Abstract
Herbivore-induced plant volatile emission is often considered to be attacker species-specific, but most experimental evidence comes from short lived herbaceous species. In a recent study we showed that black poplar (Populus nigra) trees emit a complex blend of volatiles from damaged leaves when they are attacked by generalist gypsy moth (Lymantria dispar) caterpillars. Minor nitrogenous volatiles were especially characteristic of this blend. Here we show that attack on P. nigra by a beetle species, Phratora vulgatissima (Coleoptera, Chrysomelidae), led to the emission of the same compounds as already observed after caterpillar herbivory, but with striking quantitative changes in the blend. The consequences for attraction of herbivore enemies are discussed.
Introduction
When plants are attacked by herbivorous insects, they release specific volatile blends that quantitatively and qualitatively differ from the constitutive plant volatile bouquet. These blends are frequently studied because of their attraction of herbivore enemies.Citation1 Herbivore-induced volatiles are released from the actual site of damage but can also be emitted systemically from adjacent non-damaged tissue.Citation2-4 There is convincing evidence in the recent literature that herbivore-induced plant volatile emission can be specific to the attacker species.Citation5 Insects from different feeding guilds (e.g., sucking, chewing, mining or galling insects) are especially reported to induce different volatile blends.Citation6-8 These differential plant responses are thought to be due to differences in the elicitors present in insect oral secretions, in the mode of feeding, or in the host specificity of the insects or the timing of attack.Citation9 However, most evidence for herbivore species-specific volatile emission comes from short lived herbaceous species. Woody perennials are barely studied in this context.
We recently reported that black poplar (Populus nigra) releases a very complex blend of volatiles when it is attacked by generalist gypsy moth (Lymantria dispar) caterpillars.Citation10 The blend released from damaged black poplar foliage was qualitatively and quantitatively different from that of undamaged adjacent foliage and foliage of non-damaged control trees. Female Glyptapanteles liparidis wasps, which are parasitoids of Lymantria dispar caterpillars, were attracted to the volatiles from damaged leaves, and EAG experiments and behavioral assays revealed that this species was especially attracted to minor, nitrogen-containing compounds.Citation10
As members of natural floodplain forests in Europe, black poplar trees harbor an enormous diversity of arthropods and are almost constantly under attack by different species of herbivorous insects. Whether these trees are capable of releasing herbivore species-specific volatiles thus has important consequences for herbivore enemy attraction.
To investigate whether the black poplar volatile emission patterns produced upon attack by a generalist feeding caterpillar L. dispar are themselves rather general or instead a specific tree response to this attacker species, we have now analyzed the tree response to a chewing insect that is a more specialized feeder, the blue willow beetle (Phratora vulgatissima, Chrysomelidae).
Methods
The same experimental set-up as described in Clavijo McCormick et al.Citation10 was applied but the trees were infested with Phratora vulgatissima leaf beetles (). Forty young Populus nigra trees were selected of which 20 entered the beetle-herbivory treatment and 20 functioned as non-damaged control trees. The foliage of each tree was split in 2 sections, a basipetal and an adjacent apical section, each enclosed with PET film (Toppits® Bratschlauch, Minden, Germany). Experimental herbivory only occurred in the basipetal section. In the beetle-infested trees, 20 individuals of P. vulgatissima were released in the PET bags (). Beetles were allowed to feed for 41 h before volatiles were collected and analyzed as described in.Citation10
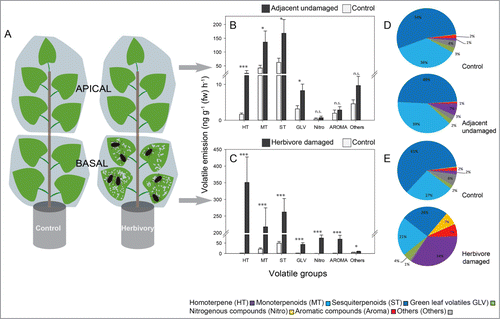
Figure 1. Emission of volatile compounds from herbivore-damaged and adjacent undamaged leaves of Populus nigra (black poplar) trees infested with blue willow beetles (Phratora vulgatissima). (A) The foliage of young trees of 20 different P. nigra genotypes was divided into basal and apical sections with PET film and 20 adult beetles were released on the basal foliage. A second set of PET film-divided trees was left as controls without beetles. After 41 h, volatiles were collected using a dynamic headspace collection system and analyzed by GC-MS/FID. (B), (C) Emission of major groups of volatiles was recorded from beetle-damaged and adjacent undamaged foliage in relation to controls- emission from corresponding regions of trees not subject to P. vulgatissima herbivory. Data are presented as mean ± SE, n = 20 (one representative of each of 20 genotypes). Asterisks indicate significant differences between beetle-infested tress and the controls: *, p< 0.05; ***, P < 0.001; Mann Whitney U-tests. (D), (E) Relative proportion of the major groups of volatiles presented with respect to the full P. nigra odor blend. A full list of all P. nigra volatiles detected is given in ref. 10.
