT-ring was almost typical in appearance (; cf. ). As in controls SMCs, the cortical cytoplasm lining the cell wall between SMC and GMC was devoid of MTs. Moreover, laterally to the inducing GMCs a typical preprophase MT-band was formed in treated SMCs ().
In control leaves, these polarizing events predominantly characterized SMCs in contact with advanced GMCs (). However, in leaves incubated with NAA and IAA they were also observed in SMCs adjacent to young GMCs (). In many SMCs, a typical preprophase MT-band was detected, although their nucleus did not occupy a polar position. Nevertheless, this can be also detected in control seedlings of Z. mays and other grasses.Citation2,35,36 The treated SMCs displayed numerous endoplasmic AFs, many of which traversed the perinuclear cytoplasm (). A well-organized AF-patch was found only in those SMCs bulging toward the advanced GMCs (; cf. ). The SMCs that were in contact with young GMCs and did not bulge toward them possessed numerous AFs randomly distributed, but not distinct AF-patches (). Collectively, these data suggest that auxin also promotes SMC polarization establishment and favors the appearance of this phenomenon to protodermal areas close to leaf meristem.
Inhibition of auxin transport restrains subsidiary cell formation
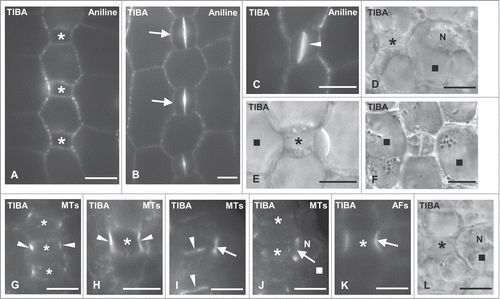
Although TIBA, at durations and concentrations used in this work did not interfere with the growth of Z. mays seedlings and especially GMC morphogenesis, subsidiary cell formation was inhibited (). The percentages of divided TIBA-treated SMCs adjacent to young GMCs, advanced GMCs, or young guard cells were 3.88%, 16.03% and 51.10% respectively (). Therefore, the number of divided SMCs, in all cases, was definitely lower compared to that of controls, whereas the number of undivided SMCs was considerably higher (). As a result, unlike to controls, in seedlings incubated with TIBA, large protodermal areas, possessing advanced GMCs or young guard cells, lacked subsidiary cells (). Notably, the symmetrical divisions in the meristem, the asymmetrical ones generating the GMCs, as well as the symmetrical GMC divisions appeared to proceed normally. This evaluation was made by tracing numerous young daughter cell walls after staining with aniline blue (). The amount of the so-called persistent GMCs, which are the undivided GMCs exhibiting distinct morphological features of guard cells was only 3.93% in 280 young stomatal complexes measured (; cf. ; see also ref. 32, 37). These data demonstrate that in Z. mays protoderm TIBA specifically inhibits the asymmetrical SMC division.
Figure 7. Protodermal areas of seedlings incubated with TIBA. (A–C) Optical sections of stomatal rows after aniline blue staining. The asterisks mark advanced GMCs, the arrows young stomatal complexes and the arrowhead in (C) a newly formed daughter cell wall of the symmetrical GMC division. Note the absence of subsidiary cells. (D) Treated SMC (square) as seen in DIC optics. The nucleus (N) is far from the inducing GMC (asterisk). (E and F). DIC optical views of a persistent GMC (E) and a young stomatal complex lacking subsidiary cells (F). The SMCs in (F) (squares) are not polarized. (G and H) Young (G) and advanced (H) treated GMCs (asterisks) after tubulin immunolabeling. The arrowheads show optical sections of the interphase MT-ring. (I and J) Treated SMC (square) following tubulin immunolabeling in external (I) and median (J) optical plane. The arrows point to sections of the preprophase MT-band of the SMC and the arrowheads to sections of the interphase MT-ring of the GMCs. N: nucleus. (K and L) Treated advanced GMC (asterisk) and one of the neighbor SMCs (square) after AFs staining (K) and in DIC optics (L). The arrows show the AF-patch of the SMC. In (L) the SMC bulges toward the "inducing" GMC and its nucleus (N) has not occupied its polar position (compare ). Treatments: TIBA (A–C) 100 μM, 48 h; (D–L) 300 μM, 48 h. Scale bars: 10 μm.
Premitotic SMC polarization was also affected in TIBA-treated leaves, where the polar migration of SMC nucleus toward the adjacent GMC was greatly inhibited (). Out of 366 SMCs adjacent to advanced GMCs, 28.96% possessed polar positioned nucleus, whereas the majority of these SMCs (71.04%) exhibited nuclei at random sites (). The respective percentage of properly positioned nucleus in SMCs adjacent to young GMCs was 18.52% out of 556 SMCs measured (). Moreover, although in controls, young guard cells were also capable of inducing polarization in their partner SMCs,Citation35,36 in many TIBA-treated stomatal complexes lacking subsidiary cells, the nucleus of SMC resided far from the polar SMC end ().
The interphase MT-ring in GMCs was typical after the application of TIBA (), however, many advanced GMCs emitted an intense tubulin fluorescence signal lining the whole surface of the lateral cell wall (). This implies that TIBA somehow interferes with GMC cortical MT reorganization during GMC transition from interphase to preprophase/prophase (for a review see ref. 2). Besides, TIBA did not considerably affect the organization of cortical MTs in SMCs. The sites of contact between SMC and GMC in treated SMCs lacked cortical MTs and the preprophase MT-band organization laterally to adjacent GMC was almost typical, regardless the position of the SMC nucleus (). In contrast, TIBA affected to some extent AF-organization. The SMCs bulging toward the neighbor GMC displayed not very well defined polar AF-patches (; cf. ).
Treatment with TIBA affected also the distribution of PIN1 carriers in Z. mays protoderm. The advanced GMCs displayed spherical conformations in the cytoplasm emitting weak PIN1 fluorescence (). Relatively few and unequally distributed PIN1 carriers were only detected on the anticlinal GMC faces (; cf. ).
