Abstract
NRT1.1 is a dual-affinity nitrate (NO3−) transporter involved in both high- and low-affinity NO3− uptake in Arabidopsis plants. In a recent study, we showed that, under cadmium (Cd) exposure, blocking the NRT1.1-mediated NO3− uptake reduces Cd entry into roots, thus lowing Cd levels in plants and improving plant growth. In addition, we also found that the Cd levels in edible parts of 11 Chinese cabbage (Brassica rapa L. ssp. pekinensis) cultivars correlated well with the NO3− uptake rates of their roots. These results suggested that the NO3− uptake of roots negatively regulate Cd uptake. Modification of NO3− uptake in crops by modulating NO3− uptake pathway might provide a biological engineering approach to reducing Cd accumulation in edible organs, thus improving food safety.
Cadmium (Cd) is highly toxic to humans. A survey showed that vegetables and rice account for almost 80% of total Cd exposure in residents of Shanghai, China's largest city.Citation1 Several strategies have been developed to minimize Cd uptake by crops from Cd concentration soil. These strategies mainly include: (1) ‘dig-and-dump’ or encapsulation of the contaminated soil, (2) chemical immobilization or extraction of Cd, and (3) phytoremediation by Cd-hyperaccumulating plants.Citation2 In addition, researchers also throw the lights to agronomic measures to minimize the Cd entry into crops, as several of the plant nutrients have many effects on Cd's availability and Cd uptake,Citation3 such as phosphate precipitating Cd2+ in soil,Citation4 Fe2+ competing with Cd2+ for IRON-REGULATED TRANSPORTER 1(IRT1).Citation5,6 Recently, we found that, in the hydroponical growth condition, the NO3− treatment resulted in higher Cd concentration and less Cd tolerance in tomato plants (Solanum lycopersicum) compared with ammonium (NH4+) treatment.Citation7 A similar result was also observed in hydroponically grown Arabidopsis (Arabidopsis thaliana) plants (). Therefore, appropriate use of nitrogen (N) fertilizers might provide a relatively inexpensive, time-saving, and effective strategy for reducing Cd entry into, and accumulation in crop. However, in another study, we found that the actual effect of the N form on Cd accumulation of the plants grown with soil was strongly associated with the pH-buffering capacity of the soil. In soil with higher pH-buffering capacity, the effect of N-form on Cd accumulation in plants was similar to that observed in the hydroponically grown plants, but in soil with lower pH-buffering capacity, application of NH4+ resulted in higher Cd levels in plants than application of NO3−, probably as a result of soil acidification by rapid nitrification of NH4+ (S.K. Fan, S.T. Du, C.W. Jin; unpublished data). This finding suggests that it could pose higher risk and difficulty to employ N-fertilization management to prevent Cd entry into crops since the pH-buffering capacity of soil varies greatly in different regions.
Figure 1. Effect of N-form on growth and Cd concentration in Arabidopsis Col-0 plants grown with solution medium. Effect of N-form on growth and Cd concentration in Arabidopsis Col-0 plants grown with solution medium. (A) and (B) The relative biomass. (C) and (D) The Cd concentration in plants from Cd treatment. At the age of 5 weeks, the plant weere transferred to Cd-free or 10 μM Cd-added growth solutions with either NO3− or NH4+ as the sole nitrogen source. The shoots and roots of plants after 7 d of treatments were harvested for biomass and Cd concentration measurements. The relative biomass was calculated as the mean of fresh weight relative to the control treatment. Error bars represent standard deviations (n = 5–8). n refers to number of biological replicates
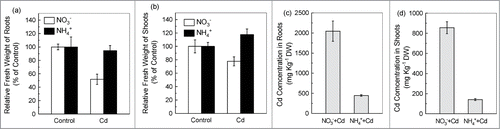
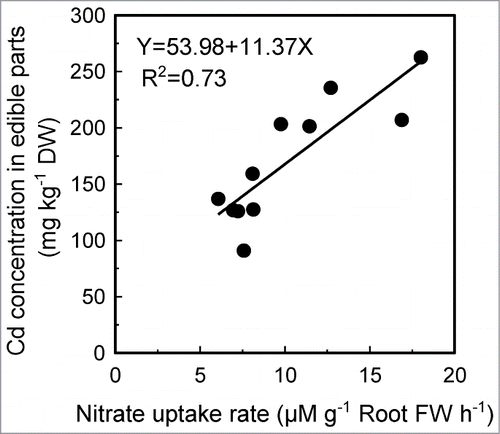
Most recently, we found that, in Arabidopsis plants, exposure of Cd reduces NO3− uptake of roots by inhibiting the activity of NITRATE TRANSPORTER 1.1 (NRT1.1), but this process, in turn, reduces Cd entry into the root cells. As a result, Cd level in plants is lower and plant growth is improved.Citation8 Therefore, inhibition of NRT1.1-mediated NO3− uptake could be recognized as a mechanism of plants for resisting Cd toxicity. This mechanism may provide a new strategy for minimizing Cd accumulation in crops grown in contaminated soil using biotechnological pathways to decrease NO3− uptake. Interestingly, in 11 Chinese cabbage (Brassica rapa L. ssp. pekinensis) cultivars, we found that their Cd levels in edible parts (shoots) correlated well with the NO3− uptake rates of roots (). The result indicates that, in addition to engineering or breeding of crops, screening the cultivar with low NO3− uptake rate in its roots may be also an available strategy for decreasing Cd accumulation in crops.