Results
Overall more than 70 compounds could be identified from the headspace of beetle-infested young black poplar trees. The rate of emission of volatiles from damaged leaves of P. nigra differed significantly from the rate of emission from the equivalent basipetal leaves of untreated control trees (). This was true for all 7 groups of volatiles, homoterpenoids, monoterpenoids, sesquiterpenoids, green leaf volatiles, nitrogenous volatiles, aromatic compounds and others (). Most volatile groups also showed differences when comparing undamaged leaves on herbivore-treated plants (adjacent to damaged leaves) to their respective controls on untreated plants.
The foliage of young black poplar trees damaged by the blue willow beetle emitted the same volatile blend qualitatively as already described when the same genotypes were fed upon by gypsy moth caterpillars.Citation9 The total emission rate of beetle-damaged foliage was ∼40% lower than that of caterpillar-damaged foliage. However, in our experiments beetle feeding caused ∼65% less leaf area loss (3.42 ± 0.52% vs. 9.85 ± 1.40%), so beetle feeding may be considered more volatile-inducing. Such a difference might be attributable to differences in feeding patterns: beetles fed toward the center of the leaf in groups of several insects, whereas caterpillars started feeding from the outer rim of leaves, mostly as individuals. Turning to groups of compounds, the proportion of aromatic compounds emitted from beetle-damaged foliage was higher than for caterpillar-damaged leaves.Citation10 In contrast, the proportion of green leaf volatiles was smaller than from caterpillar-damaged leaves.
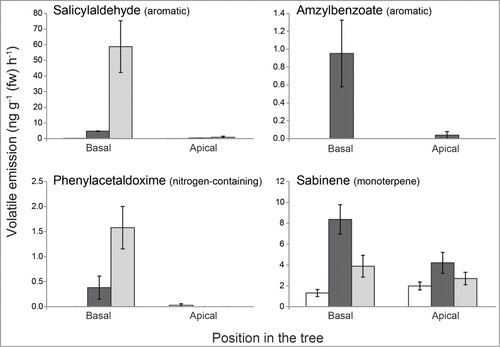
To identify the most important compounds that distinguish the caterpillar-induced from the beetle-induced volatile blends, we employed the Random Forest algorithm (Breiman 2001). Among the 10 most important compounds classifying the 2 different odor blends were mainly aromatics and monoterpenes, with salicylaldehyde being the most important (). This aromatic compound was exclusively measured in the beetle infested foliage, whereas another aromatic compound, amyl benzoate, was only released from the caterpillar-infested foliage. The nitrogen-containing aromatic compound phenylacetaldoxime,Citation11,12 derived from the aromatic amino acid phenylalanine was also among the most distinguishing compounds and mostly released from beetle-infested leaves. Caterpillar-infested leaves also emitted higher amounts of the monoterpene sabinene ().