To further investigate the implication of auxin transport in subsidiary cell formation treatments with the auxin influx inhibitor NOA were also carried out. Interestingly, NOA interfered with subsidiary cell generation and the migration of SMC nucleus toward its polar site. The amount of divided NOA-treated SMCs laterally to young or advanced GMCs was 9.21% (21 out of 228 SMCs) and 33.47% (79 out of 236 SMCs), respectively. The respective percentages in control leaves were 17.00% and 70.41%. Consequently, protodermal areas of seedlings incubated with NOA containing advanced GMCs or young guard cells frequently lacked subsidiary cells (). Although in treated SMCs adjacent to young GMCs the percentage of polarized nuclei was comparable to controls, the amount of polarized NOA-treated SMCs next to advanced GMCs was dramatically decreased. Only, 41.40% out of 157 SMCs measured exhibited a properly positioned nucleus, whereas in controls the respective percentage was 77.56%. shows treated SMCs possessing a nucleus located far from the inducing GMC. These findings suggest that auxin influx is also critical for SMC polarization and division.
Figure 8. Protodermal areas of seedlings treated with NOA (A–C), NAA + TIBA (D and E) and NAA + NOA (F). The asterisks mark advanced GMCs and the arrowheads in (B) and (E) point to SMC nucleus. (A–C) Optical sections of stomatal rows after aniline blue staining (A and C) and under DIC optics (B). Note the absence of subsidiary cells adjacent to advanced GMCs (A, B) and to young guard cells (C). (D and E) Stomatal row of NAA + TIBA-treated leaves containing advanced GMCs (D) and young stomata (E). Note the absence of subsidiary cells. (F) Protodermal region of seedlings incubated with NAA + NOA lacking subsidiary cells. Treatments: (A–C): NOA 50 μM, 72 h; (D and E): NAA 100 μM + TIBA 300 μM, 72 h; (F): NAA 100 μM + NOA 50 μM, 72 h. Scale Bars: 10 μm.
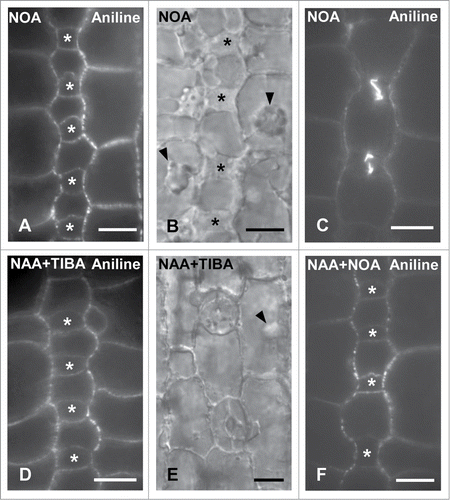
Additional control experiments were carried out in order to clarify whether auxin accumulation or its polar transport was responsible for the promotion of subsidiary cell formation observed after NAA or IAA (see above). Treatments with NAA in combination with TIBA or NOA were carried out. Under these circumstances many protodermal regions containing advanced GMCs or young guard cells were devoid of subsidiary cells (). These findings suggest that not only auxin accumulation but mainly its transport is a prerequisite for subsidiary cell formation.
Selective inhibition of the catalytic activity of PI3K blocks subsidiary cell generation
Although treatment of Z. mays seedlings with the specific PI3K inhibitor LY294002 for 24–72 h did not affect their growth or GMC morphogenesis, subsidiary cell formation was largely prevented (). The amount of newly formed subsidiary cells was greatly decreased compared to controls. Out of 474 SMCs adjacent to advanced GMCs, only 20.04% had been divided and 15.64% out of 578 SMCs laterally to young GMCs had generated subsidiary cells (). The percentage of undivided SMCs adjacent to young guard cells was 79.90% (596 out of 746 SMCs; ). Consequently, many stomatal complexes lacked subsidiary cells (). Similarly, to the results obtained with TIBA and NOA, the inhibition of asymmetrical SMC division by LY294002 treatment seems to be highly specific. The symmetrical GMC divisions were not significantly affected (). Only a small population (7.40%) of persistent GMCs () was detected in treated seedlings.
Figure 9. Protodermal areas of seedlings incubated with LY294002. (A and B) Optical sections of stomatal rows after aniline blue staining (A) and under DIC optics (B). The asterisks mark advanced GMCs, the arrows young stomatal complexes and the arrowhead a newly formed daughter cell wall of the symmetrical GMC division. Note the absence of subsidiary cells in all cases. (C and D) Persistent GMC (asterisk in C) and treated SMC (square in D) as they appear under DIC optics. The SMC nucleus (N) has not occupied a polar position near the neighboring GMC (asterisk; cf. ). (E and F) Treated SMC (square) after tubulin immunolabeling in external (E) and median (F) optical planes. The arrows point to the SMC preprophase MT-band and the arrowheads show the GMC interphase MT-ring. N: nucleus. (G) Treated SMCs (squares) after AF staining. The arrows show the AF-patch in each SMC and the asterisks the neighboring GMC. Note the local "protrusion" of the SMCs toward the neighboring GMCs. Treatments: LY294002 50 μM, (A, D–G): 48; (B and C): 72 h. Scale bars: 10 μm.
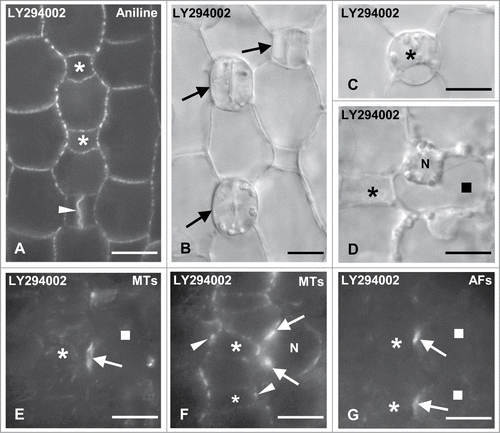
LY294002 clearly interfered with nuclear migration to the polar site of SMCs (). A random position of nucleus was detected in 80.69% SMCs adjacent to young and 83.00% SMCs laterally to advanced GMCs (), as well as in many SMCs next to young guard cells (). On the contrary, in treated SMCs neither the preprophase MT-band nor the AF-patch formation were inhibited, although the latter was not well organized ().
