Abstract
Low temperature is one of the most important environmental stresses constraining plant development and distribution. Plants have evolved complex adaptive mechanisms to face and survive freezing temperatures. Different signaling pathways regulating plant response to cold have been described, and some of them are mediated by hormones. Recently, we reported that ethylene (ET) acts as a positive regulator of plant freezing tolerance through the activation of cold-induced gene expression, including the CBF-regulon. Here, we present data demonstrating that the Arabidopsis ET overproducer mutant eto1-3 has enhanced freezing tolerance. Moreover, we also show that this mutant exhibits increased accumulation of CBF1, 2 and 3 transcripts, which should account for its tolerant phenotype. All these results constitute new genetic evidence supporting an important role for ET in plant response to low temperature by mediating the CBF-dependent signaling pathway.
Ethylene (ET) has a cardinal role integrating developmental events with external stimuli, including abiotic stresses.Citation1 Data reported by several authors have provided evidence for a positive role of ET in plant defense mechanisms against low temperature. It is well documented that exposure to cold activates ET biosynthesis in different species such as Arabidopsis or winter rye.Citation2-8 Moreover, blocking the perception or synthesis of ET severely decreases tomato and tobacco tolerance to cold, indicating that this hormone is necessary for a correct response to low temperature.Citation9 Furthermore, exogenous application of ACC, the ET precursor, significantly enhances tolerance to temperatures under 0°C in Arabidopsis, tomato and tobacco.Citation8,9 On the other hand, Yu and colleagues (2001) described that the activation of ET production elicited by the exposition to 4°C elevates the tolerance of winter rye to subzero temperatures through the accumulation of antifreeze proteins.
Recently, the analysis of ET-defective and -overproducer Arabidopsis mutants allowed us to demonstrate that ET has a key function as positive regulator of freezing tolerance and cold acclimation, an adaptive response by which plants increase their freezing tolerance after being exposed to temperatures between 10 and 4°C.Citation8 We also showed that ET participates in the reprograming of gene expression that undergoes when plants are exposed to low temperature, activating, among others, the CBF-mediated signaling pathway,Citation8 one of the main pathways controlling the cold acclimation response.Citation10 Interestingly, our results supported a prominent role in this pathway for the Arabidopsis Ψ 14-3-3 isoform RCI1A in controlling the stability of ACC synthase (ACS), the key enzymes in ET biosynthesis.Citation8
To further characterize the role of ET in plant response to low temperature, we have analyzed the constitutive freezing tolerance of the Arabidopsis ethylene overproducer mutant eto1-3.Citation11 The eto1-3 mutation produces a truncate ETO1 protein that is unable of targeting ACS5 for proteasomal degradation, which consequently provokes an increase of ET in mutant plants.Citation12 As a first step, we determined whether under our standard growing conditions eto1-3 plants also displayed increased ET levels compared to the wild-type genotype. shows that, in fact, ET content in 2-week-old eto1-3 plants grown on soil under long day conditions at 20°C is higher than in wild-type plants. Then, we assessed the freezing tolerance of 2-week-old wild-type and eto1-3 plants grown under control conditions and subsequently exposed to different subzero temperatures during 6h. Tolerance was estimated as the percentage of survivals after one week of recovery at standard growing conditions. Results showed that eto1-3 mutants are significantly more tolerant to freezing than wild-type plants (). The determined LT50 (temperature that causes 50% of lethality) values were −6,4°C and −5,5°C, respectively ().
Figure 1. Arabidopsis eto1-3 mutant plants display enhanced constitutive tolerance to freezing temperatures. (A) Levels of ET, as determined by gas chromatography, in 3-week-old Col-0 (WT) and eto1-3 plants grown on soil under long day conditions at 20°C. Data are expressed as means of 3 independent experiments with 5 plants each. Bars indicate ±SD. Asterisks indicate significant differences (P < 0.05) with WT plants. (B) Freezing tolerance of 2-week-old Col-0 (WT) and eto1-3 plants grown on soil under long day conditions at 20°C and then exposed 6 hours to the indicated freezing temperatures. Freezing tolerance was estimated as the percentage of plants surviving each specific temperature after 7 days of recovery under control conditions. Data are expressed as means of 3 independent experiments with 50 plants each. Bars indicate ±SD. Asterisks indicate significant differences (P < 0.05) with WT plants. (C) Expression levels of CBF1, CBF2 and CBF3 genes, as determined by qPCR, in 2-week-old Col-0 (WT) and eto1-3 plants grown on soil under long day conditions at 20°C. Analyses were performed in triplicate with 3 independent RNA samples. Bars indicate ±SD. Asterisks indicate significant differences (P < 0.05) with WT plants. (D) Freezing tolerance of representative WT and eto1-3 plants 7 days after being exposed to −6°C for 6 hours (h).