Table 1. The 10 most important Populus nigra volatiles that distinguish between the blends of Lymantria dispar-damaged and Phratora vulgatissima-damaged foliage as determined by the Random Forest algorithm (Breiman 2001). The volatiles are listed in decreasing relevance based upon mean decrease in accuracy (MDA). ST, sesquiterpenes
Figure 2. Specific emission of individual volatiles from the experiment described in . Volatile compounds that most discriminate between caterpillar- (Lymantria dispar) and beetle- (Phratora vulgatissima) damaged black poplar foliage (as determined by Random Forest analysis, ) were chosen. White bars = control; black bars = caterpillar damaged; gray bars = beetle damaged. Bars represent means ± SEM.
Discussion
The overall composition of the volatile blend from the blue willow beetle (P. vulgatissima) was similar to the previously observed blend from gypsy moth caterpillar (L. dispar)-damaged foliage.Citation10 Notably, nitrogen-containing compounds were found in both blends from damaged leaves, released from the actual site of beetle damage, but not in blends from adjacent undamaged leaves or leaves of undamaged control trees. However, there were major differences in the proportion of aromatic and green leaf volatiles released from beetle-damaged vs. caterpillar-damaged leaves. The greater amount of aromatic volatiles emitted from beetle-damaged foliage is largely due to the higher emission of salicylaldehyde (, ),Citation13 an aromatic compound that is also emitted from larvae of the closely related beetle species Phratora vitellinae.Citation14 However, in our study the emission came predominantly from the trees as adult P. vulgatissima beetles and their frass only released trace amounts of this compound (Unsicker SB unpublished).
Li et al. Citation15 recently documented that volatile emission in another poplar species (Populus tremula x tremuloides) differed quantitatively (but not qualitatively) when the tree was attacked by 2 different leaf chewing caterpillar species. We also found quantitative differences in volatile emission in black poplar when young trees were infested by 2 leaf chewing caterpillar species, Lymantria dispar and the poplar hawk moth Laothoe populi.15
There are many other examples in the literature of herbivore specificity in induced volatile emission pattern depending on the type of herbivore, its age, and in particular its feeding guild.Citation4,16-18 In this study, beetles created more numerous and smaller lesions in poplar leaves than the gypsy moth caterpillars did in our previous work.Citation9 Thus the overall area affected by beetle damage might be bigger even though the actual area removed is smaller. For this reason, it is difficult to directly compare the amount of volatile emission caused by insects with very different feeding modes.Citation6 The fact that poplar is host to many species of herbivores facilitates future experiments with insects from other feeding guilds such as aphids and leaf miners, to explore the issue of volatile specificity in more depth. Such specificity is readily exploited by herbivore enemies in searching for prey and hosts, depending on the diet breadth of the enemy.Citation4 Both predators and parasitoids possess sensory apparatus that can distinguish between blends based on qualitative differences or quantitative ones involving ratio-specific odor recognition.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Beate Rothe and Sandra Irmisch for help in the lab and the Max Planck Institute for Chemical Ecology greenhouse team for their help with rearing P. nigra trees.
Funding
The project was funded by the Max Planck Society.
References
- Mumm R, Dicke M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can J Zool 2010; 88:628-67; http://dx.doi.org/10.1139/Z10-032
- Arimura G, Huber DPW, Bohlmann J. Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa x deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (-)-germacrene D synthase, PtdTPS1. Plant J 2004; 37:603-16; PMID:14756770