LY294002 seems also to disturb the PIN1 carrier localization. The affected protodermal cells displayed spherical configurations, differently distributed compared to those of controls and emitting poor PIN1 fluorescence signal (). In particular, in the advanced treated GMCs PIN1 carriers were randomly located, unlike the controls where they tended to be equally distributed in all the anticlinal GMC faces (; cf. ).
Auxin and PI3K interplay promotes subsidiary cell formation
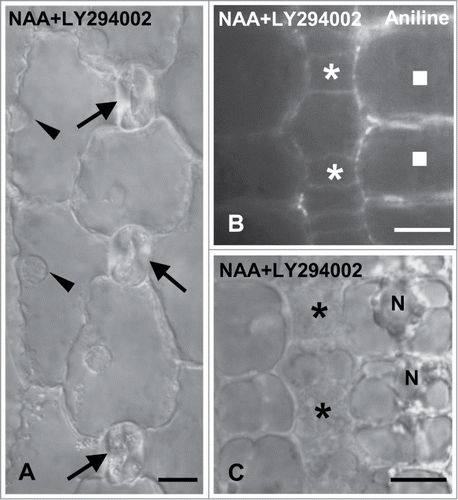
Considering the similar effect caused by inhibition of auxin transport and LY294002 on premitotic SMC polarization and asymmetrical division, it was investigated whether auxin and PI3K cooperate to generate subsidiary cells, applying exogenously NAA together with the specific PI3K inhibitor. NAA and LY294002 separately produced opposite effects on subsidiary cell formation (). Compared to SMCs solely incubated with NAA the percentage of divided SMCs adjacent to young and advanced GMCs in NAA+LY294002-treated seedlings was decreased (). Besides, opposite to the results obtained from NAA treatment, a population (17.15%) of undivided SMCs adjacent to young guard cells was observed (). Premitotic SMC polarization was also disturbed (; cf. ). The percentages of SMCs exhibiting a nucleus at its polar position were much lower than the respective ones obtained when the seedlings were treated with NAA (). These data favor the hypothesis that PI3K is implicated in transduction of the auxin signal, and that both are required for generation of SMC polarity and asymmetrical division.
Figure 10. Protodermal areas of seedlings incubated with NAA plus LY294002. (A) DIC optical view of a stomatal row. The young stomata (arrows) lack subsidiary cells. The arrowheads indicate the nucleus of each SMC that is located far from the respective stoma. (B and C) Treated SMCs (squares) after aniline blue staining (B) and in DIC optics (C). The nucleus (N) resides far from the adjacent GMCs (asterisks). Treatments: NAA 100 μM + LY294002 50 μM, 48 h. Scale bars: 10 μm.
Discussion
Auxin and the inductive stimulus in stomatal complexes
The hypothesis that the stimulus emanating from the grass GMCs, which triggers asymmetrical division in the adjacent SMCs, is a chemical substance possibly hormonal in nature has been suggested more than 50 years ago.Citation1,6,7,38 The chemical nature of the inductive stimulus is adequately supported from previous results obtained from caffeine-treated Z. mays seedlings in which the daughter cell wall separating the subsidiary cell remained incomplete, resulting in the formation of aborted SMCs. As a consequence, their adjacent GMCs were also capable of inducing divisions in the next cell compartment probably due to the transport or diffusion of the stimulus through minute perforations of the incomplete subsidiary cell wall.Citation9
The experimental data present in this work indicate for the first time that auxin might operate as the inductive stimulus that forces the asymmetrical division of Z. mays SMCs. Incubation of Z. mays seedlings with the specific auxin transport inhibitors TIBA and NOA blocked subsidiary cell formation (). On the contrary, the exogenously added NAA and IAA promoted the asymmetrical SMC division and interestingly in this case, the GMCs seem to become potent to induce divisions in SMCs at an earlier stage of their development (). Thus, in NAA- and IAA-treated leaves subsidiary cell formation occurred in protodermal regions closer to the leaf base than in the control ones. The emerging role of auxin in SMC polarization and asymmetrical division is further supported by data derived from proteomic analysis showing that the Z. mays protodermal areas close to leaf meristem are enriched in several auxin related proteins.Citation39 It is worthwhile noting that not just auxin accumulation but its polarized transport seems to be critical for the induction of subsidiary cell generation. This may be concluded, considering that auxin transport inhibitors TIBA and NOA prevented SMC polarization and subsidiary cell formation, regardless the simultaneous presence of exogenously added NAA ().
Strictly defined auxin transport routes control development and patterning of several plant tissues and organs, via managing the distribution of auxin transporters (reviews by refs. 15, 22, 27, 40, 41). Since it is known that PIN polarity primarily determines the direction of auxin flow,Citation27 a local transport of auxin from Z. mays GMCs to SMCs is further supported by the particular appearance of PIN1 efflux carriers on the lateral GMC faces. In young GMCs, they were mainly localized to the transverse GMC faces, but in the advanced ones they were found in all transverse and lateral GMC faces (). Notably, at this developmental leaf stage, in the rest protodermal cells, PIN1 carriers were mainly localized at the transverse cell faces. In this direction, the inhibition of subsidiary cell formation in TIBA-treated leaves is accompanied by disturbance of PIN1 localization in GMCs (). Therefore, it may be suggested that during GMC morphogenesis a programmed change in the direction of auxin transport takes place, which is critical for subsidiary cell formation. Since AUX1 inhibition by NOA also prevented the asymmetrical division of SMCs (), a local accumulation of auxin influx carriers is expected in Z. mays SMC polar face in control leaves, and this possibility should be investigated.
Evidence accumulated over the last years suggests that auxin is intimately involved in development of stomatal complexes of the dicotyledonous species Arabidopsis thaliana. In A. thaliana hypocotyl protoderm, auxin, acting synergistically with other hormones, promotes the asymmetrical divisions producing the GMCs.Citation42 Its crucial implication in controlling stomatal patterning of cotyledon and leaf protoderm in A. thaliana was further strengthened by recent data demonstrating that, during stomatal complex development, auxin was massively transported between different stomatal cell types. Interestingly, prior to the symmetrical division of GMCs, the levels of auxin constantly decreased in these cells.Citation19 Thus, in A. thaliana, GMCs seem to function as local auxin protodermal sources. More recently, Zhang et al.Citation43 showed that auxin interferes with stomatal development in a dose-dependent manner. Low levels of auxin in A. thaliana mesophyll promote stomatal formation, whereas elevated auxin concentrations inhibited this process. Taken, our findings and the above mentioned into consideration, it seems reasonable to extrapolate that Z. mays GMCs also act as local protodermal sources releasing auxin in the adjacent SMCs. Such release might function as an inductive stimulus specifically involved in polarization and asymmetrical division of the SMCs.