Figure 2. Correlation between NO3− uptake rate and Cd concentration in edible parts in 11 Chinese cabbage cultivars. For measurement of NO3− uptake rate, roots of hydroponically grown plants were immersed in 100 mL of a constantly agitated and aerated solution containing 0.5 mM CaSO4 and 2 mM KNO3, and the net uptake of NO3 − was measured as NO3 − depletion from the solution per unit of time. The Cd concentration in edible parts was measured in plants grown with soil containing Cd at rate of 10 mg kg−1 soil.
Because Cd is a non-essential element for plants, Cd2+ entry into root cell may rely on the transporters/channels used for acquiring bivalent cation.Citation9-11 Although knockout of those bivalent nutrient transporters, such as Fe2+ transporter AtIRT1 in Arabidopsis and Mn2+ transporter osNRAMP5 in rice, could also decrease Cd accumulation in plant tissues, but they often strongly inhibited biomass production, because these transporters are the primary determinants in uptake of relative bivalent nutrients from growth medium.Citation6,12 Therefore, biotechnological modification of these bivalent nutrient transporters to decrease Cd accumulation in crops seems not to be a wise strategy. However, the N acquisition of plants is controlled by several transporters. For example, in Arabidopsis plant, 5 NRTs and 4 NH4+ transporters (AMTs) have been currently indentified to be involved in N uptake of roots from growth medium.Citation13-16 This concept allows us to assume that the N acquisition in the plants with inhibited/blocked a certain N uptake process, may be complemented by the other N uptake processes. This is probably the reason why blocking the NRT1.1-controlled NO3− uptake only slightly affected on the plant growth in the growth condition or our previous study.Citation8 Therefore, biotechnological modifying of NO3− uptake pathway to decrease Cd entry into crop, not only can circumvent both the risk and the difficulty arisen by the N fertilizer management but also may be interfere less with crop production.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was financially supported by the Zhejiang Province Natural Science Foundation (LR13C130001 and LY14C130001), the Natural Science Foundation of China (31270041) and the Fundamental Research Funds for the Central Universities (2014QNA6006).
References
- He P, Lu Y, Liang Y, Chen B, Wu M, Li S, He G, Jin T. Exposure assessment of dietary cadmium: findings from shanghainese over 40 years, China. BMC Public Health 2013; 13:590; PMID:23773573; http://dx.doi.org/10.1186/1471-2458-13-590
- Pulford D, Watson C. Phytoremediation of heavy metal-contaminated land by trees-a review. Environm Int 2003; 29:529-40; PMID:12705950; http://dx.doi.org/10.1016/S0160-4120(02)00152-6
- Sarwar N, Saifullah, Malhi SS, Zia MH, Naeem A, Bibi S, Farid G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 2010; 90(6):925-37; PMID:20355131; http://dx.doi.org/10.1002/jsfa.3916
- Bolan NS, Adriano DC, Duraisamy P, Mani A, Arulmozhiselvan K. Immobilization and phytoavailability of cadmium in variable charge soils. I. Effect of phosphate addition. Plant Soil 2003; 250:83-94; http://dx.doi.org/10.1023/A:1022826014841
- Connolly EL, Fett JP, Guerinot ML. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 2002; 14:1347-57; PMID:12084831; http://dx.doi.org/10.1105/tpc.001263
- Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and plant growth. Plant Cell 2002; 14:1223-33; PMID:12084823; http://dx.doi.org/10.1105/tpc.001388
- Luo BF, Du ST, Lu KX, Liu WJ, Lin XY, Jin CW. Iron uptake system mediates nitrate-facilitated cadmium accumulation in tomato (Solanum lycopersicum) plants. J Exp Bot 2012; 63:3127-36; PMID:22378950; http://dx.doi.org/10.1093/jxb/ers036
- Mao QQ, Guan MY, Lu KX, Du ST, Fan SK, Lin XY, Jin CW. Inhibition of NRT1.1-controlled nitrate uptake reduces cadmium uptake in Arabidopsis. Plant Physiol 2014; 166:934-44; PMID:25106820; http://dx.doi.org/10.1104/pp.114.243766
- Verbruggen N, Hermans C, Schat H. Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 2009; 12:364-72; PMID:19501016; http://dx.doi.org/10.1016/j.pbi.2009.05.001
- Lux A, Martinka M, Vaculík M, White PJ. Root responses to cadmium in the rhizosphere: a review. J Exp Bot 2011; 62:21-37; PMID:20855455; http://dx.doi.org/10.1093/jxb/erq281
- Clemens S, Aarts MGM, Thomine S, Verbruggen N. Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 2013; 18:92-9; PMID:22981394; http://dx.doi.org/10.1016/j.tplants.2012.08.003
- Sasaki A, Yamaji N, Yokosho K, Ma JF. Nramp5 Is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012; 24:2155-67; PMID:22589467; http://dx.doi.org/10.1105/tpc.112.096925
- Yuan L, Loqué D, Kojima S, Rauch S, Ishiyama K, Inoue E, Takahashi H, von Wirén N. The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell 2007; 19:2636-52; PMID:17693533; http://dx.doi.org/10.1105/tpc.107.052134
- Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake, assimilation and remobilisation in plants: challenges for sustainable and productive agriculture. Ann Bot 2010; 105:1141-57; PMID:20299346; http://dx.doi.org/10.1093/aob/mcq028
- Wang YY, Hsu PK, Tsay YF. Uptake, allocation and signaling of nitrate. Trends Plant Sci 2012; 17:458-67; PMID:22658680; http://dx.doi.org/10.1016/j.tplants
- Léran S, Varala K, Boyer J-C, Chiurazzi M, Crawford N, Daniel-Vedele F, David L, Dickstein R, Fernandez E, Forde B, et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci 2014; 19:5-9; PMID:24055139; http://dx.doi.org/10.1016/j.tplants.2013.08.008