- Hiltpold I, Erb M, Robert CAM, Turlings TCJ. Systemic root signalling in a belowground, volatile-mediated tritrophic interaction. Plant Cell Environ 2011; 34:1267-75; PMID:21477121; http://dx.doi.org/10.1111/j.1365-3040.2011.02327.x
- Köllner TG, Lenk C, Schnee C, Köpke S, Lindemann P, Gershenzon J, Degenhardt J. Localization of sesquiterpene formation and emission in maize leaves after herbivore damage. BMC Plant Biol 2013; 13:15; PMID:23363415; http://dx.doi.org/10.1186/1471-2229-13-15
- Clavijo McCormick A, Unsicker SB, Gershenzon J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 2012; 17:303-10; PMID:22503606; http://dx.doi.org/10.1016/j.tplants.2012.03.012
- Cai X-M, Sun X-L, Dong W-X, Wang G-C, Chen Z-M. Herbivore species, infestation time, and herbivore density affect induced volatiles in tea plants. Chemoecology 2014; 24:1-14; http://dx.doi.org/10.1007/s00049-013-0141-2
- Gosset V, Harmel N, Gobel C, Francis F, Haubruge E, Wathelet JP, du Jardin P, Feussner I, Fauconnier ML. Attacks by a piercing-sucking insect (Myzus persicae Sultzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J Exp Bot 2009; 60:1231-40; PMID:19221142; http://dx.doi.org/10.1093/jxb/erp015
- Rodriguez-Saona C, Crafts-Brandner SJ, Canas LA. Volatile emissions triggered by multiple herbivore damage: Beet armyworm and whitefly feeding on cotton plants. J Chem Ecol 2003; 29:2539-50; PMID:14682532; http://dx.doi.org/10.1023/A:1026314102866
- Desurmont GA, Harvey J, van Dam NM, Cristescu SM, Schiestl FP, Cozzolino S, Anderson P, Larsson MC, Kindlmann P, Danner H, et al. Alien interference: disruption of infochemical networks by invasive insect herbivores. Plant Cell Environ 2014; 37:1854-65; PMID:24689553; http://dx.doi.org/10.1111/pce.12333
- Clavijo McCormick A, Irmisch S, Reinecke A, Boeckler GA, Veit D, Reichelt M, Hansson BS, Gershenzon J, Köllner TG, Unsicker SB. Herbivore-induced volatile emission in black poplar: regulation and role in attracting herbivore enemies. Plant Cell Environ 2014; 37:1909-23; PMID:24471487; http://dx.doi.org/10.1111/pce.12287
- Irmisch S, Andrea CM, Boeckler GA, Schmidt A, Reichelt M, Schneider B, Block K, Schnitzler JP, Gershenzon J, Unsicker SB, et al. Two herbivore-induced cytochrome P450 enzymes CYP79D6 and CYP79D7 catalyze the formation of volatile aldoximes involved in poplar defense. Plant Cell 2013; 25:4737-54; PMID:24220631; http://dx.doi.org/10.1105/tpc.113.118265
- Irmisch S, Unsicker SB, Gershenzon J, Köllner TG. Identification and characterization of CYP79D6v4, a cytochrome P450 enzyme producing aldoximes in black poplar (Populus nigra). Plant Signal Behav 2014; 8:(12), e27640; PMID:24390071
- Boeckler GA, Gershenzon J, Unsicker SB. Phenolic glycosides of the Salicaceae and their role as anti-herbivore defenses. Phytochemistry 2011; 72:1497-509; PMID:21376356; http://dx.doi.org/10.1016/j.phytochem.2011.01.038
- Köpf A, Rank NE, Roininen H, Julkunen-Tiitto R, Pasteels JM, Tahvanainen J. The evolution of host-plant use and sequestration in the leaf beetle genus Phratora (Coleoptera: Chrysomelidae). Evolution 1998; 52:517-28
- Clavijo McCormick A, Boeckler G, Kollner TG, Gershenzon J, Unsicker SB. The timing of herbivore-induced volatile emission in black poplar (Populus nigra) and the influence of herbivore age and identity affect the value of individual volatiles as cues for herbivore enemies.. BMC Plant Biol; 2014; 14:304.
- Van Poecke RMP, Roosjen M, Pumarino L, Dicke M. Attraction of the specialist parasitoid Cotesia rubecula to Arabidopsis thaliana infested by host or non-host herbivore species. Entomol Exp Appl 2003; 107:229-36; http://dx.doi.org/10.1046/j.1570-7458.2003.00060.x
- Dicke M, van Loon JJA, Soler R. Chemical complexity of volatiles from plants induced by multiple attack. Nature Chem Biol 2009; 5:317-24; PMID:19377458; http://dx.doi.org/10.1038/nchembio.169
- Delphia CM, Mescher MC, De Moraes CM. Induction of plant volatiles by herbivores with different feeding habits and the effects of induced defenses on host-plant selection by thrips. J Chem Ecol 2007; 33:997-1012; PMID:17415625; http://dx.doi.org/10.1007/s10886-007-9273-6