Auxin signaling and induction of SMC division
The view that the local change in the direction of auxin flow is involved in signal transduction that leads to the asymmetrical division of SMCs, is further supported by the results obtained after treatments with TIBA and NOA. TIBA, which disrupts PIN1 localization and blocks auxin transportCitation25,26 (see also ) and NOA which inhibits auxin influx, interfering with AUX1 carriers,Citation19,25 both resulted in a great inhibition of SMC division (). Therefore, this hormone could specifically interfere with the induction of this division. The finding that auxin signaling participates in cell cycle control, regulating specific cell cycle checkpoints during transition from G1 to S and from G2 to M phase,Citation41,44-46 corroborates this hypothesis.
Besides, PI3K in plants is an active mediator of auxin signaling and is involved in auxin-dependent processes such as root gravitropism.Citation29 In animal cells, PI3Ks have been implicated in regulation of various steps of cell division.Citation47 The participation of PI3K's catalytic activity in subsidiary cell generation of Z. mays is supported by the specific blockage of the asymmetrical division producing the subsidiary cells, after incubation of seedlings with the specific inhibitor LY294002 (). The ability of LY294002 to reverse the effect on subsidiary cell formation exerted by the exogenously added auxin allows the hypothesis that a dynamic interplay exists between PI3K and this hormone. In particular, NAA plus LY294002 treatment resulted in a remarkable reduction in the amount of subsidiary cells compared to solely NAA-treated Z. mays seedlings ().
Auxin signaling and SMC polarization
Moreover, auxin promotes polarization preceding the asymmetrical division in several cell types, including lateral root founder cells and brown algae zygotes.Citation41 In the present case, auxin signaling interferes with establishment of polarity in SMCs. The addition of exogenous auxins promotes polar migration of SMC nucleus, whereas treatments with TIBA and NOA inhibited this process (). Unlike to control seedlings, NAA and IAA treatments stimulate the polar positioning of SMC nucleus at protodermal regions close to leaf base (). The similarity of the findings obtained by TIBA, NOA, LY294002, and NAA plus LY294002 treatments in polar movement of SMC nucleus, suggests that auxin and PI3K act cooperatively in the induction of polarity in SMCs.
The migration of the nucleus toward the polar SMC site is mediated by AF cytoskeleton (reviews by ref. 2, 11, 48, 49) and interestingly, the NAA-treated SMCs adjacent to young or advanced GMCs exhibited an increased population of perinuclear AFs. Consistently, auxin signaling interferes with the organization of actin cytoskeleton. In A. thaliana leaf epidermal cells, auxin that is transported in the apoplast, modulates the activity of ROP GTPases, which in turn influence AF-organization in neighbor cells.Citation50–52 Thus, RAC/ROPs (Rho-like Rac/Rop GTPases of plants) could function as spatial switches relaying localized auxin signals.Citation53 ROP patches follow auxin gradients that establish polarity in trichoblastsCitation53 and importantly, ROPs accumulate at the polar end of Z. mays SMCs.Citation54 However, the absence of specific ROPs from Z. mays mutants produced only weak defects in SMC polarization. On the contrary, in pan1/pan1 rop2/rop2 rop9/+ triple mutants lacking partially ROP function and simultaneously, the activity of a receptor like kinase (RLK) named PAN1 (pangloss1), the premitotic SMC polarization was seriously affected.Citation54 Notably, PAN1 and its partner PAN2 also exhibit polar accumulation in SMCs of Z. mays.Citation55,56
Considering that PAN1 and ROPs cooperate on positioning SMC nucleus and that in pan1 mutants AF-patches were absent,Citation54,55 it may be suggested that PAN1 may generally interfere with cortical AF-organization. This protein also accumulates at definite regions of subsidiary cells and intervening cells of stomatal rows of Z. mays,Citation57,58 where distinct AF accumulations have also been found.Citation34,58 Thus, the AF-patches at the polar end of Z. mays SMCs, should not be considered as prime polarizing factors, since they are absent from SMCs in other grasses like Triticum turgidum, but they may be related to the local SMC bulging toward the inducing GMC.Citation12,34 Evidence favoring this hypothesis is that in TIBA- and LY294002-treated SMCs, where the polar nucleus migration and the asymmetrical division were inhibited, AF-patches were assembled. Moreover, in auxin-treated leaves, in which SMC polarization and asymmetrical division is expanded into protodermal regions close to leaf base, AF-patch was only detected in SMCs bulging toward their adjacent GMCs.
Polar MT-organization does not seem to be considerably affected in TIBA or LY294002-treated SMCs. Although these treatments block nucleus positioning and the entry of SMCs to division, the preprophase MT-band adjacent to GMC is properly assembled. Therefore, division plane in SMCs, as it is expressed by the preprophase MT-band organization, is determined regardless the inhibition of asymmetrical division. These data may confirm the hypothesis that, in SMCs of grasses, asymmetrical division and determination of the division plane are controlled by different stimuli, both of GMC origin.Citation12 SMC division seems to be triggered by auxin signaling, whereas the SMC division plane may be differently determined.