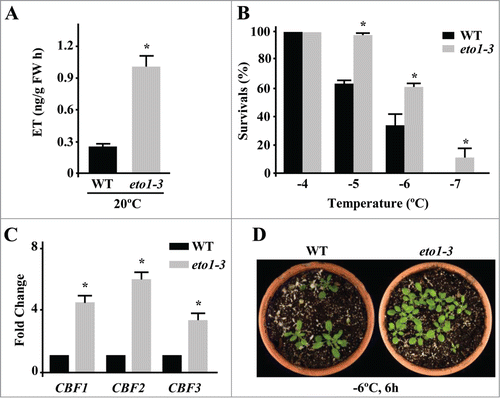
Genome wide transcriptomic analysis of Arabidopsis ACS octuple mutants, which have reduced ET content, unveiled that basal levels of this hormone are required for accurate constitutive expression of a number of cold-induced genes, including the CBF-target KIN1.Citation13 Moreover, we have reported that ET positively regulates the constitutive expression of CBF1 and CBF2, as well as that of several genes of the CBF-regulon.Citation8 Since eto1-3 plants have elevated levels of ET, we hypothesized that this mutant should have increased content of CBF transcripts. To test this possibility, the expression levels of CBF1, 2 and 3 were determined by qPCR in 2-week-old wild-type and eto1-3 plants growing under standard conditions. Results revealed that, as hypothesized, transcripts corresponding to all CBFs accumulate significantly more in the mutant than in wild-type plants (), which would account for its freezing tolerant phenotype. Consistent with these findings, we have described that an RCI1A null mutant of Arabidopsis displaying enhanced ET levels exhibits increased constitutive expression of CBF1 and CBF2, and augmented constitutive tolerance to subzero temperatures.Citation8 In contrast with the results described here, Shi and colleagues (2012) reported that eto1-3 plants display reduced tolerance to freezing temperatures. As already discussed in our previous work,Citation8 these differences must be due, in all likelihood, to the differences existing between our growing conditions (soil in pots) and those used by Shi and colleagues (2012) (MS plates). In conclusion, the characterization of eto1-3 freezing tolerance provides further genetic evidence hinting at the relevance of ET in the regulation of freezing tolerance through the control of the CBF gene expression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants BIO2010-17545 and BIO2013-47788-R from the Spanish Secretary of Research, Development and Innovation. R. Catalá is supported by a JAE-DOC contract from the CSIC.
References
- Klee HJ. Ethylene signal transduction. Moving beyond Arabidopsis. Plant Physiol 2004; 135:660-667; PMID:15208412; http://dx.doi.org/10.1104/pp.104.040998
- Field RJ. The effect of low temperature on ethylene production by leaf tissues of Phaseolus vulgaris L. Ann Bot 1981; 47:215-221
- Kacperska A, Kubacka-Zgbalska M. Is lipoxygenase involved in the formation of ethylene from ACC? Physiologia Plantarum 1985; 64:333-338; http://dx.doi.org/10.1111/j.1399-3054.1985.tb03349.x
- Harber RM, Fuchigami LH. Ethylene-induced stress resistance. 1989; Boca Raton: CRC Press.
- Ciardi JA, Deikman J, Orzolek MD. Increased ethylene synthesis enhances chilling tolerance in tomato. Physiologia Plantarum 1997; 101:333-340; http://dx.doi.org/10.1111/j.1399-3054.1997.tb01005.x
- Yu XM, Griffith M, Wiseman SB. Ethylene induces antifreeze activity in winter rye leaves. Plant Physiol 2001; 126:1232-1240; PMID:11457973; http://dx.doi.org/10.1104/pp.126.3.1232
- Wang H, Huang J, Liang X, Bi Y. Involvement of hydrogen peroxide, calcium, and ethylene in the induction of the alternative pathway in chilling-stressed Arabidopsis callus. Planta 2012; 235:53-67; PMID:21814799; http://dx.doi.org/10.1007/s00425-011-1488-7
- Catalá R, Lopez-Cobollo R, Castellano MM, Angosto T, Alonso JM, Ecker JR, Salinas J. The Arabidopsis 14-3-3 Protein RARE COLD INDUCIBLE 1A Links Low-Temperature Response and Ethylene Biosynthesis to Regulate Freezing Tolerance and Cold Acclimation. Plant Cell 2014; PMID:25122152; http://dx.doi.org/10.1105/tpc.114.127605
- Zhang Z, Huang R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol Biol 2010; 73:241-249; PMID:20135196; http://dx.doi.org/10.1007/s11103-010-9609-4
- Medina J, Catala R, Salinas J. The CBFs: three Arabidopsis transcription factors to cold acclimate. Plant Sci 2011; 180:3-11; PMID:21421341; http://dx.doi.org/10.1016/j.plantsci.2010.06.019
- Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 1990; 2:513-523; PMID:2152173; http://dx.doi.org/10.1105/tpc.2.6.513
- Wang KL, Yoshida H, Lurin C, Ecker JR. Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature 2004; 428(6986):945-50; PMID:15118728; http://dx.doi.otg/10.1038/nature02516
- Tsuchisaka A, Yu G, Jin H, Alonso JM, Ecker JR, Zhang X, Gao S, Theologis A. A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 2009; 183:979-1003; PMID:19752216; http://dx.doi.org/ 0.1534/genetics.109.107102
- Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell 2012; 24:2578-2595; PMID:22706288; http://dx.doi.org/10.1105/tpc.112.098640