The plane of SMC division might be determined by local mechanical stresses exerted on SMCs by GMCs.Citation2,12,34,59 The driving force generating such local mechanical stresses might be originated by the unequal elongation rates between the GMCs, the SMCs and the intervening cells of stomatal row. In particular, the lateral GMC cell wall seems to elongate more intensely than its SMC wall partner and the proximal to the GMC cell wall regions of the intervening stomatal row cells (unpublished data). Consequently, mechanical stresses applied to certain SMC cell wall areas may define the position of the unusual SMC preprophase MT-band. In TIBA or LY294002 treated seedlings, where GMC morphogenesis was not affected, the SMCs displayed a typical preprophase MT-band, despite the inhibition of their asymmetrical division, probably because these putative mechanical stresses are not hampered (see also ref. 12). On the contrary, in NAA- and IAA-treated seedlings where the SMC divisions occur at an early stage of GMC morphogenesis, the mechanical stresses and consequently the formation of the SMC preprophase MT-band may be generated in an opposite way. In this case, the slightly growing lateral GMC walls apply tensions to the adjacent growing SMC wall regions and the proximal regions of intervening cells of the stomatal row that display higher elongation rate at this developmental stage.
PI3K and auxin signal transduction
The findings of this study also suggest the implication of PI3K's catalytic activity in transduction routes accomplishing auxin signaling in developing Z. mays stomatal complexes. This is indicated by the extensive attenuation of SMC polarization/asymmetrical division after the inhibitory effect of LY294002 (). Moreover, LY294002 alleviated the promoting effects of the exogenously added auxin in SMC polarization and the subsequent formation of subsidiary cells (). PI3K may mediate these events influencing the recycling of PIN1 carriers since it is known that this is an actin dependent process,Citation15 and that PI3K interferes with the organization of actin cytoskeleton.Citation30,47
Alternatively, PI3K may act regulating the activity of PIN1 carriers. PINOID kinases catalyzing the phosphorylation of PIN1 proteins are activated by 3-phosphoinositide-dependent protein kinases (PDKs).Citation60 PDKs can bind to a broad range of lipids including the catalytic product of plant PI3K phosphatidyl-inositol-3-phosphate.Citation47 Other phosphoinositide kinases,Citation28 inositol triphosphate and phosphatidic acid generated from the activity of the phospholipid degrading enzymes PLCs and PLDs respectively, also mediate polar auxin transport controlling the distribution and activation of PIN carriers.Citation61,62 These data support the view that phospholipid turnover could be important for subsidiary cell generation, among others, directing polar movement of auxin. This hypothesis is in accordance with previous work demonstrating that the catalytic activity of PLCs and PLDs interferes with subsidiary cell formation in Z. mays.Citation12 PI3K apart from its potential participation in mediating auxin-induced processes, via establishment of phospholipid asymmetry across plasmalemma similarly to other phosphoinositide kinases,Citation28 may promote also signal transduction initiated by the local auxin transport from GMCs to SMCs (see also ref. 47).
In conclusion, the data presented in this article reveal for the first time that auxin signaling is implicated in polarization establishment and asymmetrical division of SMC and that PI3K actively participates in auxin signal transduction toward generation of subsidiary cells of Z. mays.
Materials and Methods
Plant material
Caryopses of Zea mays L. var Aris, kindly offered by the National Agricultural Research Foundation (Cereal Institute, Thessaloniki, Greece), were allowed to germinate on moist filter papers in darkness for 72 h at 25 ± 1°C.
Treatments
Three days-old seedlings were incubated with aqueous solutions of 200 μM IAA (Duchefa Biochemie, Haarlem, The Netherlands), 100 μM NAA (Duchefa), 100–300 μM TIBA (Alfa Aesar, Ward Hill, MA, USA), 50 μM NOA (Sigma, St. Louis, MO, USA) and 50 μM LY294002 (Sigma), as well as with NAA + TIBA, NAA + NOA; and NAA + LY294002 at concentrations mentioned above. The roots were excised and the seedlings were then placed on cotton wetted with treatments solutions. Treatments were carried out in darkness at 25 ± 1°C for 24–72 h. Following the same procedure, control seedlings were placed on cotton wetted with distilled water. The substances were derived from dimethyl sulfoxide stock solutions. The maximum dimethyl sulfoxide concentration in treatment solutions was 0.5% (v/v). To confirm that the solvent, at the concentrations used in this study, did not cause any side effect, additional control experiments were performed. In these cases the seedlings used as controls were placed on cotton wetted with 0.5% (v/v) dimethyl sulfoxide dissolved in distilled water. Experiments were repeated at least three times.
Immunocytochemistry
MT immunodetection was performed as was described in Panteris et al.Citation33 In brief, hand-made leaf strips from control or treated seedlings were fixed for 1 h with 4% (w/v) paraformaldehyde in PEM buffer [50 mM piperazine-1,4-bis(2-ethanesulfonic acid, 5 mM ethyleneglycol-O,O'-bis(2-aminoethyl)-N,N,N',N'-tetraacetic acid, 5 mM MgSO4·7H2O, pH 6.8] containing 2% (v/v) dimethyl sulfoxide and 0.1% (v/v) Triton X-100. After three washes with the same buffer the cell walls were digested with 1% (w/v) cellulase (Onozuka, Honshua, Tokyo, Japan), 1% (w/v) macerozyme (Onozuka) and 1% (w/v) driselase (Sigma) in PEM buffer pH 5.0, for 15 min. The specimens were washed again with PEM, pH 6.8 and were extracted with 5% (v/v) dimethyl sulfoxide and 1% (v/v) Triton X-100 in phosphate buffered saline (PBS) for 20 min. After rinsing with PBS the leaf strips were incubated overnight with a rat monoclonal anti-α tubulin antibody (YOL; Clone 1/34; Serotec, Oxford, UK) and afterwards with fluorescein isothiocyanate-conjugated anti-rat IgGs (Sigma) for 2 h at 37°C. Both antibodies were diluted 1:40 in PBS containing 2% (w/v) bovine serum albumin (BSA). DNA staining was performed using 10 μg/ml Hoechst 33258 (Molecular Probes, Eugene, OR, USA). Finally, the leaf strips were mounted with an anti-fade solution containing p-phenyldiamine.
Actin filament staining
Visualization of AFs was achieved using the procedure described previously in Panteris et al.Citation34 Leaf strips of control and treated seedlings were incubated for 30 min in dark with 300 μM m-maleimidobezoyl-N-hydroxysuccinimide ester (Sigma) in PEM containing 2% (v/v) dimethyl sulfoxide and 0.1% (v/v) Triton X-100. The strips were then prefixed with 1 % (w/v) paraformaldehyde in PEM for 20 min and fixed with 4% (w/v) paraformaldehyde for 1 h. Afterwards, they were washed with PEM and extracted with 5% (v/v) dimethyl sulfoxide and 1% (v/v) Triton X-100 in PEM for 20 min. The strips were finally incubated for 1 h at 37°C with Alexa Fluor 568-conjugated phalloidin diluted 10% (v/v) in PEM from a stock solution of 200 Units/ml phalloidin in methanol (Molecular Probes), and they were mounted with the anti-fade solution.
PIN1 localization in semithin sections
For PIN1 immunolocalization in semithin sections, small leaf pieces of control and treated leaves were fixed in 2% (w/v) paraformaldehyde and 0.1% (v/v) glutaraldehyde in PEM, pH 6.8, at 4°C for 1.5 h. The specimens were then washed in the same buffer and dehydrated in a graded ethanol series (10–90%) diluted in distilled water and three times in absolute ethanol, for 30 min (each step), at 0°C. The material was post-fixed with 0.25% (w/v) osmium tetroxide added to the 30% ethanol step for 2 h. The material was infiltrated with LR White (LRW; Sigma) acrylic resin diluted in ethanol, in 10% steps to 100% (1 h in each), at 4°C and finally with pure resin overnight. The samples were embedded in gelatin capsules filled with LRW resin and polymerized at 60°C, for 48 h.
Semithin sections of material embedded in LRW resin were transferred to poly-L-lysine (Sigma) coated glass slides and blocked with 5% (w/v) BSA in PBS for 5 h. After washing with PBS, anti PIN1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:20 in PBS containing 2% (w/v) BSA was applied overnight, at room temperature. Following rinsing with PBS and blocking again with BSA, the sections were incubated for 3 h, at 37°C and then they were left overnight at room temperature in fluorescein isothiocyanate-conjugated anti-goat IgGs (Sigma) diluted 1:40 in PBS containing 2% (w/v) BSA. The sections were finally mounted with the anti-fade solution.
Observation and photography
Paradermal leaf sections of control and treated seedlings were visualized with a Zeiss Axioplan microscope (Zeiss Oberkochen, Germany) equipped with a micrometric scale, a differential interference contrast (DIC) system and an Axiocam MRc5 digital camera. Some sections were incubated with a solution of 0.05% (w/v) aniline blue (Sigma) in 0.07 M K2HPO4 buffer, pH 8.5.Citation63 This handling allows the staining of newly formed daughter cell walls since they contain large amount of callose. The specimens were examined with the Zeiss Axioplan microscope which was equipped with UV source and proper filters.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by a grant from the University of Athens, Greece.
References
- Stebbins LG, Shah SS. Developmental studies of cell differentiation in the epidermis of monocotyledons. II. Cytological features of stomatal development in the Graminae. Dev Biol 1960; 2:477-500; http://dx.doi.org/10.1016/0012-1606(60)90050-6
- Galatis B, Apostolakos P. The role of the cytoskeleton in the morphogenesis and function of stomatal complexes. New Phytol 2004; 161:613-39; http://dx.doi.org/10.1046/j.1469-8137.2003.00986.x
- Facette MR, Smith LG. Division polarity in developing stomata. Curr Opin Plant Biol 2012; 15:585-92; PMID:23044038; http://dx.doi.org/10.1016/j.pbi.2012.09.013
- Pillitteri LJ, Torii KU. Mechanisms of stomatal development. Annu Rev Plant Biol 2012; 63:591-614; PMID:22404473
- Wick SM. The preprophase band. In: Lloyd CW. ed. The cytoskeletal basis of plant growth and form. Academic Press London 1991; 231-44.
- Bünning E. Polarität und inäquale teilung pflanzlichen protoplasten. Protoplasmatologia 1957; 8:1-86.
- Stebbins LG, Jain SK. Developmental studies of cell differentation in the epidermis of monocotyledons. I. Allium, Rhoeo, and Commelina. Dev Biol 1960; 2:409-26; http://dx.doi.org/10.1016/0012-1606(60)90025-7
- Pickett-Heaps JD, Northcote DH. Cell division in the formation of the stomatal complex of the young leaves of wheat. J Cell Sci 1966; 1:121-8; PMID:5929805
- Apostolakos P, Galatis B. Induction, polarity and spatial control of cytokinesis in some abnormal subsidiary cell mother cells of Zea mays. Protoplasma 1987; 140:26-42; http://dx.doi.org/10.1007/BF01273253
- Green PB, Erickson RO, Richmond PA. On the physical basis of cell morphogenesis. Ann N Y Acad Sci. 1970; 175:712-31; http://dx.doi.org/10.1111/j.1749-6632.1970.tb45187.x
- Pickett-Heaps JD, Gunning BES, Brown RC, Lemmon BE, Cleary AL. The cytoplast concept in plant cells: cytoplasmic domains and the evolution of spatially organised cell division. Am J Bot 1999; 86:153-72; PMID:21680355; http://dx.doi.org/10.2307/2656933
- Apostolakos P, Panteris E, Galatis B. The involvement of phospholipases C and D in the asymmetric division of subsidiary cell mother cells of Zea mays. Cell Motil Cytoskel 2008; 65:863-75; http://dx.doi.org/10.1002/cm.20308
- Giannoutsou E, Apostolakos P, Galatis B. Actin filament-organized local cortical endoplasmic reticulum aggregations in developing stomatal complexes of grasses. Protoplasma 2011; 248:373-90; PMID:20644970; http://dx.doi.org/10.1007/s00709-010-0180-2
- Dhonukshe P, Kleine-Vehn J, Friml J. Cell polarity, auxin transport, and cytoskeleton-mediated division planes: who comes first? Protoplasma 2005; 226:67-73; PMID:16231102; http://dx.doi.org/10.1007/s00709-005-0104-8
- Oliva M, Vernoux T, Traas J. From auxin transport to patterning. In: Chen R, Baluška F. eds. Polar auxin transport. Springer-Verlag Heidelberg 2013; 259-79.
- Prasad K, Dhonukshe P. Polar auxin transport: cell polarity to patterning. In: Chen R, Baluška F. eds. Polar auxin transport. Springer-Verlag Heidelberg 2013; 25-44.
- Forestan C, Varotto S. The role of PIN auxin efflux carriers in polar auxin transport and accumulation and their effect on shaping maize development. Mol Plant 2012; 5:787-98; PMID:22186966; http://dx.doi.org/10.1093/mp/ssr103
- Zgurski JM, Sharma R, Bolokoski DA, Schultz EA. Asymmetric auxin response precedes asymmetric growth and differentiation of asymmetric leaf1 and asymmetric leaf2 Arabidopsis leaves. Plant Cell 2005; 17:77-91; PMID:15608337; http://dx.doi.org/10.1105/tpc.104.026898
- Le J, Liu X-G, Yang K-Z, Chen X-L, Zou J-J, Wang H-Z, Wang M, Vanneste S, Morita M, Tasaka M, et al. Auxin transport and activity regulate stomatal patterning and development. Nature Commun 2014; 5:3090; http://dx.doi.org/10.1038/ncomms4090
- Tsiantis M, Brown MIN, Skibinski G, Langdale JA. Disruption of auxin transport is associated with aberrant leaf development in maize. Plant Physiol 1999; 121:1163-8; PMID:10594103; http://dx.doi.org/10.1104/pp.121.4.1163
- Nelissen H, Rymen B, Jikumaru Y, Demuynck K, Van Lijsebettens M, Kamiya Y, Inzé D, Beemster GTS. A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Curr Biol 2012; 22:1183-7; PMID:22683264; http://dx.doi.org/10.1016/j.cub.2012.04.065
- Balzan S, Gurmukh SJ, Carraro N. The role of auxin transporters in monocots development. Front Plant Sci 2014; 5:393; PMID:25177324; http://dx.doi.org/10.3389/fpls.2014.00393
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jurgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003; 115:591-602; http://dx.doi.org/10.1016/S0092-8674(03)00924-3
- Nick P. Probing the actin-auxin oscillator. Plant Signal Behav 2010; 5:94-8; PMID:20023411; http://dx.doi.org/10.4161/psb.5.2.10337
- Tsuda E, Yang H, Nishimura T, Uehara Y, Sakai T, Furutani M, Koshiba T, Hirose M, Nozaki H, Murphy AS, et al. Alkoxy-auxins are selective inhibitors of auxin transport mediated by PIN, ABCB, and AUX1 transporters. J Biol Chem 2011; 286:2354-64; PMID:21084292; http://dx.doi.org/10.1074/jbc.M110.171165
- Geldner N, Friml J, Stierhof Y-D, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 2001; 413:425-8; PMID:11574889; http://dx.doi.org/10.1038/35096571
- Wiśniewska J, Xu J, Seifertová D, Brewer PB, Růžička K, Blilou I, Rouquie D, Benková E, Scheres B, Friml J. Polar PIN localization directs auxin flow in plants. Science 2006; 312:883; PMID:16601151; http://dx.doi.org/10.1126/science.1121356
- Tejos R, Sauer M, Vanneste S, Palacios-Gomez M, Li H, Heilmann M, van Wijk R, Vermeer JEM, Heilmann I, Munnik T, et al. Bipolar plasma membrane distribution of phosphoinositides and their requirement for auxin-mediated cell polarity and patterning in Arabidopsis. Plant Cell 2014; 26:2114-28; PMID:24876254; http://dx.doi.org/10.1105/tpc.114.126185
- Joo JH, Yoo HJ, Hwang I, Lee JS, Nam KH, Bae YS. Auxin-induced reactive oxygen species production requires the activation of phosphatidylinositol 3-kinase. FEBS Lett 2005; 579:1243-8; PMID:15710420; http://dx.doi.org/10.1016/j.febslet.2005.01.018
- Li L, Saga N, Mikami K. Phosphatidylinositol 3-kinase activity and asymmetrical accumulation of F-actin are necessary for establishment of cell polarity in the early development of monospores from the marine red alga Porphyra yezoensis. J Exp Bot 2008; 59:3575-86; PMID:18703492; http://dx.doi.org/10.1093/jxb/ern207
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 1994; 269:5241-48; PMID:8106507
- Galatis B. The organization of microtubules in guard cell mother cells of Zea mays. Can J Bot 1982; 60:1148-66; http://dx.doi.org/10.1139/b82-145
- Panteris E, Apostolakos P, Galatis B. Cytoskeletal asymmetry in Zea mays subsidiary cell mother cells: a monopolar prophase microtubule half-spindle anchors the nucleus to its polar position. Cell Motil Cytoskel 2006; 63:696-709; http://dx.doi.org/10.1002/cm.20155
- Panteris E, Galatis B, Quader H, Apostolakos P. Cortical actin filament organization in developing and functioning stomatal complexes of Zea mays and Triticum turgidum. Cell Motil Cytoskel 2007; 64:531-48; http://dx.doi.org/10.1002/cm.20203
- Galatis B, Apostolakos P, Katsaros C. Synchronous organization of two preprophase microtubule bands and final cell plate arrangement in subsidiary cell mother cells of some Triticum species. Protoplasma 1983; 117:24-39; http://dx.doi.org/10.1007/BF01281781
- Galatis B, Apostolakos P, Katsaros C. Positional inconsistency between preprophase microtubule band and final cell plate arrangement during triangular subsidiary cell and atypical hair cell formation in two Triticum species. Can J Bot 1984; 62:343-59; http://dx.doi.org/10.1139/b84-053
- Galatis B. Differentiation of stomatal meristemoids and guard cell mother cells into guard-like cells in Vigna sinensis leaves after colchicine treatment. An ultrastructural and experimental approach. Planta 1977; 136:103-14; PMID:24420314; http://dx.doi.org/10.1007/BF00396185
- Bünning E. General processes of differentiation. In: Milthorpe F.L. ed. The growth of leaves. Butterworths Scientific Publications London 1956; 18-30.
- Facette MR, Shen Z, Björnsdóttir FR, Briggs SP, Smith LG. Parallel proteomic and phosphoproteomic analyses of successive stages of maize leaf development. Plant Cell 2013; 25:2798-812; PMID:23933881; http://dx.doi.org/10.1105/tpc.113.112227
- Wabnick K, Kleine-Vehn J, Balla J, Sauer M, Naramoto S, Reinöhl V, Merks RMH, Govaerts W, Friml J. Emergence of tissue polarization from synergy of intracellular and extracellular auxin signaling. Mol Syst Biol 2010; 6:447; PMID:21179019
- De Smet I, Beeckman T. Asymmetric cell division in land plants and algae: the driving force for differentiation. Nat Rev Mol Cell Biol 2011; 12:177-88; PMID:21346731; http://dx.doi.org/10.1038/nrm3064
- Saibo NJM, Vriezen WH, Beemster GTS, Van Der Straeten D. Growth and stomata development of Arabidopsis hypocotyls are controlled by gibberellins and modulated by ethylene and auxins. Plant J 2003; 33:989-1000; PMID:12631324; http://dx.doi.org/10.1046/j.1365-313X.2003.01684.x
- Zhang J-Y, He S-B, Li L, Yang H-Q. Auxin inhibits stomatal development through MONOPTEROS repression of a mobile peptide gene STOMAGEN in mesophyll. Proc Nat Acad Sci U S A 2014; 111:E3015-23; http://dx.doi.org/10.1073/pnas.1400542111
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 2002; 14:2339-51; PMID:12368490; http://dx.doi.org/10.1105/tpc.004960
- De Veylder L, Beeckman T, Inzé D. The ins and outs of the plant cell cycle. Nat Rev Mol Cell Biol 2007; 8:655-65; PMID:17643126; http://dx.doi.org/10.1038/nrm2227
- Wang L, Ruan Y-L. Regulation of cell division and expansion by sugar and auxin signalling. Front Plant Sci 2013; 4:163; PMID:23755057
- Lee Y, Munnik T, Lee Y. Plant Phosphatidylinositol 3-Kinase. In: Munnik T. ed. Lipid Signaling in Plants. Plant Cell Monographs, Springer-Verlag Heidelberg 2010; 16:95-106.
- Cleary AL. Actin in formation of stomatal complexes. In: Staiger CJ, Baluška F, Volkmann D, Barlow PW. eds. Actin: a dynamic framework for multiple plant cell functions. Kluwer Academic Publishers Dordrecht 2000; 411-26.
- Smith LG. Plant cell division: building walls in the right places. Nat Rev Mol Cell Biol 2001; 2:33-9; PMID:11413463; http://dx.doi.org/10.1038/35048050
- Xu T, Wen M, Nagawa S, Fu Y, Chen J-G, Wu M-J, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z. Cell surface- and Rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 2010; 143:99-110; PMID:20887895; http://dx.doi.org/10.1016/j.cell.2010.09.003
- Yang Z, Lavagi I. Spatial control of plasma membrane domains: ROP GTPase-based symmetry breaking. Curr Opin Plant Biol 2012; 15:601-7; PMID:23177207; http://dx.doi.org/10.1016/j.pbi.2012.10.004
- Nakamura M, Kiefer CS, Grebe M. Planar polarity, tissue polarity and planar morphogenesis in plants. Curr Opin Plant Biol 2012; 15:593-600; PMID:22906885; http://dx.doi.org/10.1016/j.pbi.2012.07.006
- Wu H-m, Hazak O, Cheung AY, Yalovsky S. RAC/ROP GTPases and auxin signaling. Plant Cell 2011; 23:1208-18; PMID:21478442; http://dx.doi.org/10.1105/tpc.111.083907
- Humphries JA, Vejlupkova Z, Luo A, Meeley RB, Sylvester AW, Fowler JE, Smith LG. ROP GTPases act with the receptor-like protein PAN1 to polarize asymmetric cell division in maize. Plant Cell 2011; 23:2273-84; PMID:21653193; http://dx.doi.org/10.1105/tpc.111.085597
- Cartwright HN, Humphries JA, Smith LG. PAN1: a receptor-like protein that promotes polarization of an asymmetric cell division in maize. Science 2009; 323:649-51; PMID:19179535; http://dx.doi.org/10.1126/science.1161686
- Zhang X, Facette M, Humphries JA, Shen Z, Park Y, Sutimantanapi D, Sylvester AW, Briggs SP, Smith LG. Identification of PAN2 by quantitative proteomics as a leucine-rich repeat-receptor-like kinase acting upstream of PAN1 to polarize cell division in maize. Plant Cell 2012; 24:4577-89; PMID:23175742; http://dx.doi.org/10.1105/tpc.112.104125
- Panteris E, Galatis B, Apostolakos P. Examination of the possible role of PAN1 protein in the ontogenesis of Zea mays stomatal complexes. In: Gkelis S, Karousou R, Kokkini S, Panteris E. eds. Program and Abstracts. 13th Panhellenic Scientific Conference, Hellenic Botanical Society, Thessaloniki 3–6 October, 2013; p. 118.
- Sutimantanapi D, Pater D, Smith LG. Divergent roles for maize PAN1 and PAN2 receptor-like proteins in cytokinesis and cell morphogenesis. Plant Physiol 2014; 164:1905-17; PMID:24578508; http://dx.doi.org/10.1104/pp.113.232660
- Kennard JL, Cleary AL. Pre-mitotic nuclear migration in subsidiary mother cells of Tradescantia occurs in G1 of the cell cycle and requires F-actin. Cell Motil Cytoskel 1997; 36:55-67; http://dx.doi.org/10.1002/(SICI)1097-0169(1997)36:1%3c55::AID-CM5%3e3.0.CO;2-G
- Zegzouti H, Anthony RG, Jahchan N, Bögre L, Christensen SK. Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc Nat Acad Sci U S A 2006; 103:6404-9; http://dx.doi.org/10.1073/pnas.0510283103
- Li G, Xue H-W. Arabidopsis PLDζ2 regulates vesicle trafficking and is required for auxin response. Plant Cell 2007; 19:281-95; PMID:17259265; http://dx.doi.org/10.1105/tpc.106.041426
- Testerink C, Munnik T. Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. J Exp Bot 2011; 62:2349-61; PMID:21430291; http://dx.doi.org/10.1093/jxb/err079
- O'Brien TP, McCully ME. The study of plant structure. Principles and selected methods. Termarcarphi Pty, Melbourne 1981; 352p.